Introduction
Peripheral nerve injury is a common cause of nerve
function loss and this damage leads to severe disability, which is
often permanent. Effective reparation of the nerve stump is a huge
challenge for surgeons. Currently, autologous nerve grafting
remains the gold standard for peripheral nerve repair (1). However, disadvantages, including
limited donor site, secondary damage, sensory loss and trauma
neuroma formation, remain (2). The
limitations and disadvantages of autologous nerve transplantation
have led to the development of tissue engineering. Various
experimental approaches and novel technologies were initiated in
the field of peripheral nerve injury repair to investigate
potential solutions (3–5). Schwann cells (SCs), the major
myelin-forming cells in the PNS, serve a vital role in nerve
regeneration by secreting a variety of neurotrophic factors and
forming the extracellular matrix (6). These characteristics of SCs mean that
they have potential to aid in the repair of the central nervous
system (7,8). SC transplantation has become one of the
best options for treating demyelinating diseases of the peripheral
and central nervous systems (9).
Neural stem cells (NSCs) are specific primitive nerve cells, which
are present in the central nervous system and are able to
differentiate into mature nerve cells, including neurons,
astrocytes and oligodendrocytes, due to their multi-differentiation
potential. NSCs may also be induced into SCs due to the low
immunogenicity, NSCs have thus disproved the long-standing theory
that neurons are unable to regenerate (10). NSCs may be expanded in vitro
on a large scale and may be kept in long-term preservation.
Furthermore, the characteristics they exhibit, including a
multi-differentiation potential, high plasticity, ability to
undergo transplant and low immunogenicity, permit them to be more
appropriate than somatic cells for use in tissue engineering
research (11,12). It has been confirmed that
transplanting NSCs into the nervous system promotes axonal
regeneration and the formation of SC outer peripheral myelin
(13). However, the base film and
nerve growth factor are also required for peripheral nerve
regeneration (14). When
transplanted into the damaged site of the peripheral nerve, NSCs
not only differentiate into SCs but also secrete certain favorable
nerve growth factors (15,16).
A number of neurotrophic factors secreted by NSCs
also help to promote regeneration of damaged nerves (17,18).
Previous studies investigating the potential of NSCs to repair the
peripheral nervous system (PNS) have provided more answers and the
mechanisms of nerve damage repair are becoming increasingly clear.
NSCs may promote cell regeneration, improve compatibility with the
surrounding tissue transplantation site and due to low
immunogenicity, they may limit or even attenuate the use of
immunosuppressive drugs (19). One
of the current challenges in neurobiology is to ensure that neural
precursor cells differentiate into specific neuron types, so that
they may be used for transplantation purposes in patients that have
experienced neuron loss.
NSCs possess the capacity of self-renewal and
multi-differentiation, allowing them to may differentiate into
neurons, astrocytes and oligodendrocytes (10). When NSCs are cultured alone under
differentiation cultivation conditions, they are prone to
differentiate into glial cells; only a small fraction develop into
neuron cells. Studies have investigated the co-culture of NSCs and
SCs to repair both central and peripheral nerve injury (20–23);
however, the specific mechanism of action is not fully
understood.
The present study aimed to investigate the
mechanisms of co-cultured NSCs and SCs in repairing peripheral
nerve injury. The effects of neurotrophic factors and SCs on NSC
survival and differentiation direction were also investigated.
Materials and methods
The animals and experimental protocols used in the
present study were approved by the Ethics Committee of Shanghai
General People's Hospital (Shanghai, China). All rats were provided
standard mouse chow and water ad libitum, and housed under a 12 h
light/dark cycle at 24°C and 50% humidity.
Culture and identification of
hippocampal NSCs of fetal rats
One female pathogen free Wistar rat (Shanghai SLAC
Laboratory Animal Co., Ltd., Shanghai, China) 14–16 days pregnant
for was anesthetized by abdominal administration of 10% chloral
hydrate (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), 300 mg/kg
body weight and fetuses removed under sterile conditions and
sacrificed post-surgery by decapitation. The fetus brains were
separated into two cerebral hemispheres and vascular membranes were
peeled and removed under a dissecting microscope. Separated
hippocampi were transferred into a 3.5-mm culture capsule filled
with pre-cooled Dulbecco's modified Eagle's medium (DMEM)/F12 (1:1;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Cell
suspension was created using TryPLE (pancreatic enzyme replacement
fluid; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for digestion
in a water bath for 40 min at 37°C, suspension solutions were
centrifuged at 350 × g for 5 min at 4°C and the supernatant was
discarded. Complete medium was then used to resuspend and count.
Cells were plated in T25 flasks at a density of 1×106
cells/ml and cultured at 37°C in a 5% CO2 incubator.
Medium was changed once every two days and passage performed every
seven days. Complete medium composition was as follows: DMEM/F12
(1:1) in serum-free medium, 2% B27 (Invitrogen; Thermo Fisher
Scientific, Inc.), 20 ng/ml epidermal growth factor (EGF;
PeproTech, Inc., Rocky Hill, NJ, USA), 20 ng/ml basic fibroblast
growth factor (bFGF; PeproTech, Inc.), L-glutamine (Gibco; Thermo
Fisher Scientific, Inc.), 1% penicillin-streptomycin
(Sigma-Aldrich; Merck KgGA). Mouse anti-rat Nestin Monoclonal
antibody (cat. no. 611826; 1:500; BD Pharmingen, San Diego, CA,
USA) immunofluorescence staining was used to identify NSCs.
Primary culture and subculture method
and identification of newborn rat SCs
A total of 10 newborn Wistar male rats (Shanghai
SLAC Laboratory Animal Co., Ltd.) were purchased at 3–5 days old
(10–12 g). All rats were provided standard mouse chow and water
ad libitum, and housed under a 12 h light/dark cycle at
24°C, 50% humidity. Rats were decapitated, the skin was disinfected
with 75% alcohol and then the bilateral sciatic nerve was dissected
under sterile conditions by removing the epineurium and connective
tissue under a dissecting microscope. Nerves were then cut into 1
mm3 fragments with ophthalmic scissors. Nerve fragments
were then mixed with digestion solution that contained 0.2% NB4
collagenase (Invitrogen; Thermo Fisher Scientific, Inc.) and 0.2%
Dispase II (neutral enzyme; Roche Applied Science, Penzberg,
Germany) at a ratio of 2:1. The solutions were put on a shaker at
37°C to digest for 45 min. During the digestion period solutions
were agitated twice. At the end of digestion, the well-digested
single-cell suspension was centrifuged at 524 × g for 5 min at 4°C
and the supernatant was discarded. Cells were resuspended and
counted with an inverted light microscope (CKX41; Olympus
Corporation, Tokyo, Japan) in SC medium containing DMEM/low
glucose, 10% fetal bovine serum (FBS; Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA), 20 ng/ml bFGF, 20 ng/ml Heregulin
(Sigma-Aldrich; Merck KGaA), 4 µM Forskolin (Sigma-Aldrich; Merck
KGaA) and 1% penicillin-streptomycin (Sigma-Aldrich; Merck KGaA).
Cells were subsequently planted in a 10-cm culture capsule at a
density of 1×106/ml and cultured at 37°C in a 5%
CO2 incubator. Passage was performed two days later. At
the time of passage, well-incubated 0.2% Dispase II was selected
and digested for 10–15 min at 37°C until the majority of SCs were
digested. Following two passages, the purity of SCs was >98%.
Rabbit anti-rat S100 (mo76101; 1:100; Dako; Agilent Technologies,
Inc., Santa Clara, CA, USA) was used for immunofluorescence
staining of SCs. Blocking was performed for 30 min at room
temperature with 5% bovine serum albumin (Beyotime Institute of
Biotechnology, Haimen, China) before incubation overnight with S100
(as above) at 4°C in the dark. Cells were subsequently incubated
with goat anti-rabbit- fluorescein isothiocyanate 488 secary
antibody (sc-2704; 1:500; Santa Cruz Biotechnology, Inc.) at 37°C
for 45 min in the dark. Cells were observed under a laser scanning
confocal microscope (C2+; Nikon Corporation, Tokyo, Japan) and
analyzed using NIS-elements AR software (V4200; Nikon
Corporation).
Detection and identification of NSCs
co-cultured with SCs
Cells were divided into three groups: i) SC group of
P2-4 generation, ii) NSC group later than P2 generation, and iii)
SC + NSC (SCs:NSCs 1:1) group. Cells were cultured for 7 days at
37°C, in an atmosphere contatining 5% CO2 (HERA cell
150i; Thermo Fisher Scientific, Inc.). Morphological changes were
observed under an inverted phase contrast microscope and documented
on a daily basis; supernatants were removed and analyzed on days 1,
3, 5 and 7. Secretion of brain-derived neurotrophic factor (BDNF)
and glial-derived neurotrophic factor (GDNF) was assessed using an
ELISA Heregulin-β-1 assay (ELISA assay kit, cat. no. EK0308-1 and
EK0363-1, rat BDNF, GDNF from Syd Labs, Inc. Natick, MA, USA) for
all three groups, followed by a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
cell viability assay (Sigma-Aldrich; Merck KGaA) to confirm cell
survival in all three groups. Differentiation medium included
DMEM/F12 (1:1), 2% B27, 20 ng/ml bFGF, 20 ng/ml EGF and 1% FBS.
Markers for cell type identification were rat anti-rabbit S100
protein (mo76101; 1:100; Dako; Agilent Technologies, Inc.) for SC
labeling, goat anti-mouse glial fibrillary acidic protein (GFAP)
monoclonal antibody (ab27952; 1:500; Abcam, Cambridge, UK) for
astrocyte labeling and rabbit anti-human microtubule associated
protein (Map2) polyclonal antibody for neuron labeling (sc-20978;
1:500; Santa Cruz Biotechnology, Inc., Dallas TX, USA).
Directional differentiation of
NSCs
Culture was subdivided into three subgroups: SCs,
NSCs and co-culture groups. All groups were seeded in the
differentiation culture media under differentiation medium. Four
target genes, BDNF, GDNF, Map2, and glial fibrillary acidic protein
(GFAP) were selected. Map2 is the specific marker for neurons,
while GFAP is the marker for astrocytes. BDNF and GDNF are two
primary neurotrophic factors secreted primarily by SCs. The cells
were seeded for 7 days and culture media were changed every other
day. On day 7 following seeding, gene expression was measured by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) for these four target genes within different groups under
appropriate serum concentrations using the QTaq™ One-Step RT-qPCR
SYBR® kit, (Takara Bio, Inc., Otsu, Japan).
In order to extract RNA, cells were harvested
(1×107 cells/10 cm plate) and 1 ml TRIzol™ (Invitrogen;
Thermo Fisher Scientific, Inc.) was added to dissociated cells and
then distributed to tubes following complete homogenization. The
tube was inverted 10 times and allowed to stand for 5 min at room
temperature.
Chloroform was added (one-fifth of the total
volume), the tube was inverted 10 times and was allowed to stand
for 5 min at room temperature. The resultant solution was
centrifuged for 15 min at 12,000 × g and 4°C, and the upper
aqueous phase (400 µl) was removed to a new tube. An equal volume
of isopropyl alcohol (400 µl) was added, incubated for 10 min
following mixing and centrifuged for 10 min at 12,000 × g
and 4°C. The supernatant was removed and replaced with 1 ml cold
75% alcohol, dissolved with diethyl pyrocarbonate (DEPC; ST036;
Beyotime Institute of Biotechnology). The resultant solution was
centrifuged for 5 min at 7,500 × g and 4°C. The supernatant
was discarded and the remaining pellet was dried for 5–10 min. The
precipitation was dissolved in 20 µl DEPC. Total RNA was quantified
using a NanoDrop 2000c Spectrophotometer (Thermo Fisher Scientific,
Inc.). The RNA quality was evaluated through 15–20 min 1.5% agarose
gel electrophoresis using 5 µl PCR product and 1 µl bromophenol
blue, followed by spectrophotometry (MJ Research; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) to determine (OD) 260/OD280.
The reverse transcription reaction was made up as follows: 1 µl
RNA, 2 µl mix (Takara Bio Inc.) and 7 µl DEPC, with a total volume
of 10 µl. The samples were then incubated at 37°C for 15 min
followed by 85°C for 5 sec. Samples were stored at 4°C for use in
future experiments. A total of 20 µl reaction product was used for
each PCR reaction, consisting of 10 µl SYBR green mix, 1 µl forward
primer, 1 µl reverse primer, 1 µl cDNA and 7 µl DEPC. For the PCR
reaction, β-actin was used as a control. The PCR reaction was
completed as follows: 95°C for 2 min to pre-denature samples, 95°C
for 10 sec to denature, 60°C for 30 sec for 40 cycles and 72°C for
30 sec. PCR was completed in a CFX-96 thermocycler (Bio-Rad
Laboratories, Inc.). Primers were synthesized for the specific
target genes as follows: GDNF, forward: 5′-CTGACTTGGGTTTGGGCTAC-3′
and reverse 5′-CCTGGCCTACCTTGTCACTT-3′, BDNF forward:
5′-GGGACCTCGGAACTCAAC-3′ and reverse: 5′-TGTATCTGCCTGGGACTG-3′,
Map2 forward: 5′-GACCACCAGGTCAGAACCAAT-3′ and reverse
5′-TGGTGTCCTGGGATAGCTCG-3′ and GFAP forward:
5′-ATGGAGCTCAATGACCGCTT-3′ and reverse:
5′-ATCTTGGAGCTTCTGCCTCAG-3′.
Statistical analysis
The transcription profile was obtained from three
independent experiments performed in duplicate. SPSS version 19.0
software was used to analyze data (IBM SPSS, Armonk, NY, USA). Data
are presented as mean ± standard deviation. One-way analysis of
variance was used for statistical analysis across all groups.
P<0.05 was considered to represent a statistically significant
difference.
Results
Morphological results of NSC
differentiation
The majority of the NSCs in the co-cultured group
differentiated into neurons, with a small population that
differentiated into astrocytes. Neuron cell bodies were translucent
with a slender shape with multiple processes, indicating that the
cells were in good shape (Fig. 1A).
Neurons were also present in the NSC group, but there were a
smaller number of cells overall and the state of the neurons was
clearly not as healthy as those grown in the co-cultured group
(Fig. 1B). For the SC group, the
morphology of the cells was the same as those cultured in SC medium
and had no differentiation trend (Fig.
1C). Following immunofluorescence staining, nerve ball cells in
the co-cultured group exhibited a gradual spread and differentiated
into neurons with positive Map2 staining (Fig. 2A), while in the NSC group, a small
number of cells differentiated into neurons, and a large proportion
of cells differentiated into astrocytes (Fig. 2B).
 | Figure 2.Immunofluorescence staining of
co-cultured and NSC groups. (A) NSC neurospheres in the co-cultured
group gradually differentiate into neurons with Map2-positive
immunofluorescence staining (green), cell nuclei stained by DAPI
(blue). (B) NSC group, a small number of cells differentiate into
neurons, Map2-positive (green), while a large proportion of cells
differentiate into astrocytes, glial fibrillary acidic protein
positive (red), cell nuclei stained by DAPI (blue). Scale bar, 50
µm. NSC, neural stem cells; Map2, microtubule associated protein;
DAPI, 4′,6-diamidino-2-phenylindole. |
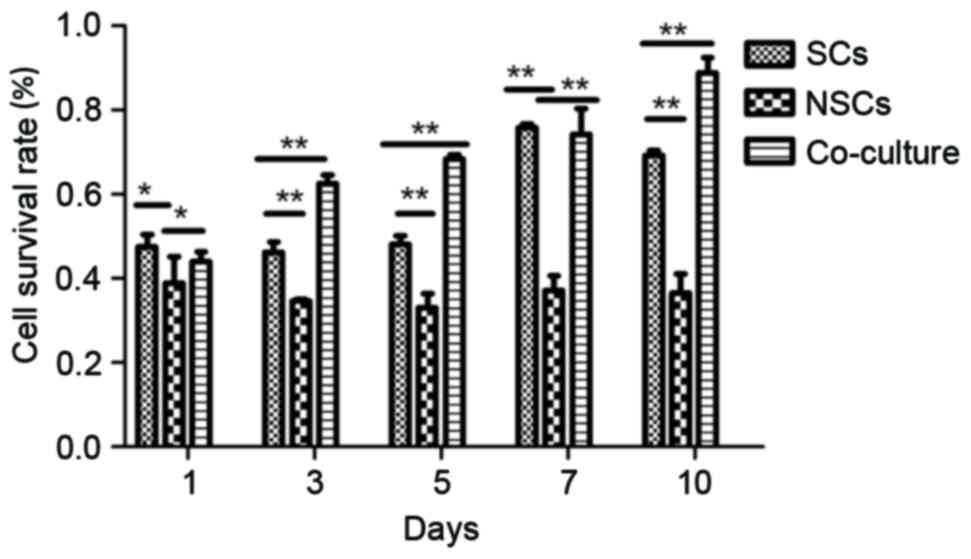
Survival cells detected by MTT
colorimetry
The survival rate in the SC group was significantly
higher than the NSC group (P<0.05), but demonstrated no
significant difference compared with the co-cultured group at days
1 and 7. However, the survival rate of the co-culture group was
significantly higher than the other groups at days 3, 5 and 10
(P<0.01). The cell survival rate of the co-culture group was
significantly higher compared with NSC groups at all points
(P<0.05; Fig. 3).
Secretion of BDNF and GDNF
The supernatant of the three groups were assessed
using an ELISA assay. Levels of neurotrophic factors secreted by
cells varied in each group on different days. For BDNF, on day 1,
there was no statistically significant difference between the three
groups, while on days 3 and 5, the amount of BDNF in the co-culture
group and SCs was significantly higher than NSCs (P<0.05;
Fig. 4A). On day 7, the amount of
BDNF secreted by the co-culture group and SCs was significantly
higher than that secreted by NSCs (P<0.05), and that in the
co-culture group was significantly higher than the SC group
(P<0.05; Fig. 4A). For GDNF, on
day 1, the amount in the co-culture group was significantly higher
than both NSC and SC groups (P<0.01), but there was no
difference between NSC and SC groups (Fig. 4B). On days 3, 5 and 7, the amount in
the co-culture group was significantly higher than the SC group
(P<0.01), and that in the SC group was higher than the NSC group
(P<0.01; Fig. 4B). Therefore, the
overall trend was as follows: The co-culture group had a higher
expression of BDNF and GDNF than the SC group, which had a higher
expression than the NSC group (Fig.
4).
mRNA expression of BDNF, GDNF, Map2,
and GFAP
Following 7 days seeding in the culture media, the
mRNA expression of four different target genes, BDNF, GDNF, Map2,
and GFAP was measured by RT-qPCR. The results demonstrated that
BDNF and GDNF gene expression in the co-culture group was
significantly higher than in the SC group, while the SCs exhibited
significantly higher expression of BDNF and GDNF than the NSC group
(all P<0.01; Fig. 5). Secondly,
the gene expression of Map2 in the co-culture group was
significantly higher than both NSC and SC groups (P<0.01), while
there was no significant difference between NSC and SC groups
(P>0.05; Fig. 5). For GFAP gene
expression, the NSC group exhibited the highest expression,
followed by SCs and then the co-culture group; there was a
significant difference between each group (each P<0.01; Fig. 5). The results suggest that co-culture
of NSCs and SCs promotes NSCs to differentiate into neurons, while
in independent cultures, NSCs are promoted to differentiate into
astrocytes.
Discussion
When damage occurs in the PNS, neuronal degeneration
stimulates a series of reactions (24). When the distal end of the extruded or
severed axons undergo Wallerian degeneration, axons and myelin
disintegrate. The resulting disintegrating matter is cleared by
macrophages and SCs (25). The SCs
differentiate and regain division and proliferation functions to
regenerate new cells that form the Bünger band, providing a
suitable microenvironment for axonal regeneration and surrounding
the newborn axons to form the myelin sheath (26). SCs are also able to secrete cell
adhesion molecules, such as laminin and fibronectin, which function
in a similar way to the extracellular matrix, thus supporting
axonal growth (27).
NSCs possess a capacity for self-renewal and
multi-differentiation. The current study demonstrated that
following co-culture with SCs, NSCs are induced to differentiate
into neurons. Furthermore, various neurotrophic factors secreted by
SCs may also promote bridging between cells, supporting the
survival of neurons (28,29). NSCs grafted to injured zones of
peripheral nerve tissue differentiate into motor neurons, the axons
of which may reach the muscle tissue. NSCs thus serve a role in
delaying muscle atrophy prior to contact between differentiated
neurons and establishment of denervated muscles (30,31).
The view that neurotrophic factors promote nerve
repair is well supported (32,33).
BDNF is the second factor to have been identified in the
neurotrophic factor family, which has 54% sequence homology to
nerve growth factor (NGF) and serves a variety of functions in the
nervous system (32,33). BDNF is a very important neurotrophic
factor for motor neurons, as it may affect the expression of
cholinergic genes and promote the survival of cultured cells
(28). GDNF closely links with its
corresponding receptor and transforming growth factor family,
promoting motor and sensory neuron survival (34–36). It
may also enhance the formation of myelin by facilitating the
migration of SCs (37).
In the present study, more cells differentiated into
neurons when NSCs were co-cultured with SCs, and these cells
possessed good morphological features including bright rounded
spots with two or three apophyses (Fig.
1). Using ELISA to assess secretion of the two factors BDNF and
GDNF, it was confirmed that under co-culture conditions, the
secretion of various neurotrophic factors was higher (P<0.05)
than under individually cultured conditions, indicating that
co-culture produced an improved nerve growth microenvironment. The
MTT assay demonstrated that cell survival in the co-culture group
is higher than in the other two groups, which is in accordance with
neurotrophic factor levels. During the experiment, it was also
observed that in the co-cultured group, not only were more neurons
formed, but the cell morphology of SCs was different compared with
the cultured alone group, and SC volume was larger with more stout
processes observed. Levels of nerve growth factors confirmed that
SCs promote NSC growth and induce differentiation of NSCs into
neurons. However, the production of NSCs during proliferation and
differentiation may also promote the growth of SCs. The present
study assessed levels of the two neurotrophic factors, BDNF and
GDNF. However, other factors such as nerve growth factor and
ciliary neurotrophic factor may also be involved in the
differentiation of NSCs. The specific mechanisms of action remain
to be confirmed in future studies.
In conclusion, the current study confirmed that
co-culturing SCs and NSCs in vitro improved the nerve
regeneration microenvironment. Positive changes to cell morphology
by interaction of the two cell types was also observed. However,
nerve function recovery is still a huge challenge in peripheral
nerve repair; the present study only verified the merits of nerve
gap bridging and increasing secretion of nerve growth factors. The
effect of co-culture of SCs and NSCs on regeneration and functional
recovery of damaged peripheral nerve may be verified through in
vivo animal experiments in the future.
Acknowledgments
The current study was supported by grants from the
Natural Science Foundation of China (Beijing, China; no. 81170925)
and K.C.Wong Education Foundation of Shanghai Jiao Tong University
School of Medicine (Shanghai, China).
References
|
1
|
Lee SK and Wolfe SW: Peripheral nerve
injury and repair. J Am Acad Orthop Sur. 8:243–252. 2000.
View Article : Google Scholar
|
|
2
|
Siemionow M, Duggan W, Brzezicki G,
Klimczak A, Grykien C, Gatherwright J and Nair D: Peripheral nerve
defect repair with epineural tubes supported with bone marrow
stromal cells: A preliminary report. Ann Plast Surg. 67:73–84.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lloyd BM, Luginbuhl RD, Brenner MJ, Rocque
BG, Tung TH, Myckatyn TM, Hunter DA, Mackinnon SE and Borschel GH:
Use of motor nerve material in peripheral nerve repair with
conduits. Microsurgery. 27:138–145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lubiatowski P, Unsal FM, Nair D, Ozer K
and Siemionow M: The epineural sleeve technique for nerve graft
reconstruction enhances nerve recovery. Microsurgery. 28:160–167.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scharpf J, Meirer R, Zielinski M, Unsal M,
Ramineni P, Nair D and Siemionow M: A novel technique for
peripheral nerve repair. Laryngoscope. 113:95–101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kanno H, Pressman Y, Moody A, Berg R, Muir
EM, Rogers JH, Ozawa H, Itoi E, Pearse DD and Bunge MB: Combination
of engineered Schwann cell grafts to secrete neurotrophin and
chondroitinase promotes axonal regeneration and locomotion after
spinal cord injury. J Neurosci. 34:1838–1855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Olson HE, Rooney GE, Gross L, Nesbitt JJ,
Galvin KE, Knight A, Chen B, Yaszemski MJ and Windebank AJ: Neural
stem cell- and Schwann cell-loaded biodegradable polymer scaffolds
support axonal regeneration in the transected spinal cord. Tissue
Eng Part A. 15:1797–1805. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bunge RP: The role of the Schwann cell in
trophic support and regeneration. J Neurol. 242:(1 Suppl 1).
S19–S21. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kanno H, Pressman Y, Moody A, Berg R, Muir
EM, Rogers JH, Ozawa H, Itoi E, Pearse DD and Bunge MB: Combination
of engineered Schwann cell grafts to secrete neurotrophin and
chondroitinase promotes axonal regeneration and locomotion after
spinal cord injury. J Neurosci. 34:1838–1855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parker MA, Anderson JK, Corliss DA,
Abraria VE, Sidman RL, Park KI, Teng YD, Cotanche DA and Snyder EY:
Expression profile of an operationally-defined neural stem cell
clone. Exp Neurol. 194:320–332. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alessandri G, Emanueli C and Madeddu P:
Genetically engineered stem cell therapy for tissue regeneration.
Ann N Y Acad Sci. 15:271–284. 2004. View Article : Google Scholar
|
|
12
|
Goldman SA and Sim F: Neural progenitor
cells of the adult brain. Novartis Found Symp. 265:66–80, 82–97.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blakemore WF: The case for a central
nervous system (CNS) origin for the Schwann cells that remyelinate
CNS axons following concurrent loss of oligodendrocytes and
astrocytes. Neuropathol Appl Neurobiol. 31:1–10. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Georgiou M, Bunting SC, Davies HA,
Loughlin AJ, Golding JP and Phillips JB: Engineered neural tissue
for peripheral nerve repair. Biomaterials. 34:7335–7343. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dong MM and Yi TH: Stem cell and
peripheral nerve injury and repair. Facial Plast Surg. 26:421–427.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mirsky R, Jessen KR, Brennan A, Parkinson
D, Dong Z, Meier C, Parmantier E and Lawson D: Schwann cells as
regulators of nerve development. J Physiol Paris. 96:17–24. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rodriguez AM, Pisani D, Dechesne CA,
Turc-Carel C, Kurzenne JY, Wdziekonski B, Villageois A, Bagnis C,
Breittmayer JP, Groux H, et al: Transplantation of a multipotent
cell population from human adipose tissue induces dystrophin
expression in the immunocompetent mdx mouse. J Exp Med.
201:1397–1405. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mosahebi A, Woodward B, Wiberg M, Martin R
and Terenghi G: Retroviral labeling of Schwann cells: In vitro
characterization and in vivo transplantation to improve peripheral
nerve regeneration. Glia. 34:8–17. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
MacDonald SC, Fleetwood IG, Hochman S,
Dodd JG, Cheng GK, Jordan LM and Brownstone RM: Functional motor
neurons differentiating from mouse multipotent spinal cord
precursor cells in culture and after transplantation into
transected sciatic nerve. J Neurosurg. 98:1094–1103. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xia L, Wan H, Hao SY, Li DZ, Chen G, Gao
CC, Li JH, Yang F, Wang SG and Liu S: Co-transplantation of neural
stem cells and Schwann cells within poly (L-lactic-co-glycolic
acid) scaffolds facilitates axonal regeneration in hemisected rat
spinal cord. Chin Med J (Engl). 126:909–917. 2013.PubMed/NCBI
|
|
21
|
Zhang X, Zeng Y, Zhang W, Wang J, Wu J and
Li J: Co-transplantation of neural stem cells and
NT-3-overexpressing Schwann cells in transected spinal cord. J
Neurotrauma. 24:1863–1877. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zeng YS, Ding Y, Wu LZ, Guo JS, Li HB,
Wong WM and Wu WT: Co-transplantation of schwann cells promotes the
survival and differentiation of neural stem cells transplanted into
the injured spinal cord. Dev Neurosci. 27:20–26. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu L, Zhou S, Feng GY, Zhang LP, Zhao DM,
Sun Y, Liu Q and Huang F: Neural stem cells enhance nerve
regeneration after sciatic nerve injury in rats. Mol Neurobiol.
46:265–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Svennigsen Fex A and Dahlin LB: Repair of
the Peripheral Nerve-Remyelination that Works. Behav Brain Sci.
3:1182–1197. 2013.
|
|
25
|
Mikami Y, Okano H, Sakaguchi M, Nakamura
M, Shimazaki T, Okano HJ, Kawakami Y, Toyama Y and Toda M:
Implantation of dendritic cells in injured adult spinal cord
results in activation of endogenous neural stem/progenitor cells
leading to de novo neurogenesis and functional recovery. J Neurosci
Res. 76:453–465. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Evans GR: Peripheral nerve injury: A
review and approach to tissue engineered constructs. Anat Rec.
263:396–404. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Madduri S and Gander B: Schwann cell
delivery of neurotrophic factors for peripheral nerve regeneration.
J Peripher Nerv Syst. 15:93–103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
An YH, Wan H, Zhang ZS, Wang HY, Gao ZX,
Sun MZ and Wang ZC: Effect of rat Schwann cell secretion on
proliferation and differentiation of human neural stem cells.
Biomed Environ Sci. 16:90–94. 2003.PubMed/NCBI
|
|
29
|
Niapour A, Karamali F, Nemati S, Taghipour
Z, Mardani M, Nasr-Esfahani MH and Baharvand H: Cotransplantation
of human embryonic stem cell-derived neural progenitors and schwann
cells in a rat spinal cord contusion injury model elicits a
distinct neurogenesis and functional recovery. Cell Transplant.
21:827–843. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Heath CA: Cells for tissue engineering.
Trends Biotechnol. 18:17–19. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shen Y, Xu J, Xu W, Xu L, Lu J and Gu Y:
Experimental study on neural stem cell transplantation delaying
denervated muscle atrophy. Zhongguo Xiu Fu Chong Jian Wai Ke Za
Zhi. 22:1051–1055. 2008.(In Chinese). PubMed/NCBI
|
|
32
|
Leibrock J, Lottspeich F, Hohn A, Hofer M,
Hengerer B, Masiakowski P, Thoenen H and Barde YA: Molecular
cloning and expression of brain-derived neurotrophic factor.
Nature. 341:149–152. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Squinto SP, Stitt TN, Aldrich TH, Davis S,
Bianco SM, Radziejewski C, Glass DJ, Masiakowski P, Furth ME and
Valenzuela DM: trkB encodes a functional receptor for brain-derived
neurotrophic factor and neurotrophin-3 but not nerve growth factor.
Cell. 65:885–893. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Henderson CE, Camu W, Mettling C, Gouin A,
Poulsen K, Karihaloo M, Rullamas J, Evans T, McMahon SB and
Armanini MP: Neurotrophins promote motor neuron survival and are
present in embryonic limb bud. Nature. 363:266–270. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matheson CR, Carnahan J, Urich JL,
Bocangel D, Zhang TJ and Yan Q: Glial cell line-derived
neurotrophic factor (GDNF) is a neurotrophic factor for sensory
neurons: Comparison with the effects of the neurotrophins. J
Neurobiol. 32:22–32. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nagano M and Suzuki H: Quantitative
analyses of expression of GDNF and neurotrophins during postnatal
development in rat skeletal muscles. Neurosci Res. 45:391–399.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Iwase T, Jung CG, Bae H, Zhang M and
Soliven B: Glial cell line-derived neurotrophic factor-induced
signaling in Schwann cells. J Neurochem. 94:1488–1499. 2005.
View Article : Google Scholar : PubMed/NCBI
|