Introduction
Acute respiratory distress syndrome (ARDS) is a
life-threatening condition with non-cardiogenic permeability
pulmonary edema characterized by the increased permeability of
pulmonary capillary endothelial cells and alveolar epithelial
cells, resulting in inflammation, hypoxemia and multiple organ
failure (1,2). Mortality rates from ARDS are estimated
to be 34–64%; however, with proper treatment, the mortality rate
from ARDS may be reduced to ~25% (2,3).
Numerous studies (4–7) have
suggested that an excessive inflammation reaction may be
responsible for this apparent mortality. The main causes of
inflammation are inflammatory cytokines, including interleukin-1
(IL-1), IL-6, tumor necrosis factor-α (TNF-α), C-reactive protein
(CRP) and other factors (7). The
release of these elevated cytokines contribute to the development
of ARDS. Given that ARDS is associated with acute pulmonary
inflammation, it is also expected to improve treatment outcomes of
anti-inflammation therapies. However, the results of clinical
studies using glucocorticoid or corticosteroid to cure ARDS remain
controversial.
Although glucocorticoids have a powerful capability
in suppressing the inflammatory process (8,9), this
effect is correlated with the magnitude and duration of
inflammation (7,10,11).
Following the administration of glucocorticoid agents, patients who
exhibited a significant reduction in TNF-α and IL-1 usually
exhibited an improved outcome (12,13);
whereas persistent elevation of inflammatory cytokines predicted a
poor outcome in patients with ARDS (11). These observations lead researchers to
questions when and what doses of glucocorticoid should be used in
the treatment of ARDS. Several reviews have investigated these
problems (7,12,14,15). One
review demonstrated that corticosteroids did not significantly
reduce hospital mortality when pooling across all trials (12); whereas another study reported that it
was not clear whether a lower dose of glucocorticoid for persistent
ARDS could reduce mortality in the long-term (16).
The present study aimed to determine the effects of
different doses and application times of glucocorticoid on the
mortality of patients with ARDS by conducting a meta-analysis of
previous randomized control trials.
Materials and methods
Literature search
All studies of interest were identified from
electronic databases, including MEDLINE (https://www.nlm.nih.gov/bsd/pmresources.html),
Embase (https://www.elsevier.com/solutions/embase-biomedical-research),
Cochrane for English literature (http://www.cochranelibrary.com/), and CNKI (http://www.cnki.net/) and VIP database for Chinese
literature up to November 2013. The following terms were used:
Glucocorticoid, corticosteroid, methylprednisolone, hydrocortisone,
acute respiratory distress, adult respiratory distress, ARDS, acute
respiratory distress syndrome and acute lung injury. In addition to
the randomized controlled trials, published reviews and relevant
bibliographies from meetings such as the American Thoracic Society,
Society of Critical Care Medicine, and the International Society of
Intensive Care and Emergency Medicine were also searched and
reviewed.
Inclusion and exclusion criteria
To avoid potential bias, the following inclusion
criteria were used: i) Designed as randomized controlled trials;
ii) participants aged ≥18 years old with a clear diagnosis of ARDS
regardless of etiology, race, nation, and sex; iii) trials should
include an intervention arm receiving glucocorticoid treatment and
a control arm receiving placebo or standard care. The dose and
cycles of the glucocorticoid treatment and data of mortality were
presented in published literature or available from authors. The
exclusion criteria were as follows: (i) Animal, infant or children
study subjects; ii) glucocorticoid therapy used to prevent the
occurrence of ARDS but not to treat it; iii) definitive diagnosis
standards of ARDS were not presented during the trials; and iv)
detailed information about glucocorticoids, including the
intervention period, dose and duration of administration were not
provided in the literature.
Data extraction and quality
assessment
Population data, number of participants,
glucocorticoid administration route, mortality and other endpoints
were independently extracted from the studies by two reviewers, and
a third reviewer was introduced when disagreements occurred.
Important missing data relevant to the study design was also
sought, including the blinding method used. Subgroup analyses
regarding the dose and duration of glucocorticoid in the treatment
of ARDS was also conducted, since the dose and duration of
glucocorticoid may be important factors that influence outcomes.
The early period of ARDS was defined as the first seven days of the
diagnosis of ARDS, and the late period as the seven days following
diagnosis of ARDS, as described by Wajanaponsan et al
(17). Low-dose or high-dose therapy
was defined according to previous studies (12,18,19).
Quality assessment of the evidence eligible for the
present meta-analysis was performed by reviewers using a scoring
method from a previous study (20).
Differences in scoring were resolved by consensus and the extracted
data were recorded carefully and independently verified by two
investigators prior to the meta-analysis.
Data analysis
Relevant data extracted and confirmed by two authors
were used for the meta-analysis. All data were typed using Review
Manager 5.2 software provided by the Cochrane Collaboration Group
(Herlev, Denmark). For dichotomous data, the Mantel-Haenszel method
was used to estimate the risk ratio (RR) with a 95% confidence
interval (CI). The inverse variance method was applied to analyze
continuous data and the mean differences had 95% CIs.
Statistical analysis
In order to reduce the heterogeneity of the included
studies, the heterogeneity of the clinic and methodology were
assessed preferentially. In order to test statistical
heterogeneity, the χ2 test was introduced. P<0.01 was
used to indicate a statistically significant difference. The
I2 test was used to evaluate the pooled variation
between all of the eligible trials. A random-effects model or a
fixed-effects model was used to perform meta-analysis, and were
selected according to the significance of the heterogeneity, as
described previously (21). In
addition, bias risk was also assessed based on the standards
reported in the Cochrane Handbook (21). Statistical tests were performed using
Stata software, version 12.0 (StataCorp LLC, College Station, TX,
USA).
Results
Study flow
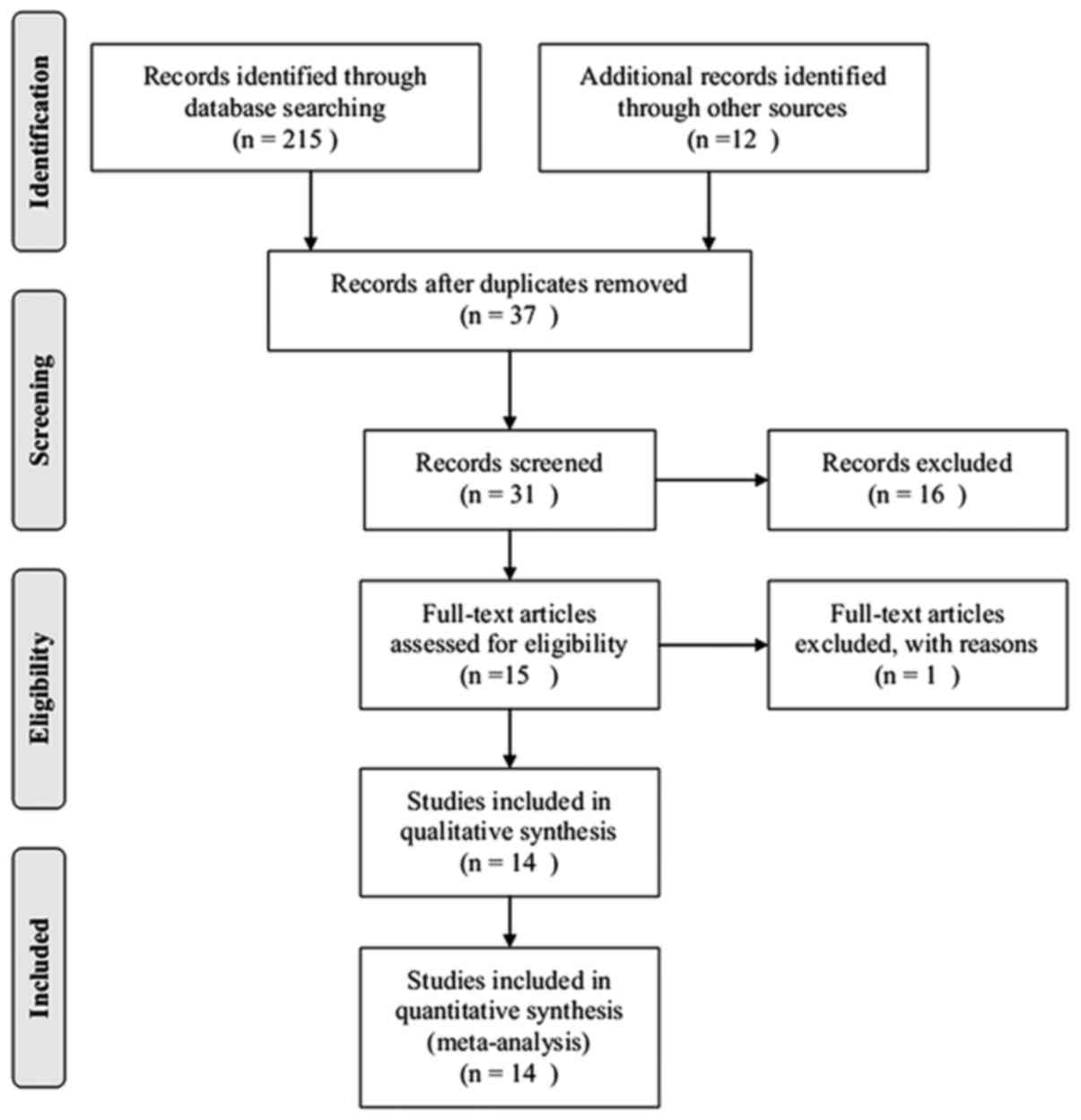
By limiting the search to human clinical studies and
excluding animal studies and basic research, 227 articles were
determined to be relevant to the topic of acute respiratory
distress syndrome and glucocorticoids, and 190 citations were
excluded after reviewing the titles and abstracts. The remaining 37
articles were reviewed in full and 14 articles (22–34) were
included for further review. In these 14 studies, two trials
(31,33) did not provide mortality data in the
text and, although the authors were contacted by e-mail, this data
remained unavailable. A total of 14 articles were eligible for the
final meta-analysis. A flowchart of the present meta-analysis is
presented in Fig. 1.
Characteristics and quality of the
studies included
According to the inclusion and exclusion criteria,
there were 14 articles (18,22–34),
included in the final analysis, two of which were Chinese (30,34). The
basic characteristics and quality of the studies included are
presented in Tables I and II. A total of 1,441 patients with ARDS
were included, with 774 cases in the treatment group and the
remaining 667 in the control. Standard care, mechanical
ventilation, and other supportive care was applied to patients in
both groups. Nine studies (24–30,34) used
low-dose therapy and high-dose therapy was studied in three trials
(22,23,32).
There were 11 studies (18,22–26,29,30,32–34) that
defined as glucocorticoid intervention at the early phase of ARDS
onset, and two studies (27,28) were considered to treat ARDS with
glucocorticoid during the late period. Furthermore, two studies
(27,28) provided data for the early and late
treatment of ARDS. Illness severity scores were not applied in the
present study.
 | Table I.Characteristics of studies
included. |
Table I.
Characteristics of studies
included.
|
| Numbers of
participants |
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Study | Treatment | Control | Number of deaths
(treatment/control) | Treatment time
(Phase of ARDS) | Regimen | Article type | Refs. |
|---|
| Hwangbo et
al | 23 | 8 | 3/7 | Early phase | Methylprednisolone,
8 mg kg−1d−1 for 7 days, low-dose | Not RCT | 18 |
| Weigelt et
al | 39 | 42 | 18/13 | Early phase | Methylprednisolone,
120 mg kg−1d−1 for 2 days, high-dose | RCT | 22 |
| Bernard et
al | 50 | 49 | 30/31 | Early phase | Methylprednisolone,
120 mg kg−1d−1 for 1 day, high-dose | RCT | 23 |
| Marik et
al | 14 | 16 | 1/3 | Early phase | Hydrocortisone, 10
mg/kg, once, low-dose | RCT | 24 |
| Confalonieri et
al | 23 | 23 | 0/7 | Early phase | Hydrocortisone, 240
mg/d for 7 days, low-dose | RCT | 25 |
| Annane et
al | 85 | 92 | 45/62 | Early phase | Hydrocortisone, 200
mg/d for 7 days, low-dose | RCT | 26 |
| Steinberg, et
al | 89 | 91 | 18/24 | Early and late
phase | Methylprednisolone,
2 mg kg−1d−1 for 25 days, low-dose | RCT | 27 |
| Meduri et
al | 63 | 28 | 15/12 | Early and late
phase | Methylprednisolone,
1 mg kg−1d−1 for 28 days, low-dose | RCT | 28 |
| Mikami et
al | 15 | 16 | 1/0 | Early phase | Prednisolone, 40 mg
for 3 days, low-dose | RCT | 29 |
| Wan et
al | 38 | 43 | 5/3 | Early phase | Dexamethasone, 1 mg
kg−1d−1 for 3 days, low-dose | RCT | 30 |
| Ferguson et
al | 135 | 88 | Not available | Early phase | Corticosteroids,
not available | RCT | 31 |
| Azevedo et
al | 133 | 133 | 20/40 | Early phase | Methylprednisolone,
1 g for 3 days, high-dose | RCT | 32 |
| Seam et
al | 55 | 24 | Not reported | Early phase | Methylprednisolone,
not available | RCT | 33 |
| Liu et
al | 12 | 14 | 2/7 | Early phase | Hydrocortisone, 300
mg/d for 7 days, low-dose | RCT | 34 |
 | Table II.Quality assessment of studies
included. |
Table II.
Quality assessment of studies
included.
| Study | Randomization | Allocation | Blinding | No baselines
heterogeneity | Clear criteria of
inclusion and exclusion | Clear regimen | Clear
endpoints | Follow-up | Total score | Refs. |
|---|
| Hwangbo et
al | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 5 | 18 |
| Weigelt et
al | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 6 | 22 |
| Bernard et
al | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | 23 |
| Marik et
al | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 6 | 24 |
| Confalonieri et
al | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 6 | 25 |
| Annane et
al | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | 26 |
| Steinberg et
al | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | 27 |
| Meduri et
al | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 5 | 28 |
| Mikami et
al | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 5 | 29 |
| Wan et
al | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 6 | 30 |
| Ferguson et
al | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 5 | 31 |
| Azevedo et
al | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | 32 |
| Seam et
al | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 6 | 33 |
| Liu et
al | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 5 | 34 |
As shown in Table
II, the quality of the studies included was acceptable for the
comparison of the effect of glucocorticoid therapy on the mortality
of ARDS, although some studies (18,25,29–31,34)
failed to provide a clear method of blinding, and a few studies
(18,24,34)
revealed limitations in the sample size, anti-inflammatory agents
and in the risk of bias of glucocorticoid stopping for perceived
improvement.
Meta-analysis
Initially, a meta-analysis of the mortality,
incident infection, days remained alive and off mechanical
ventilation, lung injury scores, multiple organ failure and
PaO2/FiO2 ratio was planned. However, due to
the limited data available, only a pooled analysis of the
mortality, incident infection and days alive and off mechanical
ventilation was conducted. Subgroup analysis of the mortality data
accordingly to the different doses and duration of glucocorticoid
therapy was also included.
Effect of glucocorticoid on mortality
in ARDS patients
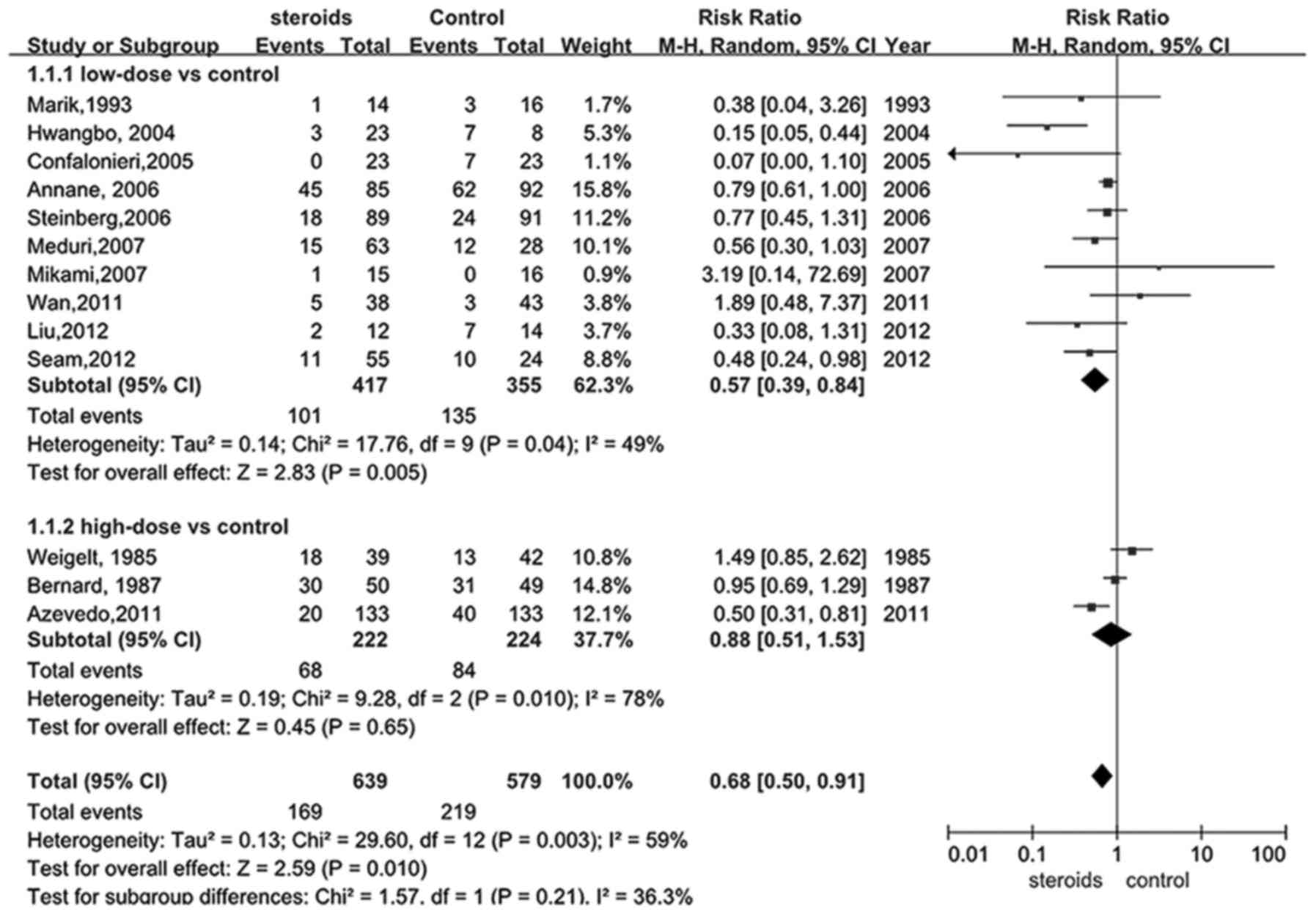
A total of 12 trials (18,22–30,32,34) were
selected to assess whether glucocorticoid treatment was beneficial
to patients with ARDS by reducing mortality. The main findings are
depicted in Fig. 2, estimating a
value of 0.68 for the RR of overall mortality (95% CI, 0.50–0.91).
Supported by the pooled analysis, a significant difference was
identified in the glucocorticoid intervention group for lowering
the overall mortality when compared with the control group
(P<0.05).
As previously described, studies of low-dose and
high-dose glucocorticoid treatment with a threshold value of 2
mg/kg/d were identified. As presented in Fig. 2, there were 9 articles (18,24–30,34)
comparing the impact of low-dose treatment of glucocorticoid on
mortality rates of ARDS patients with the controls. Significantly
reduced mortality was identified in the low-dose intervention group
compared with the control, and the combined RR was 0.57 with a 95%
CI between 0.39 and 0.84 (P<0.05), indicating that treating ARDS
with low-dose administration of glucocorticoid may sufficiently
decrease the mortality of ARDS by a relative ratio of 0.57.
However, this result was no longer beneficial when comparing
low-dose glucocorticoid with the control in late steroid rescue
studies (P>0.05). Only three articles (22,23,32)
studied the effect of high-dose glucocorticoid on the mortality
rates of ARDS, and the pooled data (Fig.
2) failed to support the hypothesis that high-dose
glucocorticoid was able to significantly benefit ARDS patients,
though a plausible trend was identified (RR, 0.88; 95% CI,
0.51–1.53, P>0.05).
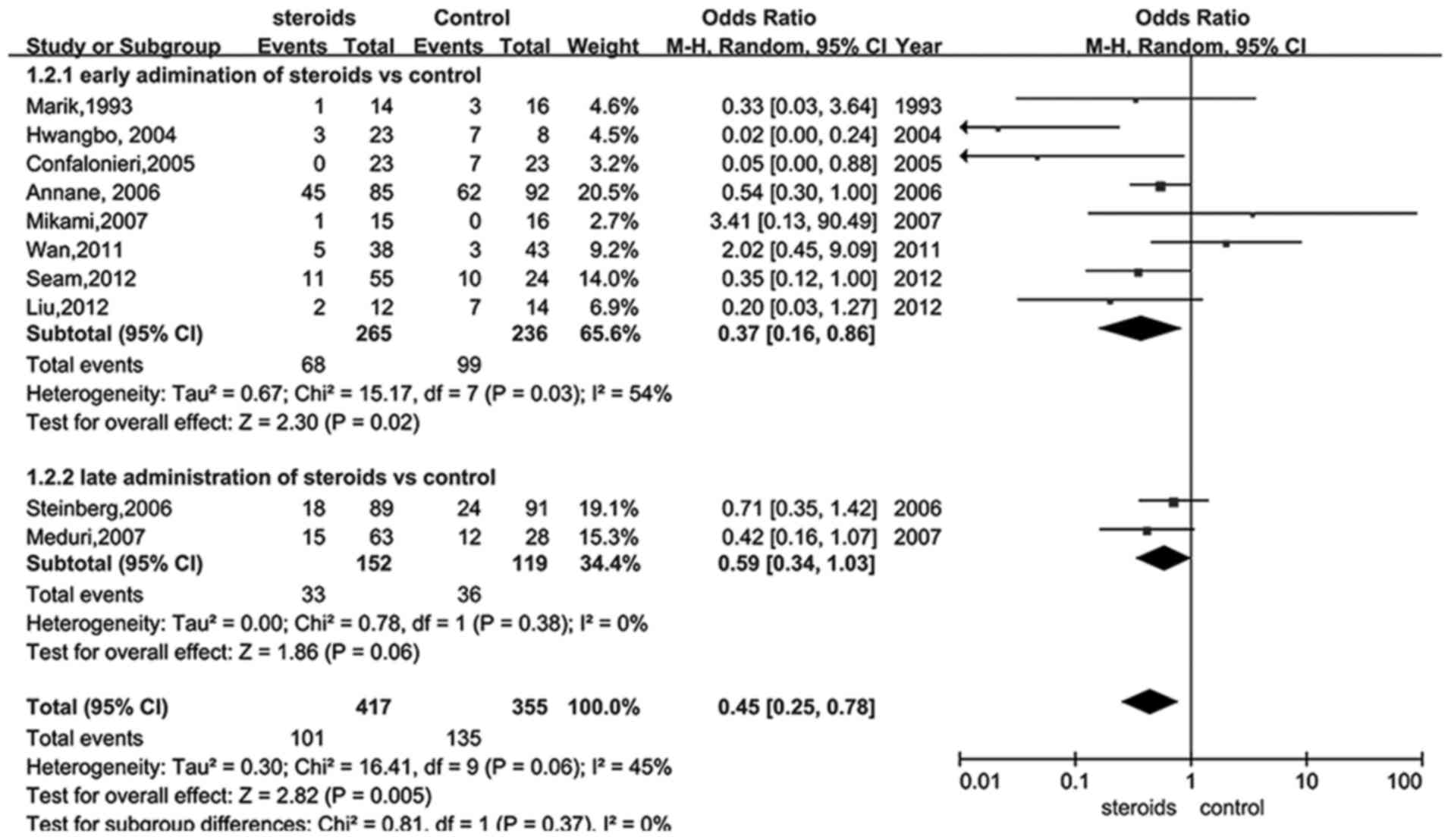
Given that the administration time of glucocorticoid
was able to influence the outcome, a subgroup analysis of
administration of glucocorticoid at early and late periods of ARDS
was performed using the data from 13 trials (18,22–30,32–34).
Data presented in Fig. 3 suggested a
significant reduction of mortality in the early treatment group
(RR, 0.37; 95% CI, 0.16–0.86, P<0.05), whereas glucocorticoid
therapy during the late period of ARDS was demonstrated to be
insufficient to significantly reduce mortality (RR, 0.59; 95% CI,
0.34–1.03, P>0.05). Furthermore, sensitivity analysis indicated
that the low dose of glucocorticoid provided an improved outcome
when excluding studies of low quality (RR, 0.40; 95% CI, 0.21–0.74,
P=0.004).
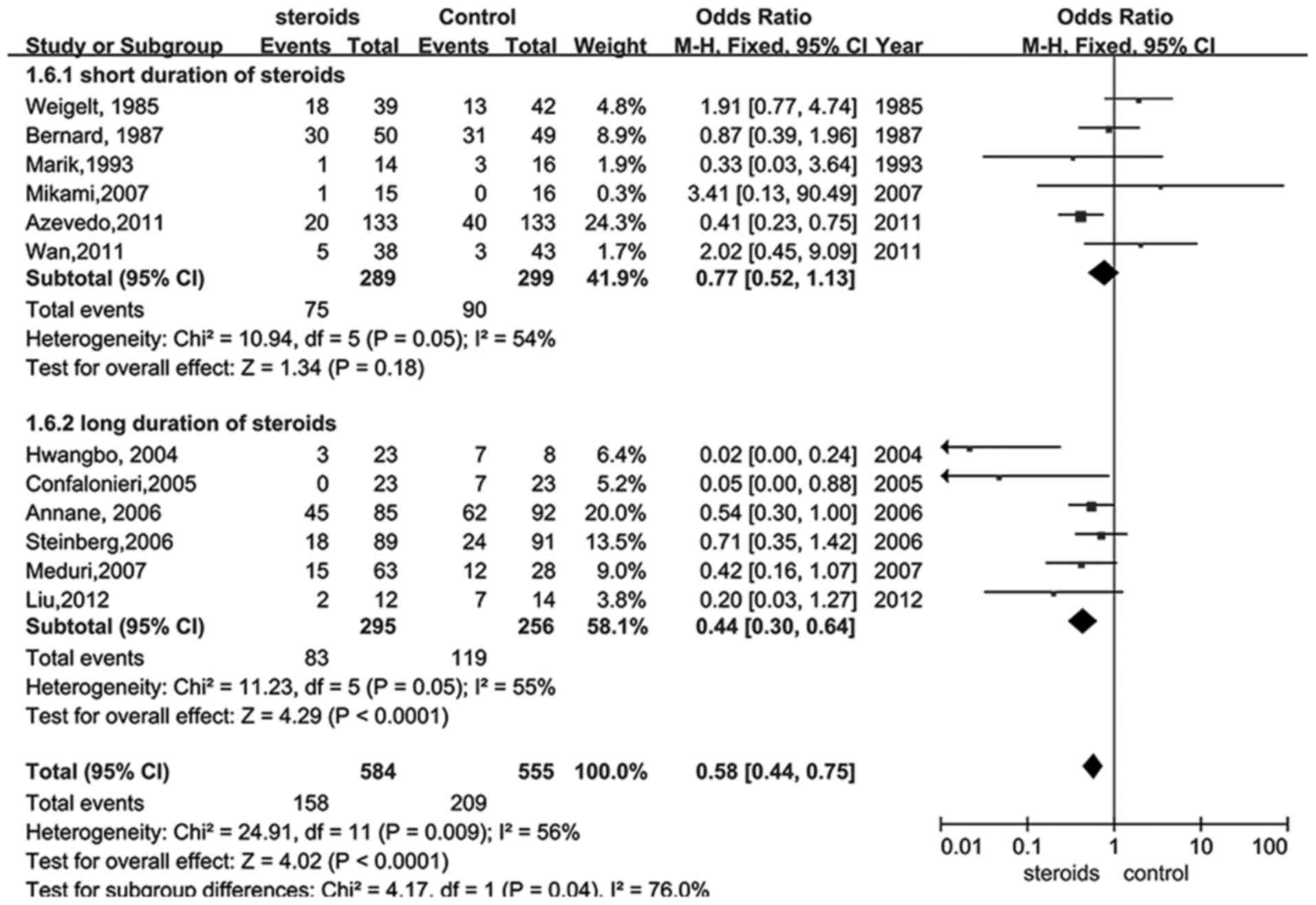
Glucocorticoid therapy was also analyzed with regard
to the bias of glucocorticoid termination for perceived benefit. A
total of 13 trials (18,22–30,32–34) were
selected in which participants received glucocorticoid
administration for 7 days or less and compared with those that
received treatment for more than 7 days. The results illustrated
that a longer duration of treatment with glucocorticoid provided a
better outcome (RR, 0.44; 95% CI, 0.30–0.64, P<0.05; Fig. 4), whereas shorter therapy did not
exhibit statistical significance (RR, 0.77; 95% CI, 0.52–1.13,
P>0.05; Fig. 4).
Effect of glucocorticoid treatment on
days remaining alive and off mechanical ventilation in patients
with ARDS
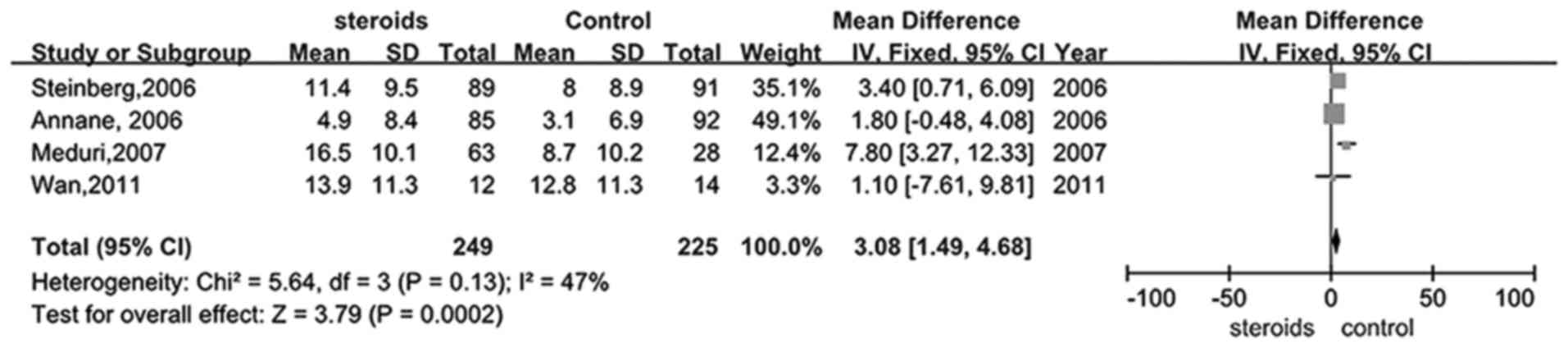
Five trials (26–28,30,33)
studied whether glucocorticoid therapy was able to increase the
number of days remaining alive and off mechanical ventilation in
patients with ARDS. Mechanical ventilation-free days were
significantly increased in the treatment group when compared with
the control (RR, 3.08; 95% CI, 1.49–4.68, P<0.05; Fig. 5).
Effect of glucocorticoid therapy on
the PaO2/FiO2 ratio in patients with
ARDS
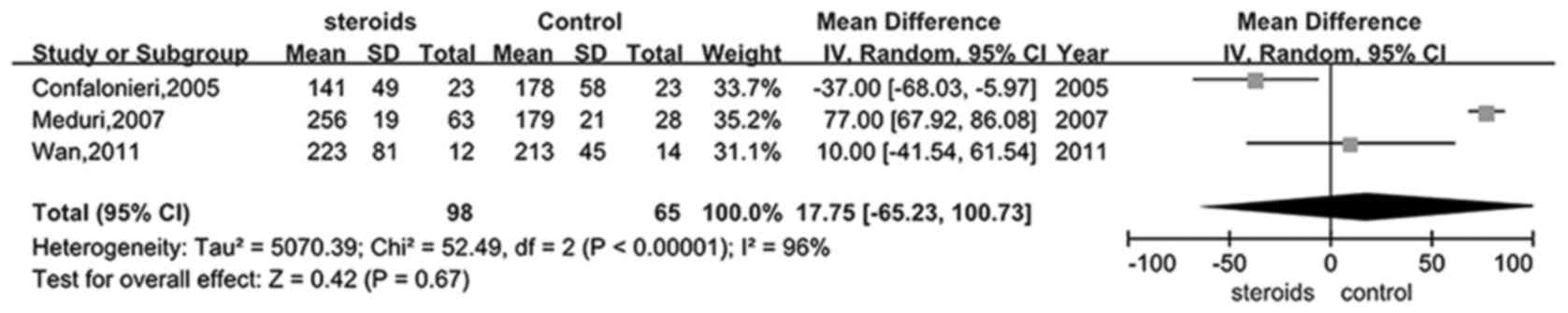
Only four trials (25,28,30,31)
provided information on the PaO2/FiO2 ratio
in the glucocorticoid and control groups. It was shown that there
was evident heterogeneity between the studies, therefore a random
model was selected to perform this analysis. Analysis of the
PaO2/FiO2 ratio in the treatment and control
groups did not exhibit a significant difference (RR, 17.75; 95% CI,
−65.23–100.73, P>0.05; Fig. 6),
favoring the application of glucocorticoid in the ARDS
treatment.
Effect of glucocorticoid treatment on
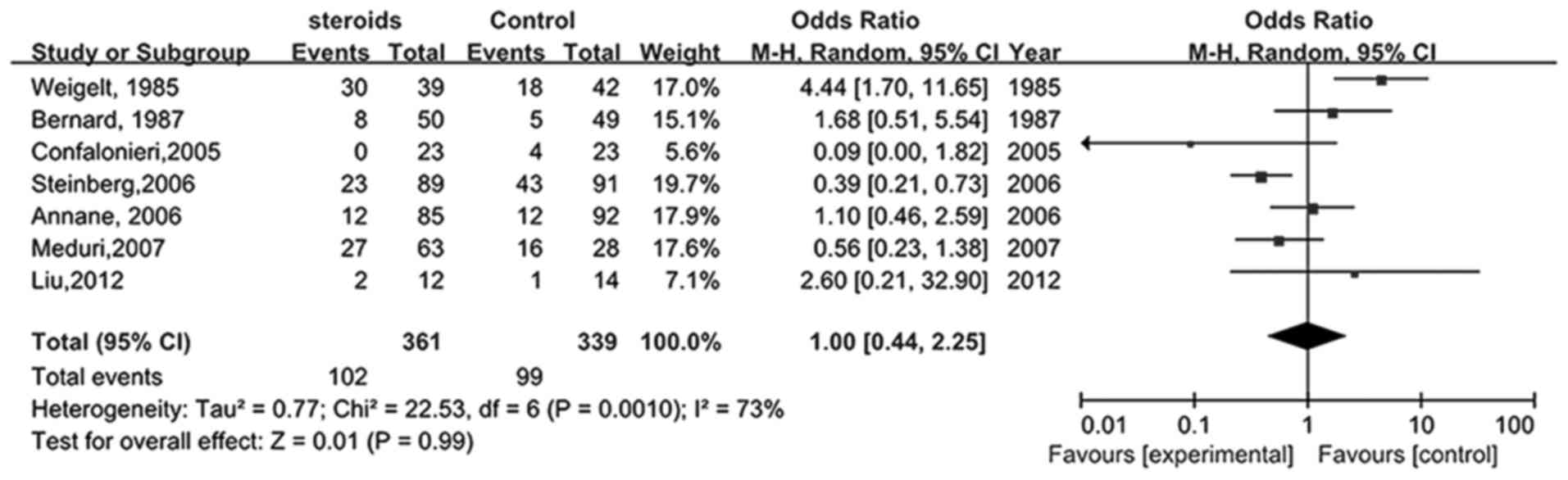
incident infections in patients with ARDS
Seven studies (22,23,25–28,34)
compared the influence of glucocorticoids on novel infections in
patients with ARDS. As shown in Fig.
7, the pooled RR was 1.00 (95% CI, 0.44–2.25), indicating that
the risk of infection for patients receiving glucocorticoid therapy
was not significantly different when compared with the control
group (P>0.05).
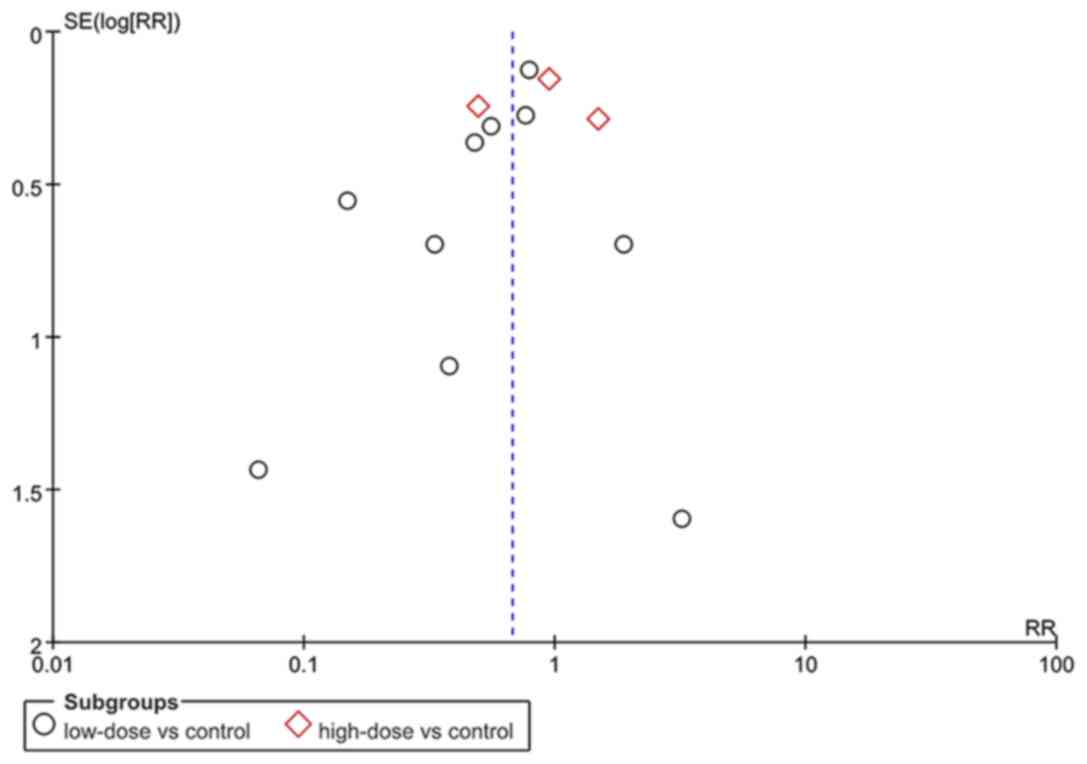
Publication bias
An analysis was also performed in order to evaluate
the possible publication bias in the studies included, and the
results were negative (Fig. 8).
Discussion
The present meta-analysis suggested that an evident
reduction in the mortality of patients with ARDS was observed
following low-dose glucocorticoid treatment during the early stages
of ARDS. A favorable outcome was identified in the subgroup
analysis of the effect of glucocorticoid on the 28-day mortality in
the treatment group. However, a high-dose of glucocorticoid and
administration of glucocorticoid during the late period of ARDS did
not significantly improve the outcome of patients with ARDS,
although trends were identified in favor of the glucocorticoid
treatment. The present study identified that the duration of
glucocorticoid therapy was a factor affecting the effect of
treatment as administration of glucocorticoid for >7 days
significantly reduced the mortality of ARDS patients. The results
also suggested that the number of days the patients remained alive
and off mechanical ventilation as well as the ratio of
PaO2/FiO2 improved after the administration
of glucocorticoid without significantly increasing the incidence of
infection after the onset of ARDS.
A number of reviews (7,12,16,20,35,36)
on the effect of therapeutic glucocorticoid in ARDS have been
published recently; however, the recommendations from these studies
did not reach a consensus. The meta-analysis performed by Peter
et al (20) included five
trials that assessed whether steroids were able to significantly
improve outcomes. The results demonstrated that steroid therapy was
associated with a clear trend towards reduced mortality.
Nevertheless, a definitive role of steroid use in treating ARDS was
not established. This result may be explained by the design of the
analysis as a subgroup analysis of mortality based on the dose and
duration of the therapy was not performed and the sample size of
the patients was relatively small. In addition, a review by
Thompson (16) investigated the role
of glucocorticoid in early and late ARDS, and indicated that
short-duration and high-dose of steroids in patients with or at
risk for ARDS revealed a trend for a worse outcome, whereas the
impact of a low-dose on the mortality rate in persistent patients
with ARDS was unclear. Another review by Thompson (35), indicated that a short duration of
high-dose glucocorticoid treatment was not effective for early
ARDS. These results should be viewed with caution due to the common
features of patients, treatment regimens and of the method of
measuring the outcomes (28-day mortality for example) of their
included studies, which all contributed to an increase in the bias.
By contrast, Lamontagne et al (12) performed a meta-analysis in order to
determine the impact of steroids on mortality rates from ARDS,
acute lung injury and severe pneumonia, and they concluded that
low-dose steroids administrated at the first two weeks of the
illness may reduce mortality. Furthermore, a study by Meduri et
al (36) included five trials
and concluded that prolonged glucocorticoid therapy significantly
improved the outcomes of patients with ARDS, and had a distinct
survival benefit in preventing ARDS if initiated properly. Another
review by Meduri et al (7)
demonstrated a similar result that glucocorticoid therapy was
associated with a significant risk reduction in mortality and
improved mechanical ventilation-free days. The findings of the
present meta-analysis were in accordance with the study by
Lamontagne et al (12),
although heterogeneity of study subjects existed. Low-dose
glucocorticoid and early administration was demonstrated to be
effective in improving the survival outcome and other factors
associated with lung function or treatment efficacy in patients
with ARDS. These findings indicated that a proper dose of
glucocorticoid at the beginning and even prior to the onset of ARDS
may provide a significant improvement not only in preventing the
development of ARDS but also in reducing ARDS-associated
mortality.
The apparent differential effect of an early and low
dose of glucocorticoid therapy in ARDS, which was observed in the
present study, has been reported previously (12). However, the underlying mechanism for
this condition remains unclear. As explained previously, cytokines
including TNF-α, IL-1 and IL-6 have been indicated to have a
pathophysiological role in the development of ARDS. Early
administration of glucocorticoid at a low-dose may suppress the
release of these factors and decrease their levels. However, to
date the efficacy of low-dose steroids for the remission of
inflammation and improvement of survival has not been fully
elucidated in ARDS (1). The optimal
time for administering glucocorticoid remains under investigation.
Steroid-associated benefits may start at the first two weeks of
ARDS whereas later administration may cause a loss of the benefits
of the steroids (37,38). In the studies included in the present
meta-analysis, the initial use of glucocorticoid ranged from <1
day to a few days after ARDS was diagnosed, making the treatment
outcome on the basis of different beginning time of treatment.
Glucocorticoid administration was not demonstrated
to increase the incidence of infection, however, a trend of an
increasing risk of infection was observed. Instead, glucocorticoid
administration increased the number of days the patients remained
alive and off mechanical ventilation and improved the
PaO2/FiO2 ratio, indicating an economical
benefit by decreasing the cost of supportive care as well as an
improved lung function of exchanging gas and providing oxygen for
the whole body. These observations indicate the efficacy of early
administration of low dose glucocorticoids in improving the
clinical outcomes without significantly increasing the incidence of
infection if they are used in the treatment of ARDS.
Although the present study attempted to avoid
possible bias and to reduce heterogeneity by selecting evidence and
performing this meta-analysis, there are still a few limitations
that remain. Firstly, only a low number of studies were used for
the analysis of mortality, incident infection rate and
PaO2/FiO2 ratio. In the subgroup analysis of
mortality a further reduced number of patients and data were
selected, thus increasing the risk of selective bias. Secondly,
apart from the definition of ARDS the agents used for treatment and
doses of agents were not consistent with the studies, thus
challenging the confirmed efficacy of glucocorticoid determined by
the present study. For the facilitation of the meta-analysis, a low
or high dose of treatment agent was introduced based on the
equality effect of steroids. This may underestimate or overestimate
the effect of steroids as it failed to adequately compare their
respective effects at exactly the same dose and agent. Thirdly, it
remains unclear whether glucocorticoids are safe as some studies
that were included presented limited information on the incidence
of infections. In summary, more randomized controlled trials with a
larger number of events are required in order to confirm the
results of the present study. Although there were limitations to
the present study, the present study was able to provide reliable
and clinically useful results.
In conclusion, early administration of low-dose
glucocorticoids during the early period of ARDS onset is
recommended based on reduced mortality, improvements in the
PaO2/FiO2 ratio and mechanical
ventilation-free days without increasing the risk of incident
infection.
References
|
1
|
Koh Y: Update in acute respiratory
distress syndrome. J Intensive Care. 2:22014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Del Sorbo L and Slutsky AS: Acute
respiratory distress syndrome and multiple organ failure. Curr Opin
Crit Care. 17:1–6. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guérin C, Reignier J, Richard JC, Beuret
P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, et
al PROSEVA Study Group, : Prone positioning in severe acute
respiratory distress syndrome. N Engl J Med. 368:2159–2168. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mondrinos MJ, Kennedy PA, Lyons M,
Deutschman CS and Kilpatrick LE: Protein kinase C and acute
respiratory distress syndrome. Shock. 39:467–479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carnesecchi S, Pache JC and
Barazzone-Argiroffo C: NOX enzymes: Potential target for the
treatment of acute lung injury. Cell Mol Life Sci. 69:2373–2385.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carnesecchi S, Dunand-Sauthier I, Zanetti
F, Singovski G, Deffert C, Donati Y, Cagarelli T, Pache JC, Krause
KH, Reith W and Barazzone-Argiroffo C: NOX1 is responsible for cell
death through STAT3 activation in hyperoxia and is associated with
the pathogenesis of acute respiratory distress syndrome. Int J Clin
Exp Pathol. 7:537-551.eCollection 20142014.
|
|
7
|
Meduri G, Bell W, Sinclair S and Annane D:
Pathophysiology of acute respiratory distress syndrome.
Glucocorticoid receptor-mediated regulation of inflammation and
response to prolonged glucocorticoid treatment. Presse Med.
40:e543-e5602011.
|
|
8
|
Vandevyver S, Dejager L, Tuckermann J and
Libert C: New insights into the anti-inflammatory mechanisms of
glucocorticoids: An emerging role for
glucocorticoid-receptor-mediated transactivation. Endocrinology.
154:993–1007. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nixon M, Andrew R and Chapman KE: It takes
two to tango: Dimerisation of glucocorticoid receptor and its
anti-inflammatory functions. Steroids. 78:59–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Headley AS, Tolley E and Meduri GU:
Infections and the inflammatory response in acute respiratory
distress syndrome. Chest. 111:1306–1321. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meduri GU, Headley S, Kohler G, Stentz F,
Tolley E, Umberger R and Leeper K: Persistent elevation of
inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1
beta and IL-6 levels are consistent and efficient predictors of
outcome over time. Chest. 107:1062–1073. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lamontagne F, Briel M, Guyatt GH, Cook DJ,
Bhatnagar N and Meade M: Corticosteroid therapy for acute lung
injury, acute respiratory distress syndrome and severe pneumonia: A
meta-analysis of randomized controlled trials. J Crit Care.
25:420–435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rocco PR, Souza AB, Faffe DS, Pássaro CP,
Santos FB, Negri EM, Lima JG, Contador RS, Capelozzi VL and Zin WA:
Effect of corticosteroid on lung parenchyma remodeling at an early
phase of acute lung injury. Am J Respir Crit Care Med. 168:677–684.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Confalonieri M, Annane D, Antonaglia C,
Santagiuliana M, Borriello EM and Meduri GU: Is prolonged low-dose
glucocorticoid treatment beneficial in community-acquired
pneumonia? Curr Infect Dis Rep. 15:158–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matthay MA, Song Y, Bai C and Jones KD:
The acute respiratory distress syndrome in 2013. Translational
Respiratory Medicine. 1:1–6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thompson BT: Corticosteroids for ARDS.
Minerva Anestesiol. 76:441–447. 2010.PubMed/NCBI
|
|
17
|
Wajanaponsan N, Reade MC and Milbrandt EB:
Steroids in late ARDS? Crit Care. 11:3102007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hwangbo B, Lee HS, Lee JM, Kim MS, Kim HY,
Choi YJ and Zo JI: Low dose steroid therapy at an early phase of
acute respiratory distress syndrome after thoracic surgery. Chest
Journal. 126:719S-a-719S2004. View Article : Google Scholar
|
|
19
|
Fremont RD and Rice T: Low-dose steroids
in ARDS. Chest. 132:10952007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peter JV, John P, Graham PL, Moran JL,
George IA and Bersten A: Corticosteroids in the prevention and
treatment of acute respiratory distress syndrome (ARDS) in adults:
Meta-analysis. BMJ. 336:1006–1009. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Higgins J and Altman DG: Assessing risk of
bias in included studies. Cochrane handbook for systematic reviews
of interventions. Wiley-Blackwell; Chichester: pp. 187–241. 2008,
View Article : Google Scholar
|
|
22
|
Weigelt JA, Norcross JF, Borman KR and
Snyder WH III: Early steroid therapy for respiratory failure. Arch
Surg. 120:536–540. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bernard GR, Luce JM, Sprung CL, Rinaldo
JE, Tate RM, Sibbald WJ, Kariman K, Higgins S, Bradley R, Metz CA,
et al: High-dose corticosteroids in patients with the adult
respiratory distress syndrome. N Engl J Med. 317:1565–1570. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marik P, Kraus P, Sribante J, Havlik I,
Lipman J and Johnson DW: Hydrocortisone and tumor necrosis factor
in severe community-acquired pneumonia. A randomized controlled
study. Chest. 104:389–392. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Confalonieri M, Urbino R, Potena A,
Piattella M, Parigi P, Puccio G, Porta R Della, Giorgio C, Blasi F,
Umberger R and Meduri GU: Hydrocortisone infusion for severe
community-acquired pneumonia: A preliminary randomized study. Am J
Respir Crit Care Med. 171:242–248. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Annane D, Sébille V and Bellissant E;
Ger-Inf-05 Study Group, : Effect of low doses of corticosteroids in
septic shock patients with or without early acute respiratory
distress syndrome. Crit Care Med. 34:22–30. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Steinberg KP, Hudson LD, Goodman RB, Hough
CL, Lanken PN, Hyzy R, Thompson BT and Ancukiewicz M: National
Heart, Lung, and Blood Institute Acute Respiratory Distress
Syndrome (ARDS) Clinical Trials Network: Efficacy and safety of
corticosteroids for persistent acute respiratory distress syndrome.
N Engl J Med. 354:1671–1684. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meduri GU, Golden E, Freire AX, Taylor E,
Zaman M, Carson SJ, Gibson M and Umberger R: Methylprednisolone
infusion in early severe ARDS: results of a randomized controlled
trial. Chest. 131:954–963. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mikami K, Suzuki M, Kitagawa H, Kawakami
M, Hirota N, Yamaguchi H, Narumoto O, Kichikawa Y, Kawai M, Tashimo
H, Arai H, Horiuchi T and Sakamoto Y: Efficacy of corticosteroids
in the treatment of community-acquired pneumonia requiring
hospitalization. Lung. 185:249–255. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wan MH, Li J, Gong HL, Xue P, Zhu L, Chen
GY, Xia Q and Wen-Fu T: Clinical observation on the effect of
dexamethasone and Chinese herbal decoction for purgation in severe
acute pancreatitis patients. Chin J Integr Med. 17:141–145. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ferguson N, Adhikari N, Cook D, Hand L,
Henzler D, Zhou Q, Meade M and Lamontagne F: Corticosteroid use in
ARDS patients enrolled in the oscillation for ARDS treated early
(oscillate) trial. Am J Respir Crit Care Med. 183:A11692011.
|
|
32
|
Azevedo AF, Miranda-Filho DB,
Henriques-Filho GT, Leite A and Ximenes RA: Randomized controlled
trial of pulse methyl prednisolonex placebo in treatment of
pulmonary involvement associated with severe leptospirosis.
[ISRCTN74625030]. BMC Infect Dis. 11:1862011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Seam N, Meduri GU, Wang H, Nylen ES, Sun
J, Schultz MJ, Tropea M and Suffredini AF: Effects of
methylprednisolone infusion on markers of inflammation, coagulation
and angiogenesis in early acute respiratory distress syndrome. Crit
Care Med. 40:495–501. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu L, Li J, Huang YZ, Liu SQ, Yang CS,
Guo FM, Qiu HB and Yang Y: The effect of stress dose glucocorticoid
on patients with acute respiratory distress syndrome combined with
critical illness-related corticosteroid insufficiency. Zhonghua Nei
Ke Za Zhi. 51:599–603. 2012.(In Chinese). PubMed/NCBI
|
|
35
|
Thompson BT: Glucocorticoids and acute
lung injury. Crit Care Med. 31 Suppl 4:S253–S257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meduri GU, Golden E, Freire AX, Taylor E,
Zaman M, Carson SJ, Gibson M and Umberger R: Methylprednisolone
infusion in early severe ARDS: Results of a randomized controlled
trial. Chest. 131:954–963. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Suter PM: Lung inflammation in ARDS -
friend or foe? N Engl J Med. 354:1739–1742. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Annane D: Glucocorticoids for ARDS: Just
do it! Chest. 131:945–946. 2007. View Article : Google Scholar : PubMed/NCBI
|