Introduction
Although improvements of cardiac surgical procedures
and perioperative management have significantly decreased patient
mortality during cardiac surgery, cardiogenic shock remains the
main reason for perioperative death (1,2).
Cardiogenic shock progresses rapidly with mortality rates as high
as 40–80%, because of a decrease in cardiac output, peripheral
tissue hypoperfusion and microcirculation disturbance, resulting in
fatal systemic inflammatory response syndrome (SIRS) and multiple
organ dysfunction syndrome (MODS) (3–7).
Therefore, basic and clinical research on cardiogenic shock has
been a research hotspot in the field of cardiovascular medicine
(8). Following the principles of
improving oxygen supply and reducing oxygen consumption, the
treatment goal of cardiogenic shock is to maintain hemodynamic
stability, prevent systemic damage from low perfusion, and gain the
opportunity for etiological treatment (9). However, when both drug and support
means cannot reverse the trend of deterioration, mechanical assist
devices (MADs) remain the only option for patients. Presently, MADs
include the intra-aortic balloon pump (IABP), extracorporeal
membrane oxygenation (ECMO) and ventricular assist device (VAD)
(10,11). Among which, the IABP is the most
frequently used and has saved countless patients with advanced
heart disease over the past 50 years (12). Theoretically, IABP can improve
diastolic perfusion pressure, coronary blood flow and myocardial
oxygen supply. It can also reduce left ventricular afterload,
reduce ventricular work, reduce oxygen consumption, and promote
heart function recovery. However, since 2012, a large number of
clinical trials demonstrated that IABP cannot reduce cardiogenic
shock 30-day mortality of patients with acute myocardial infarction
(13). Whether IABP can reduce the
mortality of patients with cardiogenic shock during the
perioperative period of cardiac surgery remains to be determined.
There is no accurate timing for IABP application in these patients.
In the present study, patients with perioperative cardiogenic shock
from cardiac surgery were followed. The influence of different IABP
treatment timings and different severe degrees of cardiogenic shock
were observed for patient prognosis and mortality.
Patients and methods
Patients
According to the diagnostic and exclusion criteria
of the IABP SHOCK-II clinical trial, a total of 197 patients were
included in this study. All patients experienced cardiogenic shock
during the perioperative period of cardiac surgery and accepted
IABP treatment from January 2011 to October 2015, when admitted to
the Second Affiliated Hospital of Harbin Medical University.
Experimental grouping
Patients were divided into five groups on the basis
of different application timing of IABP (time interval from
cardiogenic shock to implementation of IABP): 0–60 min group (83
patients), 61–120 min group (41 patients), 121–180 min group (22
patients), 181–240 min group (22 patients) and >240 min group
(29 patients). The highest vasoactive-inotropic score (VIS) of
patients was calculated before the application of IABP, and they
were divided into five groups according to VIS score: 0–10 group
(25 patients), 11–20 group (62 patients), 21–30 group (60
patients), 31–40 group (23 patients) and >40 group (27
patients).
Experimental indexes
The leading index was 30-day mortality. The
secondary indexes were mortality of patients in the different
VIS-score groups, duration of mechanical ventilation, duration of
ICU stay, duration of hospital stay, total hospitalization charges,
daily hospitalization charges and the application rate of
continuous renal replacement therapy (CRRT). The risk factors
related to mortality and the occurrence of IABP complications were
also analyzed.
Analysis of cause of death
Factors related to intervention: EuroSCORE,
operation time, anesthesia time, IABP treatment timing, duration of
IABP-support and the application of CRRT.
Factors related to drugs: The VIS score of patients
before the application of IABP.
The baseline characteristics of patients: Age,
gender, smoking, drinking, diabetes mellitus (DM), hypertension,
body mass index (BMI), lacunar infarction, cerebral infarction,
percutaneous coronary intervention (PCI), stent implantation,
unstable angina, preoperative cardiac function classification,
preoperative ejection fraction (EF) value, preoperative
interventricular septal thickness (IVST) and preoperative left
ventricular posterior wall thickness (LVPWT).
The occurrence of IABP related complications: The
occurrence of IABP complications were analyzed including
thrombocytopenia; bleeding; lower limb ischemia; thrombosis and
embolism; vascular injury; and mechanical complications, such as
balloon burst and catheter fracture.
Statistical analysis
SAS software version 9.1.3 (SAS Institute, Cary, NC,
USA) and GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA,
USA) computer data processing software were used. A χ2
test was used to analyze enumeration data and ANOVA was applied for
measurement data. Student's t-test was implemented for comparisons
between two groups. For measurement data unsuitable for Student's
t-test, a nonparametric test was used. Single factor and
multifactor logistic regression analysis were applied for the
analysis of risk factors for death.
Results
Baseline characteristics of
patients
Between January 2011 and October 2015, 6,189
patients underwent open cardiac surgery in our center. Among them,
197 were enrolled in this study. The study included 130 males
(65.99%) and 67 females (34.01%); mean age was 58.68±9.42 years,
mean BMI was 24.614±3.86, mean duration of hospital stay was
34.83±19.11 days. IABP support time was 3–456 h (mean support time
was 112.04±78.73 h). The vast majority of patients had cardiac
function ranging from moderate to poor (two patients were New York
Heart Association (NYHA) class II, 113 patients were NYHA class III
and 82 patients were NYHA class IV. Mean operating time was
267.79±80.40 min, the average anesthesia time was 312.06±85.39 min.
The overall hospital mortality of the entire cohort was 53.81%.
Most patients accepted coronary artery bypass surgery in this
study, followed by valve replacement (Table I).
 | Table I.The distribution of type of surgery
for IABP inserted cardiogenic shock patients. |
Table I.
The distribution of type of surgery
for IABP inserted cardiogenic shock patients.
| Surgery | Case no. | Percent |
|---|
| CABG | 108 | 54.82 |
| CABG + other | 24 | 12.187 |
| Valve
replacement | 51 | 25.89 |
| Pericardiectomy | 8 | 4.06 |
| Bentall | 3 | 1.52 |
| Congenital heart
disease surgery | 2 | 1.02 |
| Atrial myxoma
resection | 1 | 0.51 |
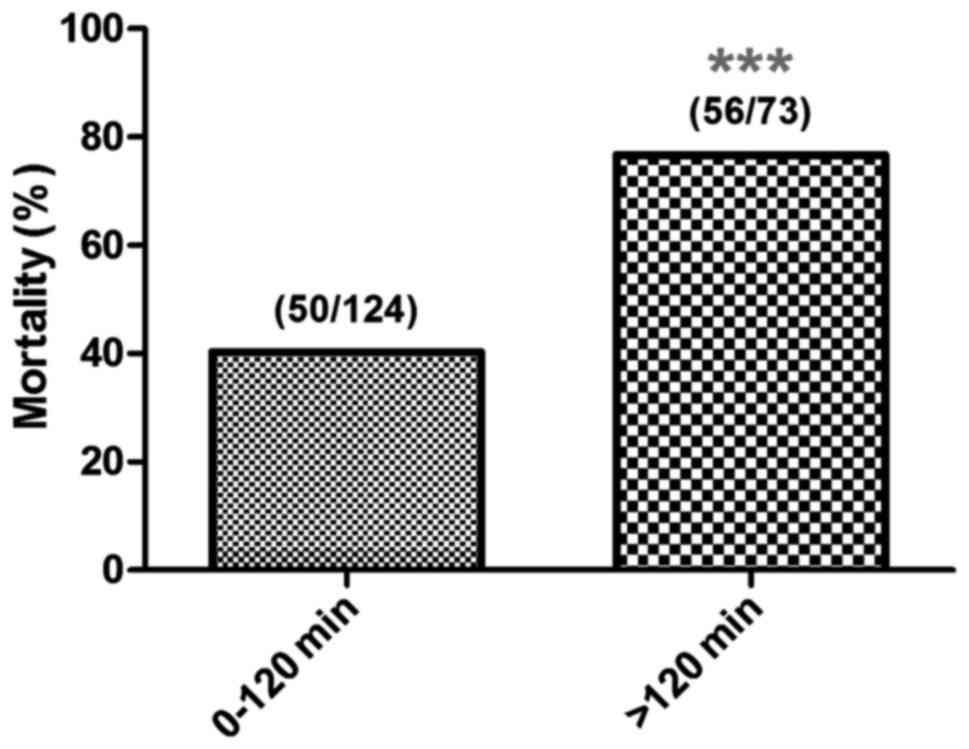
The influence of IABP application
timing on patient prognosis
The relationship between IABP application timing and
30-day mortality: Mortality was ~40% when IABP support began within
120 min from onset of shock. Mortality rates increased to over 70%
when IABP support began after 120 min. Mortality rate was ~80% when
IABP support began 240 min later (P<0.05, P<0.01 and
P<0.001). According to the obvious trend of mortality, patients
were divided into two groups based on different application timing,
the 0–120 and >120 min groups. We found that compared with the
>120 min group, IABP support beginning within 120 min from
cardiogenic shock significantly reduced the 30-day mortality (40.3
and 76.7%, respectively; P<0.001) (Figs. 1 and 2).
Secondary indexes
Different IABP application timing had no effect on
indexes such as hospitalization duration, duration of ICU stay,
duration of invasive mechanical ventilation (through endotracheal
intubation or tracheotomy), total hospitalization charges and daily
hospitalization charges (P>0.05). The proportion of patients
that received CRRT in the 0–60, 61–120, 121–180, 181–240 and
>240 min groups were 15.66, 26.83, 31.82, 31.82 and 51.72%,
respectively (Fig. 3). The
increasing trend was statistically significant (P<0.001). These
results demonstrated that, when timing of IABP application was
later, tissue perfusion was worse which increased the likelihood of
patients having severe renal insufficiency. The daily
hospitalization charges in the 0–120 min group were lower than in
the >120 min group by 1,300 yuan (P<0.01), while the invasive
mechanical ventilation time, total mechanical ventilation time,
duration of hospital stay, duration of ICU stay and total
hospitalization charges were not statistically different between
the two groups (P>0.05) (Table
II).
 | Table II.The difference between secondary
indexes. |
Table II.
The difference between secondary
indexes.
| Groups | 0-120 min | >120 min | P-value |
|---|
| N | 124 | 73 |
|
| Duration of
hospital stay (days) | 36.88 (19.84) | 31.34 (17.37) | 0.049 |
| Duration of ICU
stay (h) | 200.14
(170.22) | 212.78
(171.05) | 0.616 |
| Invasive mechanical
ventilation time (h) | 184.28
(197.19) | 206.04
(180.89) | 0.446 |
| Total mechanical
ventilation time (h) | 222.27
(211.904) | 231.34
(190.227) | 0.763 |
| Total
hospitalization charges (yuan) | 217741.67
(99665.91) | 229460.59
(124098.73) | 0.468 |
| Daily
hospitalization charges (yuan) | 6799.13
(2942.2) | 8127.4
(3583.77) | 0.005 |
Duration of hospital stay after IABP
in the 0–120 and >120 min group
The duration of hospital stay after IABP in the
0–120 min group was 16.81 days, which was significantly higher than
in the 120 min group (10.45 days) (Fig.
4). When patients accepted IABP treatment 120 min after
occurrence of cardiogenic shock, the effect was unsatisfactory, and
most patients died prematurely. According to the following formula:
Total hospitalization charges = duration of hospital stay × daily
hospitalization charges. Although the daily hospitalization cost of
patients in the 120 min group was higher, premature death occurred
after IABP, which shortened the duration of hospital stays.
Therefore, the advantages of IABP are not reflected in economic
indicators. By analyzing data of surviving patients, early IABP
intervention reduced hospital charges (Fig. 5).
Analysis of the factors related to
death
EuroSCORE
The EuroSCORE of all 197 patients in this study was
6.48±3.16 (1–17), there were no statistical differences
between the 0–120 and >120 min groups (P>0.05). This
suggested that the operative risks of patients in both groups were
similar. Single logistic regression analysis indicated that
mortality and EuroSCORE had no significant correlation (P>0.05)
(Fig. 6).
The relationship between IABP application timing
and VIS score
VIS score = dopamine dose (µg/kg·min) + dobutamine
dose (µg/kg·min) + 100 × epinephrine dose (µg/kg·min) + 10 ×
milrinone (µg/kg·min) + 10,000 × vasopressin dose (U/kg·min) + 100
× norepinephrine dose (µg/kg·min) + 10 × phenylephrine dose
(µg/kg·min) (18). VIS score
reflects the dosage of vasoactive drugs and positive inotropic
drugs. Higher score shows a higher level of critically ill
patients. In this study, VIS score before IABP treatment was
25.42±15.80. By statistical analysis, we found that the VIS score
in the 0–120 min group was significantly lower than in the >120
min group (P<0.01). Therefore, later IABP application was
related to higher VIS score (Fig.
7). VIS score in surviving patients was also significantly
lower than in patients who died (P<0.001) (Fig. 8).
The relationship between VIS score and
mortality
According to the highest VIS score before IABP
treatment, the patients were divided into five groups: 0–10, 11–20,
21–30, 31–40 and 40+ group and the mortality rates were 32.00,
38.71, 56.67, 78.26 and 85.19%, respectively. Mortality increased
with VIS score (P<0.001) (Fig.
9). Early treatment of IABP may therefore delay or reverse the
rise of VIS score, which may reduce the adverse effects of
hypotension on perfusion of peripheral organs, and prevent the
occurrence of MODS, thereby reducing patient mortality.
Analysis of risk factors
Single factor logistic regression analysis showed
that the death of patients was related to the insertion timing of
IABP (P<0.001) and the VIS score (P<0.001) before the
insertion of IABP (Table III).
Multifactor logistic regression analysis indicated that the
independent risk factors of death in patients with cardiogenic
shock during cardiac surgery were related to the IABP insertion
timing and VIS score before balloon insertion (Table IV). Furthermore, every 1 h delay of
IABP application increased the patient's risk of death by 1.373.
Every increase of VIS score by 10 points, increased the patient's
risk of death by 1.047 (Table V).
Mortality had no significant correlation with age, gender, smoking,
drinking, DM, hypertension, BMI, lacunar infarction, cerebral
infarction, PCI, stent implantation, unstable angina, preoperative
cardiac function classification, preoperative EF value,
preoperative IVST and preoperative IVPWT (P>0.05).
 | Table III.Risk factors associated with
mortality. |
Table III.
Risk factors associated with
mortality.
| Variables | B | WALD | P-value | OR | CI lower | CI upper |
|---|
| Gender |
|
|
|
|
|
|
| M |
|
|
|
|
|
|
| F | 0.455 | 2.216 | 0.137 | 1.577 | 0.866 | 2.872 |
| Age | 0.011 | 0.563 | 0.453 | 1.012 | 0.982 | 1.042 |
| Smoking |
|
|
|
|
|
|
| N |
|
|
|
|
|
|
| Y | −0.083 | 0.080 | 0.777 | 0.921 | 0.519 | 1.632 |
| Drinking |
|
|
|
|
|
|
| N |
|
|
|
|
|
|
| Y | −0.257 | 0.588 | 0.443 | 0.774 | 0.401 | 1.491 |
| DM |
|
|
|
|
|
|
| N |
|
|
|
|
|
|
| Y | −0.265 | 0.707 | 0.401 | 0.767 | 0.414 | 1.423 |
| Hypertention |
|
|
|
|
|
|
| N |
|
|
|
|
|
|
| Y | 0.129 | 0.204 | 0.652 | 1.138 | 0.649 | 1.994 |
| BMI | 0.016 | 0.172 | 0.678 | 1.016 | 0.941 | 1.098 |
| Lacunar
infarction |
|
|
|
|
|
|
| N |
|
|
|
|
|
|
| Y | 0.333 | 0.733 | 0.392 | 1.395 | 0.651 | 2.991 |
| Cerebral
infarction |
|
|
|
|
|
|
| N |
|
|
|
|
|
|
| Y | 0.877 | 2.571 | 0.109 | 2.404 | 0.823 | 7.025 |
| Previous PCI |
|
|
|
|
|
|
| N |
|
|
|
|
|
|
| Y | −0.665 | 1.725 | 0.189 | 0.514 | 0.191 | 1.387 |
| Cardiac function
(NYHA class III–IV) |
|
|
|
|
|
|
| N |
|
|
|
|
|
|
| Y | 0.107 | 0.136 | 0.713 | 1.113 | 0.629 | 1.969 |
| CS-IABP (min) | 0.007 | 17.733 | 0.000 | 1.007 | 1.004 | 1.010 |
|
CS_IABP(1) (0–60) | −0.105 | 0.053 | 0.817 | 0.900 | 0.368 | 2.200 |
|
CS_IABP(2) (61–120) | −0.299 | 0.384 | 0.535 | 0.742 | 0.289 | 1.907 |
|
CS_IABP(3) (121–180) | 1.475 | 5.640 | 0.018 | 4.371 | 1.294 | 14.768 |
|
CS_IABP(4) (181–240) | 1.232 | 4.263 | 0.039 | 3.429 | 1.064 | 11.043 |
|
CS_IABP(5) (240+) | 1.595 | 7.547 | 0.006 | 4.929 | 1.579 | 15.380 |
| CS_IABP
(0–120) |
|
|
|
|
|
|
| CS_IABP
(120+) | 1.584 | 22.775 | 0.000 | 4.875 | 2.544 | 9.345 |
| VIS score | 0.056 | 17.812 | 0.000 | 1.058 | 1.031 | 1.086 |
| EF value | 0.018 | 1.989 | 0.158 | 1.018 | 0.993 | 1.043 |
| IVST | 0.218 | 3.696 | 0.055 | 1.244 | 0.996 | 1.554 |
| LVPWT | 0.124 | 1.346 | 0.246 | 1.131 | 0.918 | 1.394 |
 | Table IV.The independent predictors associated
with mortality. |
Table IV.
The independent predictors associated
with mortality.
| Variables | B | WALD | P-value | OR | CI lower | CI upper |
|---|
| CS IABP | 0.317 | 9.953 | 0.002 | 1.373 | 1.127 | 1.671 |
| VIS | 0.045 | 12.515 | 0.000 | 1.047 | 1.020 | 1.073 |
 | Table V.Complications. |
Table V.
Complications.
| Complication | Number | Percent |
|---|
| Thrombocytopenia
(need platelet transfusion) | 19 | 9.64 |
| Ischemia or
embolism | 7 | 3.55 |
| Vascular
impairment | 1 | 0.51 |
| Severe
bleeding | 0 | 0 |
Complications
The main complications included thrombocytopenia,
peripheral ischemia, thrombosis, embolism and vascular impairment.
Two patients underwent thrombectomy (removal of thrombus) of the
femoral artery, followed by remission of disease (Table V). There were no IABP complication
related deaths in this study.
Discussion
For evaluation of any kind of treatment, two of the
most important premises are treatment timing and indications on
whether it is reasonable. This includes IABP. Regarding therapeutic
effect of IABP, several studies over the past 50 years have shown
that IABP is a means of assisting circulation, but it cannot solve
primary heart problems. However, if the right patients for IABP
treatment are chosen at the optimal time, they could be greatly
benefitted because the mechanism and effect of IABP is certain
(15). The IABP counterpulsation is
key for patients with severe cardiac function after primary disease
is resolved, which is sufficient to recover the function of stunned
myocardium in the reversible stage (16). Because IABP improved tissue perfusion
to avoid progression of SIRS and MODS, patients had a chance to
recover during the most dangerous part of the perioperative phase.
The application of IABP in cardiac surgery has increased (17), and the rate of application of IABP in
the cardiac surgery department of our hospital increased from 1.72%
in 2011 to 4.78% in 2014.
At present, many clinical studies on cardiac surgery
do not support the preventive application of IABP (19,20), and
guidelines recommend IABP only as a means for circulation in
patients with refractory cardiogenic shock. The present study did
not adopt a preventive application strategy. Although all selected
patients met the criteria for cardiogenic shock, there were
differences in the severity of conditions. We adopted VIS score to
assess the critical conditions of patients (18,21,22), and
EuroSCORE to evaluate baseline status of patients (23,24). The
results showed that the overall EuroSCORE in patients was 6.48±3.16
points (out of 17 points). Therefore, the patients in the study
were high-risk. VIS score was initially used in newborns and
infants after cardiac surgery but in recent years has gradually
been used in adults and adolescents (25) during the perioperative period of
cardiac surgery to evaluate degree of critical illness. Relative to
the complexity of APACHE II, the calculation method of VIS score is
simpler and quicker (26). We found
that the mortality rate of patients was as high as 85.19% when VIS
score was over 40 points, therefore we do not recommend IABP for
patients with VIS score >40 and where cardiogenic shock occurred
over 240 min, as the ideal recovery time was missed because of
ischemic myocardium. Not only is the effect of IABP at this time
not ideal, but may increase the risk of complications.
Unfortunately, the application of IABP within 120
min after cardiogenic shock did not reach the expected effect in
economic benefits. Through further analysis, we found that many
patients with premature death and survival time shortened because
of starting IABP treatment 120 min after cardiogenic shock, missed
the optimal timing of treatment. This offset the advantage of early
IABP application which can save on total charges. Patient survival
is however, the most important outcome, and length of hospital stay
and hospital expenses cannot be compared with survival rate.
Moreover, we found that IABP treatment 120 min after cardiogenic
shock reduced the total and daily hospitalization charges of
surviving patients. Without the interference of shorter survival
time because of death, the evaluation of the advantages of IABP is
more reasonable and saves on charges.
IABP support from 3–456 h (1–19 days), an average of
112.04±78.73 h in this study, was comparable with the results (24 h
to 11 days) of most studies (27).
Complications occurred only in a minority of patients (13.7%) as in
other clinical studies (28–31), and there were no serious bleeding
events related to IABP, or IABP complication related deaths.
Therefore, IABP is relatively safe for patients with cardiac shock
during the perioperative period of cardiac surgery. Because of the
efficacy of IABP technology itself and its circulation support
being very precise, focus should be placed on selecting the optimal
timing in different patients, and how to improve the treatment
effect of IABP. Assessing the patient's condition by VIS score
alone is not sufficient and many factors should also be considered.
For example, the area of myocardial infarction, cardiac function,
organ function, effect of cardiac surgery and microcirculation.
Damage to heart function because of large areas of infarction are
irreversible regardless of drugs, intervention, circulatory assist
devices and conventional surgery. Therefore, new evaluation systems
which are more rapid, accurate and comprehensive are required, to
help judge IABP intervention timing accurately and choose the
appropriate patients, so as to improve the therapeutic effect of
IABP, thereby improving the prognosis of patients with cardiogenic
shock during the perioperative period of cardiac surgery.
References
|
1
|
Zhang J, Lang Y, Guo L, Song X, Shu L, Su
G, Liu H and Xu J: Preventive use of intra-aortic balloon pump in
patients undergoing high-risk coronary artery bypass grafting: a
retrospective study. Med Sci Monit. 21:855–860. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ho CH, Chen ZC, Chu CC, Wang JJ and Chiang
CY: Temporal trends of in-hospital mortality in patients treated
with intra-aortic balloon pumping: a Nationwide Population Study in
Taiwan, 1998–2008. PLoS One. 10:e01315752015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cao J, Liu W, Zhu J and Zhao H: Risk
factors and clinical characteristics of in-hospital death in acute
myocardial infarction with IABP support. Int J Clin Exp Med.
8:8032–8041. 2015.PubMed/NCBI
|
|
4
|
Wan YD, Sun TW, Kan QC, Guan FX, Liu ZQ
and Zhang SG: The effects of intra-aortic balloon pumps on
mortality in patients undergoing high-risk coronary
revascularization: a meta-analysis of randomized controlled trials
of coronary artery bypass grafting and stenting era. PLoS One.
11:e01472912016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ranucci M, Ballotta A, Castelvecchio S, De
Vincentiis C, Biondi A, Parisi A, Menicanti L and Frigiola A:
Surgical and Clinical Outcome REsearch (SCORE) Group: Perioperative
heart failure in coronary surgery and timing of intra-aortic
balloon pump insertion. Acta Anaesthesiol Scand. 54:878–884. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kucuker A, Cetin L, Kucuker SA, Gokcimen
M, Hidiroglu M, Kunt A, Saglam F and Sener E: Single-centre
experience with perioperative use of intraaortic balloon pump in
cardiac surgery. Heart Lung Circ. 23:475–481. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cove ME and MacLaren G: Clinical review:
mechanical circulatory support for cardiogenic shock complicating
acute myocardial infarction. Crit Care. 14:2352010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ferguson JJ III, Cohen M, Freedman RJ Jr,
Stone GW, Miller MF, Joseph DL and Ohman EM: The current practice
of intra-aortic balloon counterpulsation: results from the
Benchmark Registry. J Am Coll Cardiol. 38:1456–1462. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Graf T and Thiele H: Mechanical support in
cardiogenic shock. Herz. 40:224–230. 2015.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Giridharan GA, Lee TJ, Ising M, Sobieski
MA, Koenig SC, Gray LA and Slaughter MS: Miniaturization of
mechanical circulatory support systems. Artif Organs. 36:731–739.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bruti G, Kolyva C, Pepper JR and Khir AW:
Measurements of intra-aortic balloon wall movement during inflation
and deflation: effects of angulation. Artif Organs. 39:E154–E163.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gilotra NA and Stevens GR: Temporary
mechanical circulatory support: a review of the options,
indications, and outcomes. Clin Med Insights Cardiol. 8:(Suppl 1).
75–85. 2015.PubMed/NCBI
|
|
13
|
Thiele H, Zeymer U, Neumann FJ, Ferenc M,
Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K,
Fuernau G, et al: IABP-SHOCK II Trial Investigators: Intraaortic
balloon support for myocardial infarction with cardiogenic shock. N
Engl J Med. 367:1287–1296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ihdayhid AR, Chopra S and Rankin J:
Intra-aortic balloon pump: indications, efficacy, guidelines and
future directions. Curr Opin Cardiol. 29:285–292. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brugts JJ and Caliskan K: Short-term
mechanical circulatory support by veno-arterial extracorporeal
membrane oxygenation in the management of cardiogenic shock and
end-stage heart failure. Expert Rev Cardiovasc Ther. 12:145–153.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feola M, Haiderer O and Kennedy JH:
Intra-aortic balloon pumping (IABP) at different levels of
experimental acute left ventricular failure. Chest. 59:68–76. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kapelios CJ, Terrovitis JV, Siskas P,
Kontogiannis C, Repasos E and Nanas JN: Counterpulsation: a concept
with a remarkable past, an established present and a challenging
future. Int J Cardiol. 172:318–325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nguyen HV, Havalad V, Aponte-Patel L,
Murata AY, Wang DY, Rusanov A, Cheng B, Cabreriza SE and Spotnitz
HM: Temporary biventricular pacing decreases the
vasoactive-inotropic score after cardiac surgery: a substudy of a
randomized clinical trial. J Thorac Cardiovasc Surg. 146:296–301.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu PJ, Cassiere HA, Dellis SL, Kohn N,
Manetta F and Hartman AR: Propensity-matched analysis of the effect
of preoperative intraaortic balloon pump in coronary artery bypass
grafting after recent acute myocardial infarction on postoperative
outcomes. Crit Care. 18:5312014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kucuker A, Cetin L, Kucuker SA, Gokcimen
M, Hidiroglu M, Kunt A, Saglam F and Sener E: Single-centre
experience with perioperative use of intraaortic balloon pump in
cardiac surgery. Heart Lung Circ. 23:475–481. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gaies MG, Gurney JG, Yen AH, Napoli ML,
Gajarski RJ, Ohye RG, Charpie JR and Hirsch JC:
Vasoactive-inotropic score as a predictor of morbidity and
mortality in infants after cardiopulmonary bypass. Pediatr Crit
Care Med. 11:234–238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu JG, Pensiero A, Aponte-Patel L, de
Villa Velez B, Rusanov A, Cheng B, Cabreriza SE and Spotnitz HM:
Short-term reduction in intrinsic heart rate during biventricular
pacing after cardiac surgery: a substudy of a randomized clinical
trial. J Thorac Cardiovasc Surg. 146:1494–1500. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lundemoen S, Kvalheim VL, Svendsen OS,
Mongstad A, Andersen KS, Grong K and Husby P: Intraaortic
counterpulsation during cardiopulmonary bypass impairs distal organ
perfusion. Ann Thorac Surg. 99:619–625. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Geissler HJ, Hölzl P, Marohl S,
Kuhn-Régnier F, Mehlhorn U, Südkamp M and de Vivie ER: Risk
stratification in heart surgery: Comparison of six score systems.
Eur J Cardiothorac Surg. 17:400–406. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Garcia RU, Walters HL III, Delius RE and
Aggarwal S: Vasoactive inotropic score (VIS) as biomarker of
short-term outcomes in adolescents after cardiothoracic surgery.
Pediatr Cardiol. 37:271–277. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ishibashi N, Miyasho K, Kitamura T, Ookuma
T, Kashitani N, Beika N, Yamashita T and Ujike Y: Hemodynamic
effects of intravenous calcium administration on septic shock
patients: a retrospective study. Acta Med Okayama. 69:197–204.
2015.PubMed/NCBI
|
|
27
|
Su D, Yan B, Guo L, Peng L, Wang X, Zeng
L, Ong H and Wang G: Intra-aortic balloon pump may grant no benefit
to improve the mortality of patients with acute myocardial
infarction in short and long term: an updated meta-analysis.
Medicine (Baltimore). 94:e8762015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Svitek V, Mand'ák J and Harrer J:
Intra-aortic balloon counterpulsation in cardiac surgery patients -
experiences of Department of Cardiac Surgery, Charles University,
Faculty of Medicine in Hradec Králove, University Hospital Hradec
Králové, Czech Republic. Rozhl Chir. 87:68–73. 2008.(In Slovak).
PubMed/NCBI
|
|
29
|
Kovács E, Becker D, Daróczi L, Gálfy I,
Hüttl T, Laczkó A, Paukovits T, Vargha P and Szabolcs Z: Analysis
of vascular complications of IABP therapy in open-heart surgery
patients 1999–2004. Magy Seb. 59:105–111. 2006.(In Hungarian).
PubMed/NCBI
|
|
30
|
Lauwers E, Meese G, Adriaensen H, Amsel B
and Van der Mast M: Perioperative intra-aortic balloon
counterpulsation in cardiosurgery: a retrospective study. Acta
Anaesthesiol Belg. 41:41–45. 1990.PubMed/NCBI
|
|
31
|
Meco M, Gramegna G, Yassini A, Bellisario
A, Mazzaro E, Babbini M, Pediglieri A, Panisi P, Tarelli G,
Frigiola A, et al: Mortality and morbidity from intra-aortic
balloon pumps. Risk analysis. J Cardiovasc Surg (Torino). 43:17–23.
2002.PubMed/NCBI
|