Introduction
Chronic hypoxia-induced pulmonary arterial
hypertension (PAH) is an important pathogenesis mechanism in the
progress of chronic obstructive pulmonary disease to chronic
pulmonary heart diseases (1). PAH
can be caused by chronic hypoxia-induced pulmonary vasoconstriction
and reconstruction but the exact mechanism is unclear (1). There are not enough effective clinical
interventions. Under hypoxia, mitochondrial electron transport
chain (ETC) regulates the production of mitochondrial-derived
oxygen-free radicals reactive oxygen species (ROS) (2), especially H2O2,
which is permeable and can activate the transcription factor, low
oxygen-induced factor hypoxia-inducible factor (HIF)-1α (3), causing the mitochondrial ATP-sensitive
potassium (mitoKATP) channel of pulmonary artery smooth muscle
cells (PASMCs) to be open and activated (4). It gives a positive feedback on ROS
level and the expression of HIF-1α (5), promoting the proliferation of PASMCs
and inhibiting their apoptosis (6)
and taking part in the remodeling process of chronic hypoxic
pulmonary blood vessels. In addition, ~6% of human microRNAs
(miRNAs) including the miR-210 have HIF binding sites in the
promoter region, with which HIF can combine to regulate the
expression of miRNA (7). It is
confirmed that the expression of HIF-1α and miR-210 increases and
forms a positive feedback loop (8)
when human mesenchymal stem cells are under hypoxia. The highly
expressed miR-210 under hypoxia stress is able to make target
regulation to the mitochondrial iron-sulfur protein integrin (ISCU)
of tumor cells, vascular endothelial cells and many other cells,
affecting the circulation of Krebs, electron transport, ion
metabolism and the production of ROS, thus affecting the function
of mitochondria (9). Previous
studies confirmed that the ETC complex II inhibitor can activate
mitoKATP and mitoKATP-specific openers can also inhibit the
activity of complex II (10). Those
two are closely related, suggesting that ETC complex II may be a
component of mitoKATP, composition or an regulatory factor
(10).
Based on the above, we found in in vitro cell
experiments that the mechanism of the mitoKATP regulating
HIF-1α/miR-210/ISCU signaling axis forming a positive feedback loop
in PAH is involved in the remodeling process of chronic hypoxic
pulmonary blood vessels (11).
However, to the best of our knowledge, there are few studies in
vivo on animals. The current study examined the relationship
between the mitoKATP and the signaling axis from the PAH rat model
to provide strong evidence for clinical treatment.
Materials and methods
Animals and model building
Two hundred healthy adult SPF SD rats, each weighing
150–200 g were fed normally. The rats were housed in a temperature
controlled room (21 ± 2°C) on a 12:12-h light:dark cycle (lights on
at 06:00) with free access to water and food. The rats were
randomly divided into five groups: A normal control, a mimic
miR-210 agent (mimic-210) intervention, a miR-210 inhibitor
(anti-210) intervention, a chronic PAH group and an anti-210
intervention PAH groups, with 40 rats in each group.
Establishment of the chronic PAH rat
model
The rats were placed in an open normobaric
low-oxygen cabin in which the oxygen concentration was kept at
10.0±0.3% and the carbon dioxide concentration was kept <3%. The
low-oxygen condition was kept for 8 h daily, 4 weeks continuously.
The intervention in rats with mimic-210 and anti-210 (both from
R&D Systems, Minneapolis, MN, USA) was made by the nasal
topical drug delivery method (compared with systemic drug delivery,
nasal topically drug delivery is lower in dosage, works better in
targeting and miRNA positioning, and can reduce side effects on
other organs). Several studies have shown that small interfering
RNA (siRNA) delivered topically though nasal cavity or trachea can
have a significant target gene effect in the lungs (12).
Approval for the animal studies was received from
Wuhan Central Hospital Affiliated to Huazhong University (Hubei,
China).
Research methods
The rat PASMCs in each group were acutely isolated
(each PASMC was obtained using the enzyme digestion method and its
shrinkage was observed by phenylephrine to identify its
physiological activity). The flow cytometry method was used to
detect immunofluorescent activity of mitoKATP and ROS, the RT-qPCR
assay was used to detect the gene of HIF-1α/miR-210/ISCU and
western blot analysis was used to detect the protein of HIF-1α and
ISCU. The gene and protein expressions were detected again after
mitoKATP-specific opener diazoxide and blocker 5-HD (both from
R&D Systems) was given via tail vein and took effect on each
group of rats, respectively. The indicators above were detected
again after ISCU recombinant protein was given via tail vein and
ISCU siRNA (both from R&D Systems) via nasal feeding and took
effect on each group of rats, respectively.
Detection methods
Flow cytometry
FITC-labeled anti-human R-123, ROS monoclonal
antibody, immunoglobulin G (IgG) isotype control with corresponding
fluorescence tags and a BD FACSAria sorting flow cytometer (both
from BD Biosciences, Frankin Lakes, NJ, USA). Separately 10 µl of
different fluorescently labeled R-123, ROS antibodies and isotype
control was added to the tubes of the PASMC suspension. Three sets
of peripheral blood were added with anticoagulant (50 µl each set)
and was incubated at 25°C, in the dark for 15 min. Hemolysin (1 ml)
was added and reacted at room temperature in the dark for 15 min
and washed with phosphate-buffered saline (PBS), followed by
centrifugation at 1,200 × g for 5 min, and 500 µl of PBS was added
for cell suspension. Flow cytometry and the CellQuest software
(Version 3.1, BD Biosciences, San Jose, CA, USA) were used to
detect and corresponding fluorescence-tagged IgG staining cells
isotype control was used as a negative control. ROS is the oxygen
sensing function indicator of the mitochondrial respiratory chain.
R-123 fluorescence intensity is proportional to the mitochondrial
membrane potential, indirectly detecting the activity of
mitoKATP.
RT-qPCR assay
Primer sequences were synthesized by Shanghai
Shenggong Biological Engineering Technology & Services Co.,
Ltd. (Shanghai, China). The fluorescence quantitative PCR
instrument 7900HT was purchased from Applied Biosystems (Foster
City, CA, USA). Conventional TRIzol reagent was used to extract
total RNA, and the ultraviolet spectrophotometric method was used
to detect the concentration and purity. The reverse transcription
kit indicated the synthesis of cDNA, designed primer sequences and
amplified PCR (Table I). The
reaction system consisted of 2X Taq Master Mix (25 µl), forward and
reverse primers (10 µM) of 2 µl, template DNA (4 µl) and
ddH2O (17 µl) to make a total reaction volume of 50
µl.
 | Table I.Primer sequences used in this
study. |
Table I.
Primer sequences used in this
study.
| Genes | Forward primer | Reverse primer | Band length (bp) |
|---|
| HIF-1α |
5′-CGTTCCTTCGATCAGTTGTC-3′ |
5′-TCAGTGGTGGCAGTGGTAGT-3′ | 143 |
| miR-210 |
5′-CGCCTGTGCGTGTGACAGCG-3′ |
5′-GTGCAGGGTCCGAGGT-3′ | 71 |
| ISCU |
5′-GGCAAACCAGGCAGAGCCAGAG-3′ |
5′-GGATGGTACGGCCCGAGGTG-3′ | 95 |
| β-actin |
5′-CTGGAACGGTGAAGGTGACA-3′ |
5′-AAGGGACTTCCTGTAACAATGCA-3′ | 140 |
The amplification conditions were pre-denaturation
at 94°C for 4 min, denaturation at 94°C for 30 sec, annealing at
56°C for 30 sec, extension at 72°C for 1 min for total of 35
cycles, with another extension at 72°C for 10 min. The agarose gel
electrophoresis was performed by loading 5 µl of PCR product in
each well, with an voltage of 110V for 40 min. The gel image was
captured under ultraviolet analyzer and the results were expressed
as a ratio of the target gene and reference gene,
2−ΔΔCq.
Western blot analysis
Total protein was extracted according to protein
extraction kit (BCA protein). The BCA protein assay kit was used
for the determination of protein concentration to make up 5.0 mg/ml
of concentration to store at −80°C. SDS-PAGE electrophoresis method
(Beijing Liuyi Instrument Factory, Beijing, China) was used to
separate HIF-1α and the ISCU proteins. The gel was stained and
placed in fast dye Coomassie brilliant blue to analyze with the gel
imaging system (Syngene, Frederick, MD, USA) for the grayscale
value, while using β-actin as the internal control.
Statistical analysis
SPSS 19.0 statistical software (Chicago, IL, USA)
was used for inputting and analysis, all the quantitative data were
expressed as mean ± standard deviation. The single factor ANOVA
analysis was used for group comparison, and LSD or Bonferroni test
was used for pairwise comparison; qualitative data were expressed
as a number or percentage (%), χ2 test was used for
group comparison; P<0.05 was considered to indicate a
statistically significant difference.
Results
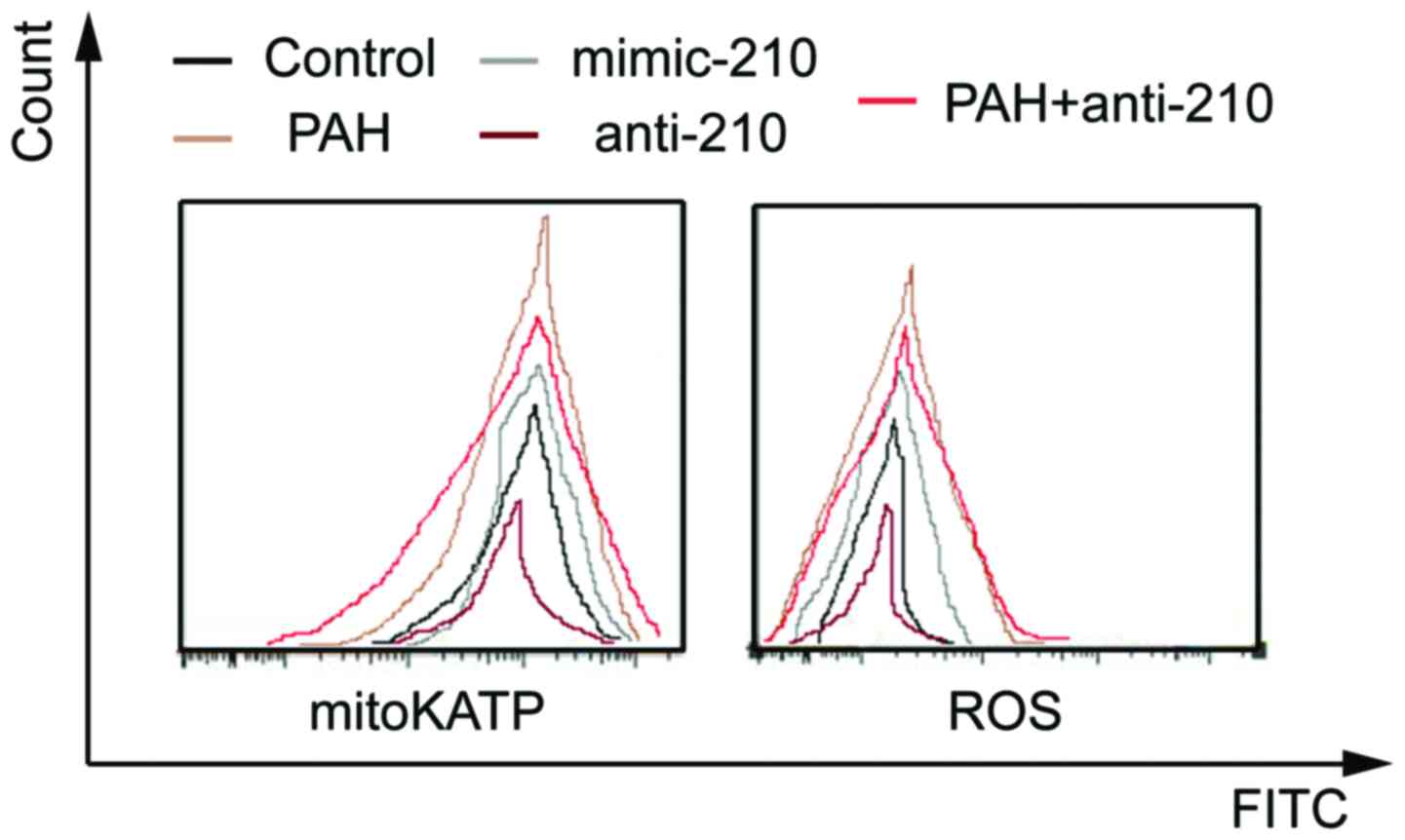
Comparison of the activity of mitoKATP
and ROS
The activity of mitoKATP and ROS of the mimic-210
group was significantly higher than that of the control group while
that of the anti-210 group was significantly reduced (P<0.05).
The activity of mitoKATP and ROS of the chronic PAH group was
significantly higher than that of the control group while that of
the anti-210 intervention PAH group was significantly reduced
(P<0.05) (Fig. 1).
The activity of ROS in all the groups increased
after given mitoKATP specific opener diazoxide via the tail vein.
ROS activity in all the groups reduced significantly after given
blocker 5-HD (P<0.05). The activity of mitoKATP and ROS in all
the groups reduced after given ISCU recombinant protein via the
tail vein (P<0.05). In addition, the activity of mitoKATP and
ROS in all the groups significantly increased after given ISCU
siRNA via nasal feeding (P<0.05).
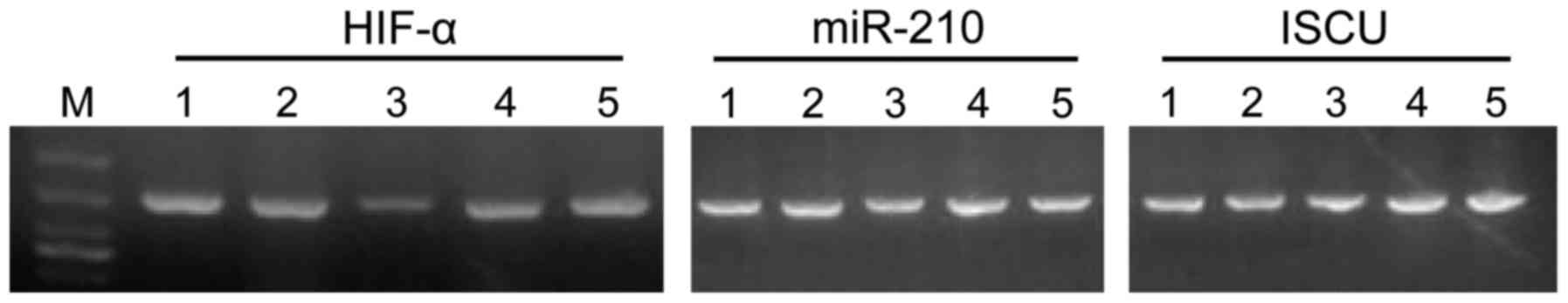
Gene level of HIF-1α/miR-210/ISCU
The levels of HIF-1α/ miR-210/ISCU of the mimic-210
group were significantly higher than that of the control group
while that of the anti-210 group was significantly reduced
(P<0.05). The gene level of HIF-1α/miR-210/ISCU of the chronic
PAH group was significantly higher than that of the control group
while that of the anti-210 intervention PAH group was significantly
reduced (P<0.05) (Fig. 2).
The gene level of HIF-1α/miR-210/ISCU in all the
groups increased after diazoxide was given. The gene level of
HIF-1α/miR-210/ISCU in all the groups decreased following 5-HD,
with a statistically significant difference (P<0.05). The gene
level of HIF-1α/miR-210/ISCU in all the groups decreased following
ISCU recombinant protein, whereas the gene level of
HIF-1α/miR-210/ISCU in all the groups increased following ISCU
siRNA, with a statistically significant difference (P<0.05).
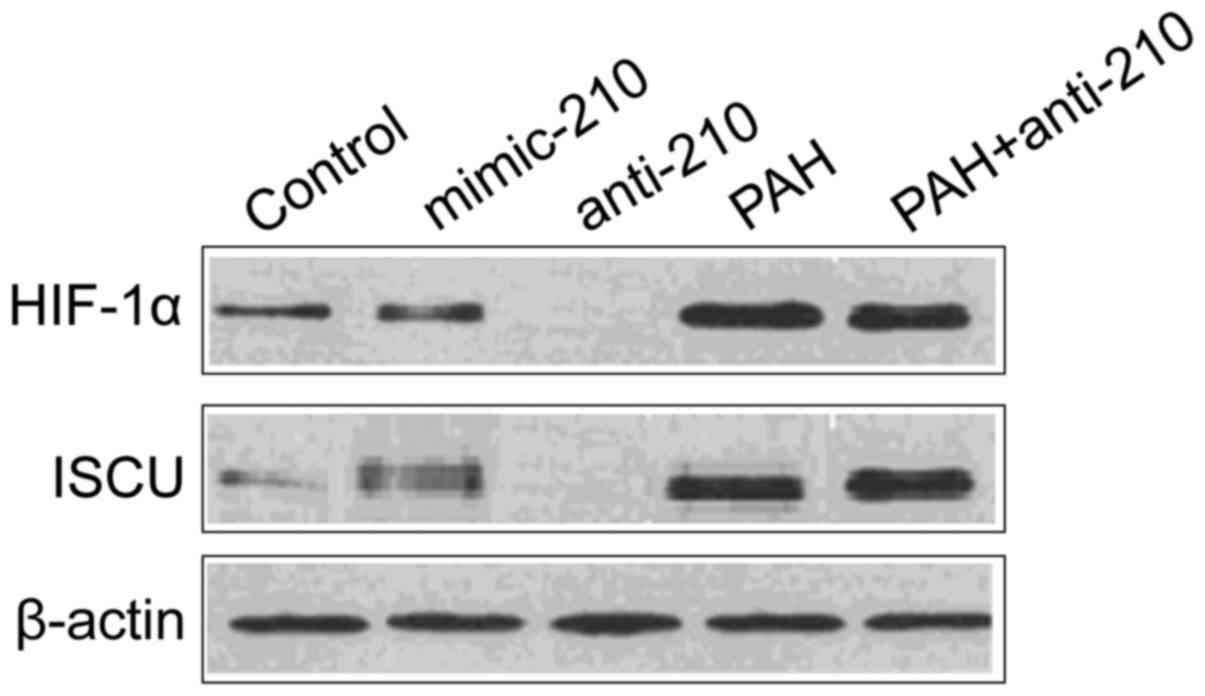
Protein level of HIF-1α and ISCU
The protein levels of HIF-1α and ISCU of the
mimic-210 group were significantly higher than that of the control
group while that of the ant-210 group was significantly reduced
(P<0.05); the protein level of HIF-1α and ISCU of the chronic
PAH group was significantly higher than that of the control group
while that of the anti-210 intervention PAH group was significantly
reduced (P<0.05) (Fig. 3).
The protein level of HIF-1α and ISCU in all the
groups significantly increased after diazoxide was given, whereas
the protein levels of HIF-1α and ISCU in all the groups were
significantly reduced after 5-HD was given (P<0.05). The protein
level of HIF-1α in all the groups significantly reduced after ISCU
recombinant protein was given (P<0.05). In addition, the protein
level of HIF-1α in all the groups significantly increased after
ISCU siRNA was given (P<0.05).
Discussion
Our previous experiments in cell lines has confirmed
that activated mitoKATP under hypoxia can increase the production
of ROS, which can activate the HIF (13). The mitoKATP of PASMCs opens and
regulates the signaling pathway of ROS/HIF/miR-210/ISCU under
hypoxia, forming a positive feedback loop that continually induce a
high expression of miR-210, thus repetitively stimulate the
proliferation of PASMCs. The opening and activating of mitoKATP can
cause an influx of potassium ions and mitochondrial swelling, being
an important factor that affects the mitochondrial membrane
potential. It can protect a variety of cells such as nerve cells
and heart muscle cells and promote cell survival and inhibit
apoptosis (14). Previous findings
have shown that miR-210 has an anti-apoptotic function of hypoxic
PASMCs (15). We assume that the
opening and activating of mitoKATP changes oxygen-free radical
levels by affecting the oxygen sensing function of mitochondrial
respiratory chain complex, thus activating increased HIF and
regulating the HIF/miR-210/ISCU signaling pathway while ISCU
affects the activity of mitoKATP by affecting the activity of ETC
compound II. In this way, a positive feedback loop is formed,
repeatedly stimulating the proliferation of PASMCs to promote the
remodeling of pulmonary blood vessels.
Through establishment of the PAH rat model and in
vivo study, we found that the activity of mitoKATP and ROS and
the gene, protein levels of HIF-1α/miR-210/ISCU of the mimic-210
group were significantly higher than that of the control group,
while that of the anti-210 group was significantly reduced. These
indicators of the chronic PAH group were all significantly higher
than those of the control group while those of the anti-210
intervention PAH group was significantly reduced. The indicators of
all the groups significantly increased after mitoKATP-specific
opener diazoxide was given, and the indicators of all the groups
significantly reduced after blocker 5-HD was given. The indicators
of all the groups significantly reduced after ISCU recombinant
protein was given, the indicators of all the groups significantly
increased following ISCU siRNA. Thus, the mechanism of mitoKATP
regulating HIF-1α/miR-210/ISCU signaling axis and formation of a
positive feedback loop exists in the PAH rat model.
We further explored, giving mitoKATP-specific
inhibitors in vivo, pulmonary arterial pressure and
pathological changes in reversible pulmonary hypertension rats
(16). In this way, we completely
explained the mechanism of mitoKATP regulating HIF-1α/miR-210/ISCU
signaling axis and the formation of a positive feedback loop in
chronic PAH, providing theoretical basis for filtering new chronic
hypoxic PAH therapeutic targets.
Currently there are no studies on chronic hypoxic
PASMC mitoKATP regulating miR-210 and its target gene ISCU. This
study explored that the chronic hypoxic PASMC mitoKATP regulating
HIF/miR-210/ISCU signaling axis and forms a positive feedback loop,
repeatedly stimulating the proliferation and migration of PASMCs.
Since they horizontally regulate the expression of genes after
transcription, miRNAs can regulate the function of mitochondria at
the genetic level, providing experimental basis for chronic hypoxic
pulmonary hypertension gene therapy. This in vivo study on
the function of mitoKATP regulating HIF-1α/miR-210/ISCU signaling
axis and formation of a positive feedback loop based on previous
research has improved value and significance due to the complexity
of in vivo experiments.
Acknowledgements
This study was supported by the Health and Family
Planning Commission of Hubei Province (no. WJ2015MB140).
References
|
1
|
Zhang S, Liu B, Fan Z, Wang D, Liu Y, Li
J, Wang N, Liu Y and Zhang B: Targeted inhibition of survivin with
YM155 promotes apoptosis of hypoxic human pulmonary arterial smooth
muscle cells via the upregulation of voltage-dependent
K+ channels. Mol Med Rep. 4:3415–3422. 2016.
|
|
2
|
Zhang B, Chu W, Wei P, Liu Y and Wei T:
Xanthohumol induces generation of reactive oxygen species and
triggers apoptosis through inhibition of mitochondrial electron
transfer chain complex I. Free Radic Biol Med. 89:486–497. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Comito G, Calvani M, Giannoni E, Bianchini
F, Calorini L, Torre E, Migliore C, Giordano S and Chiarugi P:
HIF-1α stabilization by mitochondrial ROS promotes Met-dependent
invasive growth and vasculogenic mimicry in melanoma cells. Free
Radic Biol Med. 51:893–904. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu HL, Zhang ZX, Chen CS, Cai C, Zhao JP
and Wang X: Effects of mitochondrial potassium channel and membrane
potential on hypoxic human pulmonary artery smooth muscle cells. Am
J Respir Cell Mol Biol. 42:661–666. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vriend J and Reiter RJ: Melatonin and the
von Hippe-Lindau/HIF-1 oxygen sensing mechanism: a review. Biochim
Biophys Acta. 1865:176–183. 2016.PubMed/NCBI
|
|
6
|
Dong L, Li Y, Hu H, Shi L, Chen J, Wang B,
Chen C, Zhu H, Li Y, Li Q, et al: Potential therapeutic targets for
hypoxia-induced pulmonary artery hypertension. J Transl Med.
12:392014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang X, Ren H, Zhao T, Ma W, Dong J, Zhang
S, Xin W, Yang S, Jia L and Hao J: Single nucleotide polymorphism
in the microRNA-199a binding site of HIF1A gene is associated with
pancreatic ductal adenocarcinoma risk and worse clinical outcomes.
Oncotarget. Feb 8–2016.(Epub ahead of print).
|
|
8
|
Guo S, Bai R, Liu W, Zhao A, Zhao Z, Wang
Y, Wang Y, Zhao W and Wang W: MicroRNA-210 is upregulated by
hypoxia-inducible factor-1α in the stromal cells of giant cell
tumors of bone. Mol Med Rep. 12:6185–6192. 2015.PubMed/NCBI
|
|
9
|
Cawley K, Logue SE, Gorman AM, Zeng Q,
Patterson J, Gupta S and Samali A: Disruption of microRNA
biogenesis confers resistance to ER stress-induced cell death
upstream of the mitochondrion. PLoS One. 8:e738702013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cuong DV, Kim N, Joo H, Youm JB, Chung JY,
Lee Y, Park WS, Kim E, Park YS and Han J: Subunit composition of
ATP-sensitive potassium channels in mitochondria of rat hearts.
Mitochondrion. 5:121–133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin Y, Xie WP and Wang H: Hypoxic
pulmonary hypertension and novel ATP-sensitive potassium channel
opener: the new hope on the horizon. Zhongguo Ying Yong Sheng Li
Xue Za Zhi. 28:510–523. 2012.(In Chinese). PubMed/NCBI
|
|
12
|
Moschos SA, Spinks K, Williams AE and
Lindsay MA: Targeting the lung using siRNA and antisense based
oligonucleotides. Curr Pharm Des. 14:3620–3627. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo Y, Yang T, Lu J, Li S, Wan L, Long D,
Li Q, Feng L and Li Y: Rb1 postconditioning attenuates liver warm
ischemia-reperfusion injury through ROS-NO-HIF pathway. Life Sci.
88:598–605. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zuo X, Zong F and Wang H, Wang Q, Xie W
and Wang H: Iptakalim, a novel ATP-sensitive potassium channel
opener, inhibits pulmonary arterial smooth muscle cell
proliferation by downregulation of PKC-α. J Biomed Res. 25:392–401.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin Y, Pang T, Nelin LD, Wang W, Wang Y,
Yan J and Zhao C: MKP-1 is a target of miR-210 and mediate the
negative regulation of miR-210 inhibitor on hypoxic hPASMC
proliferation. Cell Biol Int. 39:113–120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Tang Y and Zhang YL: Hypoxic
pulmonary hypertension (HPH) and iptakalim, a novel ATP-sensitive
potassium channel opener targeting smaller arteries in
hypertension. Cardiovasc Drug Rev. 23:293–316. 2005. View Article : Google Scholar : PubMed/NCBI
|