Introduction
Kidney transplantation is the most effective
treatment for end-stage renal disease in patients who require
dialysis, helping to improve their quality of life and prolong
their survival (1). There are
several immunosuppressive agents currently used for renal
transplantation, which are effective at preventing acute rejection
throughout the transplantation process. However, the process still
has some adverse effects, including ischemia/reperfusion injury
(IRI), which occurs after blood flow recovery and restoration to
tissues and organs. It has previously been identified that IRI is
associated with an increased incidence of acute rejection and
decreased long-term allograft survival, and is considered to be one
of the leading causes of early allograft dysfunction (2,3).
Therefore, it is necessary to adopt effective measures to reduce or
alleviate IRI during kidney transplantation.
Ozone is a powerful oxidizing gas, which is commonly
used as a disinfectant in the water and food industries (4). Although ozone is a potentially toxic
agent, it can modulate many biochemical pathways at low and
controlled non-toxic doses (5).
Medical ozone therapy involves delivering a mixture of gaseous
ozone and oxygen to the body (6). It
has previously been identified that ozone therapy can be useful in
treating inflammation-mediated diseases, such as infected wounds,
burns, and advanced ischemic diseases (7). Ozone therapy also results in resistance
to oxidative stress by inducing antioxidant systems (8,9).
Furthermore, it has been reported that ozone oxidative
preconditioning (OOP), a type of ozone therapy (10) has a protective effect against IRI in
the liver and kidney (11,12). To our knowledge, the current study is
the first to investigate the effect of OOP on IRI in a homologous
kidney transplantation in a rat model.
Materials and methods
Animal preparation
The experimental protocol used in the present study
was approved by the Animal Ethics Review Committee of Wuhan
University (Wuhan, China), and the procedures were conducted in
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals from the National Institutes of Health. A
total of 36 clean male, 7-week-old, Sprague Dawley (SD) rats
(250–300 g) were purchased from Huazhong University of Science and
Technology, all of them were kept in an air-filtered, homeothermal
(20–22°C), and light-controlled (light between 7:00 a.m. and 7:00
p.m.) room, and allowed free access to a standard diet.
Experimental protocol
Kidney donor rats were randomly divided into three
groups, 12 rats in each, with 6 donors and 6 recipients. In the OOP
and kidney transplantation (OOP+KT) group, donor rats (n=6)
received 15 OOP treatments by transrectal insufflations (1 mg/kg),
once a day, at an ozone concentration of 50 µg/ml, before their
left kidneys were transplanted into recipient rats. In the kidney
transplantation (KT) group, recipient rats each received a left
kidney from a donor rat that had not undergone OOP treatment. The
experiments that follow were only on the donated kidneys in groups
OOP+KT (n=6) and KT (n=6). In the sham (S) group (n=12), the rats'
abdomens were opened and closed without transplantation; six
kidneys from six rats were used in the subsequent experiments.
Surgical procedure
In all groups, donors were injected
intraperitoneally with atropine (0.01 mg/kg), buprenorphine (0.04
mg/kg) and diazepam (10 mg/kg), all drugs were purchased from
Sigma-Aldrich; Merck Millipore (Darmstadt, Germany). After 10 min,
they were anesthetized with pentobarbital (45 mg/kg). Then, the
donor's blood vessels and ureter were fully separated. The kidneys
were flushed through the aorta with 3 ml of 4°C cold Ringer's
lactate solution (Shanghai Baxter Healthcare Co., Ltd., Shanghai,
China) with heparin (50 units/ml) until homogeneously pale. Only
the left kidney of each rat was harvested, leaving the renal
arteriovenous, ureter and parts of bladder around the ureter
openings intact. Each kidney was placed in cold Ringer's lactate
solution at 4°C for 180 min. Recipient rats underwent a left
nephrectomy prior to accepting the donated kidneys. Orthotopic
renal transplantation was then performed. The donor SD rat kidney
was implanted orthotopically into a recipient SD rat with
end-to-end anastomosis of renal artery, vein and ureter. Finally,
recipient rats underwent right nephrectomy. During the surgery,
body temperature was monitored and constantly kept between 35°C and
37°C. After transplantation, the rats were placed on a warm blanket
with free access to water and standard laboratory chow.
Preservation of kidneys
After all the recipient rats were anesthetized with
atropine (0.01 mg/kg), buprenorphine (0.04 mg/kg) and diazepam (10
mg/kg), blood of recipient rats was drawn for analysis 24 h
following kidney transplantation. The renal allografts were
harvested for subsequent experiments. While under anesthesia, the
animals were sacrificed by cervical dislocation. All rats underwent
a laparotomy and nephrectomy to harvest the kidneys. Kidneys were
then fixed in 10% phosphate-buffered formalin or immediately
frozen, and stored at −80°C for subsequent experiments.
Serum assays
To assess creatinine (Cr) and blood urea nitrogen
(BUN) serum levels, blood samples were collected, centrifuged and
kept at −20°C until analysis. The samples were examined using an
Olympus AU 2700 Analyzer (Olympus Corporation, Tokyo, Japan),
following standard techniques.
Histological examination
The kidney was fixed in 10% neutral-buffered
formalin, embedded in paraffin wax and cut into 4-µm thick sections
according to standard procedure. Sections were deparaffinzed and
hydrated gradually and examined using hematoxylin and eosin
staining and immunohistochemistry. Morphological assessment was
performed by an experienced renal pathologist who was unaware of
the treatment that had been carried out in each case, images were
captured using a BX53F light microscope (Olympus Corporation), at a
magnification of ×400. A grading scale of 0–4, as outlined by
Jablonski et al (13), was
used for the histopathological assessment of isogeneic renal
transplantation-induced damage of the proximal tubules.
Periodic acid-Schiff staining
Paraffin sections were routinely dewaxed to water.
Serial sections (4-µm) were washed with distilled water, incubated
in 0.5–1% (v/v) aqueous periodate for 5–10 min, and washed a
further three times with distilled water. The sections were then
incubated in Schiff's reagent (Fuzhou Maixin Biotechnology
Development Co., Ltd., Fuzhou, China) for 10–30 min. After
staining, the sections were washed three times with sulfite, then
Rinsed with running water for 10 min, and then washed with
distilled water once. The sections were then counterstained with
hematoxylin to identify nuclei.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
To observe cell apoptosis induced by ischemia, an
in situ apoptosis detection kit (Promega Corporation,
Madison, WI, USA) was used. The TUNEL assay was performed based on
the manufacturer's instructions. Briefly, after being fixed in 4%
paraformaldehyde/phosphate-buffered saline (PBS; pH 7.4) solution
at 4°C overnight, the whole specimens were extensively washed with
1X PBS solution three times, then immersed into 70% ethanol for at
least 24 h at 20°C. After being washed a further three times with
PBS solution, the samples were immersed into a permeabilization
buffer for 15 min on ice then washed again with PBS solution.
Subsequently they were incubated in 50 ml reaction buffer (5 ml
terminal deoxynucleotidyl transferase enzyme and 45 ml Labeling
Safe Buffer) (Promega Corporation) for 90 min at 37°C. The labeling
procedure was stopped by washing with PBS solution. The image was
analyzed using a Zeiss LSM 510 Confocal laser scanning microscope
(Carl Zeiss AG, Oberkoden, Germany) with a 488 nm excitation line
and a 530 nm emission filter. Five high-power fields of vision in
the distribution areas of the apoptotic cells in each slide were
randomly selected, and the average number of apoptotic cells per
100 cells was calculated. The apoptotic index (AI) was expressed as
a percentage.
Measurement of malondialdehyde (MDA),
superoxide dismutase (SOD), glutathione (GSH) and catalase (CAT)
levels in the kidney
Renal tissue MDA concentration was measured using
the thiobarbituric acid method. Levels of lipid peroxides were
measured as an indicator of MDA production rates (assay kit;
Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Absorbance was measured at 532 nm using a spectrometer. SOD
activity in renal tissue was measured using a commercialized
chemical assay kit (Nanjing Jiancheng Bioengineering Institute) by
the xanthine oxidase method. Absorbance was determined at 550 nm
using a spectrometer. GSH content was detected using a colorimetric
GSH detection kit (Nanjing Jiancheng Bioengineering Institute).
Briefly, the renal homogenate was mixed with reagent A, reagent B
and reagent C. Absorbance of the yellow solution was measured at
412 nm. CAT activity in renal tissue was measured using a
commercialized chemical assay kit (Nanjing Jiancheng Bioengineering
Institute) by the visible light method. Absorbance was determined
at 405 nm using a spectrometer. All protein concentrations of renal
tissue homogenate samples were determined using the Coomassie Blue
method (assay kit; Nanjing Jiancheng Bioengineering Institute).
Immunohistochemistry
The expression levels of nuclear factor erythroid
2-related factor 2 (Nrf-2) and heme oxygenase 1 (HO-1) were
evaluated using immunohistochemical staining. Briefly, 5-µm
sections were deparaffinized, and endogenous peroxidase activity
was blocked with 3% hydrogen peroxide at 37°C for 10 min. Then, the
sections were treated with 10% normal goat serum (Wuhan Boster
Biological Technology, Ltd., Wuhan, China) in Tris-buffered saline
for 30 min at 37°C. Subsequently, they were incubated overnight at
4°C with a rabbit polyclonal anti-rat antibody to Nrf-2 (catalogue
no. sc-722, 1:300; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
and a rabbit polyclonal anti-rat antibody to HO-1 (catalogue no.
10701-1-AP, 1:1,000; Wuhan Sanying Biotechnology; Proteintech Group
Inc., Wuhan, China). After washing three times with PBS, these
sections were incubated with the horseradish peroxidase
(HRP)-conjugated anti-rabbit secondary antibody (catalogue no.
BA1054, 1:5,000, Wuhan Boster Biological Technology, Ltd., Wuhan,
China) for 30 min at room temperature, after which the color
reagent 3,3′-diaminobenzidine was added. For the negative control
group, the same procedures were performed with the exception of
adding the primary antibody.
Western blot analysis
Proteins were extracted and purified from renal
tissue as previously described (14). In brief, protein samples were
prepared for gel electrophoresis and separated on 12.5% sodium
dodecyl sulfate-polyacrylamide gels (40 µg/lane), then transferred
to a nitrocellulose membrane (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The membrane was blocked with 5% nonfat dry milk in
Tris-buffered saline with Tween (TBST) buffer, then incubated with
primary antibodies overnight at 4°C. After rinsing with TBST buffer
extensively, the blots were incubated with secondary antibodies,
and developed with the use of an enhanced chemiluminescence kit
(Pierce Protein Biology; Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Finally, the blots were captured on light-sensitive
imaging film (Kodak, Rochester, NY, USA) for analysis. The
following antibodies were used: A rabbit polyclonal antibody to
Nrf-2 (catalogue no. sc-722, 1:300; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), a rabbit polyclonal antibody to HO-1 (catalogue
no. 10701-1-AP, 1:1,000; Wuhan Sanying Biotechnology, Wuhan,
China), and a rabbit polyclonal antibody to GAPDH (catalogue no.
BA-2913, 1:300; Wuhan Boster Biological Technology, Ltd., Wuhan,
China). GAPDH was used to show equal amounts of protein loading in
each lane. HRP-conjugated anti-rabbit (BA-1054, 1:5,000; Wuhan
Boster Biological Technology, Ltd., Wuhan, China) or anti-mouse
secondary antibodies (BA-1051, 1:5,000, Wuhan Boster Biological
Technology, Ltd., Wuhan, China). The expression levels following
the western blot analysis of each protein was assessed for the
three groups using ImageJ software. To quantify the results, the
average gray value of each protein was calculated.
Statistical analysis
All the data were statistically analyzed using SPSS
18.0 statistical software (SPSS Inc., Chicago, IL, USA) and
presented as the mean ± standard deviation. Statistical differences
were analyzed using the Student's t-test and P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of OOP on renal function after
kidney transplantation
The renal functional parameters of rats were
detected 24 h after renal transplantation. Rats subjected to
isogeneic renal transplantation showed a significant increase in
BUN and Cr levels compared with sham-operated rats (P<0.05). The
negative effects on renal function induced by renal transplantation
were significantly reduced in the OOP+KT group compared with the KT
group, in relation to levels of BUN or serum Cr (P<0.05;
Fig. 1).
Effect of OOP on morphological
features of renal cells
Histopathological examination revealed that
morphological lesions existed in the allograft kidney tissue after
isogeneic renal transplantation. Renal injury was evidenced by loss
of brush borders, congestion, tubular cell swelling and tubular
dilation. However, this renal damage was attenuated by OOP
(Figs. 2 and 3). Furthermore, the Jablonski grade
analysis of severe acute tubular necrosis results showed that the
OOP+KT group had significantly lower levels of damage compared with
the KT group (Table I). In addition,
OOP significantly reduced the AI in the OOP+KT group compared with
the KT group (P<0.05; Table I and
Fig. 4).
 | Table I.Effect of OOP on Jablonski grade and
apoptosis index in renal tissue (n=6). |
Table I.
Effect of OOP on Jablonski grade and
apoptosis index in renal tissue (n=6).
| Group | S | KT | OOP+KT |
|---|
| Jablonski
grade | 0.31±0.27 |
3.16±0.35a |
2.43±0.29a,b |
| Apoptosis index,
% | 1.58±0.35 |
27.45±2.46a |
17.80±2.73a,b |
Levels of SOD, MDA, GSH and CAT in
renal tissue
As shown in Table
II, the level of MDA content, which was indicated by the level
of lipid peroxidation, was significantly higher in the KT and
OOP+KT groups than in the S group (P<0.05). However, the level
of MDA in the OOP+KT group was significantly lower than in the KT
group (P<0.05). Meanwhile, the levels of SOD, GSH and CAT in the
kidney tissue were significantly decreased after kidney
transplantation in the KT group and the OOP+KT group compared with
the S group (P<0.05). However, the levels of SOD, GSH and CAT
after kidney transplantation were significantly higher in the
OOP+KT group compared with the KT group (P<0.05).
 | Table II.Effect of OOP on the protein
expression levels of SOD, MDA, GSH and CAT in renal tissue 24 h
after renal transplantation (n=6). |
Table II.
Effect of OOP on the protein
expression levels of SOD, MDA, GSH and CAT in renal tissue 24 h
after renal transplantation (n=6).
| Groups | SOD, U/mg | MDA, nmol/mg | GSH, nmol/mg | CAT, U/mg |
|---|
| S | 85.83±7.24 | 3.40±1.30 | 10.10±0.94 | 71.93±6.43 |
| KT |
39.36±8.23a |
6.26±1.87a |
3.29±1.65a |
35.75±5.93a |
| OOP+KT |
57.42±9.02a,b |
4.83±1.92a,b |
7.46±1.49a,b |
57.55±7.31a,b |
Expression levels of Nrf-2 and
HO-1
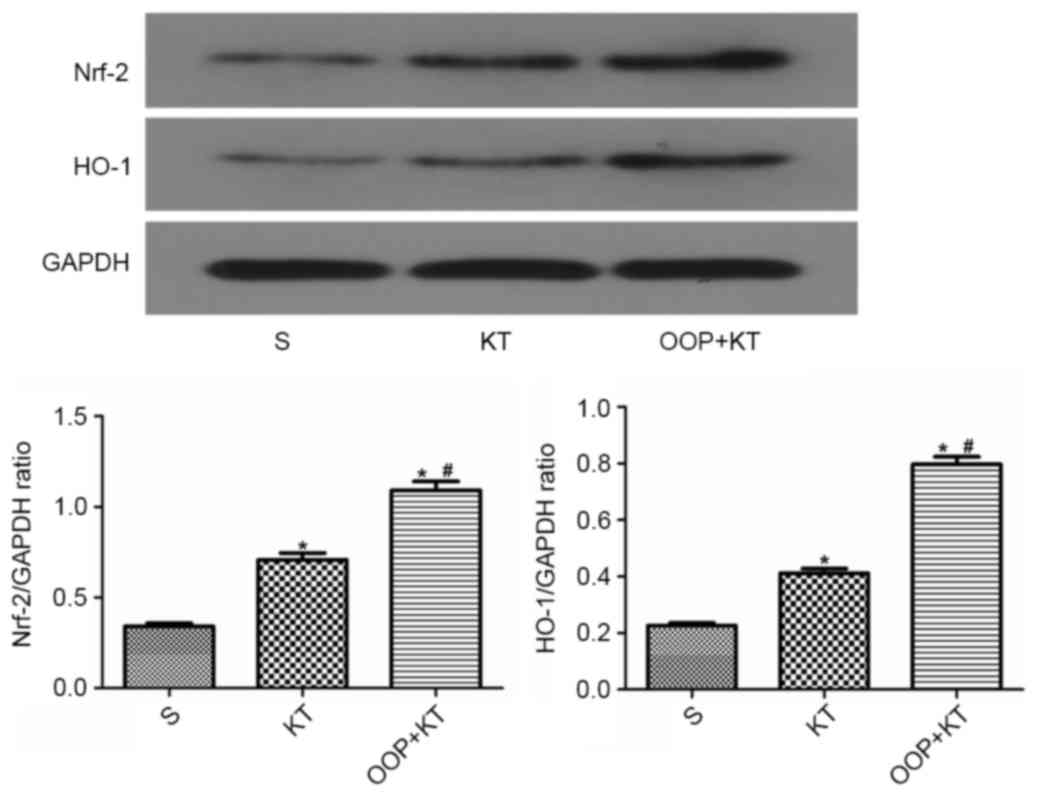
The results of the immunohistochemistry analysis
showed that the expression levels of Nrf-2 and HO-1 in the OOP+KT
group were higher than those in the KT group (Figs. 5 and 6). Furthermore, the western blot analysis
confirmed that the expression levels of Nrf-2 and HO-1 in the
OOP+KT group were significantly higher than those in the KT group
(P<0.05; Fig. 7).
Discussion
Compared with dialysis treatment, kidney
transplantation is often seen as preferable for patients with
end-stage kidney disease, and previous research confirms that renal
transplant patients have a longer survival time, with a better
quality of life, than dialysis patients (15,16).
However, despite a low occurrence of acute allograft rejection due
to the application of novel anti-immunity drugs, some detrimental
factors still exist during the perioperative period of renal
transplantation, such as IRI and chronic graft rejection (17).
IRI is a pathological process that involves
oxidative stress, intracellular calcium overloading, inflammation
reaction and cell apoptosis. Oxidative stress is one of the most
important components of IRI, and is primarily caused by excessive
reactive oxygen species (ROS) being generated in ischemic tissue
after reperfusion (18). Naturally
generated antioxidant enzymes can counteract the cellular effects
of oxygen free radicals under normal conditions (18,19), but
excessive ROS generation during the period of ischemia reperfusion
cannot be effectively controlled by this system (20). It has been reported that inflammation
cytokines and chemokines are released during oxygen radical
generation and lipid peroxidation. Together with adhesion
molecules, these attract inflammatory cells, such as monocytes and
neutrophils, which in turn help to release more ROS and aggravate
renal damage (2). Therefore,
effective measures or treatments to attenuate oxidative stress or
help balance the oxidative and anti-oxidative systems may be useful
in alleviating IRI during renal transplantation.
In the present study, the effects of OOP on kidney
transplantation were investigated in a rat model. It was found that
pretreatment with a controlled concentration of ozone before kidney
transplantation could decrease oxidative stress injury,
demonstrated by a significantly reduced renal tissue concentration
of MDA in the OOP+KT group compared with the KT group. Furthermore,
compared with the KT group, indicators of anti-oxidative stress
significantly increased in the allograft kidney tissue, such as
SOD, GSH and CAT. These results were consistent with a prior study,
which identified that OOP improves organ stress by enhancing
endogenous protective mechanisms (21).
Nrf2 belongs to the cap ‘n’ collar family, a small
group of transcription factors that contain a unique basic leucine
zipper motif (22,23). Nrf2 principally regulates
transcriptional activation through an antioxidant responsive
element (ARE) (24), which is
considered to be a cis-acting regulatory element in promoter
regions of many cytoprotective genes. Under basal conditions, Nrf2
is constantly targeted for Keap-1-mediated ubiquitination and
subsequent proteasomal degradation to maintain low Nrf2 protein
levels (25). However, upon
activation, Nrf2 can activate downstream antioxidant proteins,
phase II metabolizing/detoxifying enzymes and phase III
ATP-dependent drug efflux pump-encoded genes (26–28). It
has been reported that Nrf2 activates numerous types of enzymes
with antioxidation and detoxifying activities that serve a key
function in the protection of cells against various environmental
stresses, such as electrophiles, ROS and reactive nitrogen species
(29). In the present study, the
expression level of Nrf2 in the OOP+KT group was significantly
higher than that in the KT group. A proposed explanation for this
is the low levels of OOP in the KT group, which could have helped
to trigger the activation of Nrf2/Keap-1. Furthermore, a prior
study indicates that overexpression of Nrf2 increases ARE
transcriptional activity and enhances the expression of several
ARE-dependent antioxidant and cytoprotective enzymes, including
HO-1, glutamate cysteine ligase, glutathione peroxidase and NAD(P)H
quinone oxidoreductase 1 (30). In
the present study, the expression level of HO-1 in the OOP+KT group
was significantly higher than that in the KT group, which could
help to explain why the OOP+KT group displayed less severe
oxidative stress injury than the KT group. In addition, it has
previously been proposed that Nrf2-dependent HO-1 expression could
inhibit the activation of nuclear factor κB, stimulated by tumor
necrosis factor alpha and monocyte chemoattractant protein-1
secretion in endothelial cells (31).
In addition, the present results suggest that OOP
could reduce the rate of apoptosis in renal tubular epithelium
cells caused by oxidative stress injury during the process of renal
transplantation. It has been demonstrated that excessive ROS
overwhelming the scavenging capacity of the endogenous antioxidant
system can lead to cellular damage (32), such as blocking cellular
mitochondrial respiration (33), or
facilitating the formation of mitochondrial transition pores, which
help to increase the release of apoptosis-related proteins after
ischemia reperfusion (34,35). A previous study outlines several
measures to prevent oxidative stress-induced apoptosis, such as
increasing the activity of the endogenous antioxidant system,
applying exogenous antioxidant enzymes or free radical scavengers,
or decreasing the lipid peroxidation byproducts (36). In this study, it was indicated that
OOP reduces the apoptosis or necrosis of renal tubular epithelium
cells, which may be related to the strengthening of endogenous
antioxidant systems, such as the Nrf2/HO-1 pathway.
In conclusion, this study demonstrated that OOP
could mitigate oxidative stress injury and apoptosis of renal
tubular epithelium cells during kidney transplantation in a rat
model, which may be related to activation of the Nrf2/HO-1
signaling pathway and reduction of renal tubular epithelial cell
apoptosis. However, the long-term effect of OOP on renal
transplantation has not been studied here, such as the differences
in survival rate between groups. Furthermore, the effects of OOP on
the immune response and inflammation between different strains of
rats, especially in the acute immunological rejection model, need
to be investigated further.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81400753) and the Natural
Science Foundation of Hubei Province (grant no. 2014CFB362).
References
|
1
|
Wolfe RA, Ashby VB, Milford EL, Ojo AO,
Ettenger RE, Agodoa LY, Held PJ and Port FK: Comparison of
mortality in all patients on dialysis, patients on dialysis
awaiting transplantation, and recipients of a first cadaveric
transplant. N Engl J Med. 341:1725–1730. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Perico N, Cattaneo D, Sayegh MH and
Remuzzi G: Delayed graft function in kidney transplantation.
Lancet. 364:1814–1827. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peeters P, Terryn W, Vanholder R and
Lameire N: Delayed graft functioning in renal transplantation. Curr
Opin Crit Care. 10:489–498. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gulmen S, Kurtoglu T, Meteoglu I and
Okutan H: Ozone therapy as an adjunct to vancomycin enhances
bacterial elimination in methicillin resistant Staphylococcus
aureus mediastinitis. J Surg Res. 185:64–69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bocci V: Is it true that ozone is always
toxic? The end of a dogma. Toxicol Appl Pharmacol. 216:493–504.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sagai M and Bocci V: Mechanisms of action
involved in ozone therapy: Is healing induced via a mild oxidative
stress? Med Gas Res. 1:292011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bocci VA: Scientific and medical aspects
of ozone therapy. State of the art. Arc Med Res. 37:425–435. 2006.
View Article : Google Scholar
|
|
8
|
Inal M, Dokumacioglu A, Ozcelik E and Ucar
O: The effects of ozone therapy and coenzyme Q10 combination on
oxidative stress markers in healthy subjects. Ir J Med Sci.
180:703–707. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guven A, Gundogdu G, Sadir S, Topal T,
Erdogan E, Korkmaz A, Surer I and Ozturk H: The efficacy of ozone
therapy in experimental caustic esophageal burn. J Pediatr Surg.
43:1679–1684. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bakkal BH, Gultekin FA, Guven B, Turkcu
UO, Bektas S and Can M: Effect of ozone oxidative preconditioning
in preventing early radiation-induced lung injury in rats. Braz J
Med Biol Res. 46:789–796. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen H, Xing B, Liu X, Zhan B, Zhou J, Zhu
H and Chen Z: Ozone oxidative preconditioning protects the rat
kidney from reperfusion injury: The role of nitric oxide. J Surg
Res. 149:287–295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ajamieh HH, Menéndez S, Martínez-Sánchez
G, Candelario-Jalil E, Re L, Giuliani A and Fernández OS: Effects
of ozone oxidative preconditioning on nitric oxide generation and
cellular redox balance in a rat model of hepatic
ischaemia-reperfusion. Liver Int. 24:55–62. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jablonski P, Howden BO, Rae DA, Birrell
CS, Marshall VC and Tange J: An experimental model for assessment
of renal recovery from warm ischemia. Transplantation. 35:198–204.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park KM, Cho HJ and Bonventre JV:
Orchiectomy reduces susceptibility to renal ischemic injury: A role
for heat shock proteins. Biochem Biophys Res Commun. 328:312–317.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Laupacis A, Keown P, Pus N, Krueger H,
Ferguson B, Wong C and Muirhead N: A study of the quality of life
and cost-utility of renal transplantation. Kidney Int. 50:235–242.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schnuelle P, Lorenz D, Trede M and Van Der
Woude FJ: Impact of renal cadaveric transplantation on survival in
end-stage renal failure: Evidence for reduced mortality risk
compared with hemodialysis during long-term follow-up. J Am Soc
Nephrol. 9:2135–2141. 1998.PubMed/NCBI
|
|
17
|
Menke J, Sollinger D, Schamberger B,
Heemann U and Lutz J: The effect of ischemia/reperfusion on the
kidney graft. Curr Opin Organ Transplant. 19:395–400. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haugen E and Nath KA: The involvement of
oxidative stress in the progression of renal injury. Blood Purif.
17:58–65. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Edelstein CL, Ling H and Schrier RW: The
nature of renal cell injury. Kidney Int. 51:1341–1351. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Castaneda MP, Swiatecka-Urban A, Mitsnefes
MM, Feuerstein D, Kaskel FJ, Tellis V and Devarajan P: Activation
of mitochondrial apoptotic pathways in human renal allografts after
ischemia reperfusion injury. Transplantation. 76:50–54. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rodriguez ZZ, Guanche D, Alvarez RG,
Rosales FH, Alonso Y and Schulz S: Preconditioning with
ozone/oxygen mixture induces reversion of some indicators of
oxidative stress and prevents organic damage in rats with fecal
peritonitis. Inflamm Res. 58:371–375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Itoh K, Igarashi K, Hayashi N, Nishizawa M
and Yamamoto M: Cloning and characterization of a novel erythroid
cell-derived CNC family transcription factor heterodimerizing with
the small Maf family proteins. Mol Cell Biol. 15:4184–4193. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sykiotis GP and Bohmann D:
Stress-activated cap‘n’collar transcription factors in aging and
human disease. Sci Signal. 3:re32010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang HC, Nguyen T and Pickett CB:
Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates
antioxidant response element-mediated transcription. J Biol Chem.
277:42769–42774. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang KM, Chen HH, Wang TC, Chen IL, Chen
YT, Yang SC, Chen YL, Chang HH, Huang CH, Chang JY, et al: Novel
oxime-bearing coumarin derivatives act as potent Nrf2/ARE
activators in vitro and in mouse model. Eur J Med Chem. 106:60–74.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Giudice A and Montella M: Activation of
the Nrf2-ARE signaling pathway: A promising strategy in cancer
prevention. Bioessays. 28:169–181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maher JM, Dieter MZ, Aleksunes LM, Slitt
AL, Guo G, Tanaka Y, Scheffer GL, Chan JY, Manautou JE, Chen Y, et
al: Oxidative and electrophilic stress induces multidrug
resistance-associated protein transporters via the nuclear
factor-E2-related factor-2 transcriptional pathway. Hepatology.
46:1597–1610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y and Gordon GB: A strategy for
cancer prevention: Stimulation of the Nrf2-ARE signaling pathway.
Mol Cancer Ther. 3:885–893. 2004.PubMed/NCBI
|
|
29
|
Cominacini L, Mozzini C, Garbin U, Pasini
A, Stranieri C, Solani E, Vallerio P, Tinelli IA and Pasini Fratta
A: Endoplasmic reticulum stress and Nrf2 signaling in
cardiovascular diseases. Free Radic Biol Med. 88:233–242. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aboonabi A and Singh I: Chemopreventive
role of anthocyanins in atherosclerosis via activation of Nrf2-ARE
as an indicator and modulator of redox. Biomed Pharmacother.
72:30–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Osburn WO, Karim B, Dolan PM, Liu G,
Yamamoto M, Huso DL and Kensler TW: Increased colonic inflammatory
injury and formation of aberrant crypt foci in Nrf2-deficient mice
upon dextran sulfate treatment. Int J Cancer. 121:1883–1891. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chong ZZ, Li F and Maiese K: Oxidative
stress in the brain: Novel cellular targets that govern survival
during neurodegenerative disease. Prog Neurobiol. 75:207–246. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamamoto T, Maruyama W, Kato Y, Yi H,
Shamoto-Nagai M, Tanaka M, Sato Y and Naoi M: Selective nitration
of mitochondrial complex I by peroxynitrite: Involvement in
mitochondria dysfunction and cell death of dopaminergic SH-SY5Y
cells. J Neural Transm (Vienna). 109:1–13. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim GW, Kondo T, Noshita N and Chan PH:
Manganese superoxide dismutase deficiency exacerbates cerebral
infarction after focal cerebral ischemia/reperfusion in mice:
Implications for the production and role of superoxide radicals.
Stroke. 33:809–815. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Murakami K, Kondo T, Kawase M, Li Y, Sato
S, Chen SF and Chan PH: Mitochondrial susceptibility to oxidative
stress exacerbates cerebral infarction that follows permanent focal
cerebral ischemia in mutant mice with manganese superoxide
dismutase deficiency. J Neurosci. 18:205–213. 1998.PubMed/NCBI
|
|
36
|
Huang T, Cheng AG, Stupak H, Liu W, Kim A,
Staecker H, Lefebvre PP, Malgrange B, Kopke R, Moonen G and Van De
Water TR: Oxidative stress-induced apoptosis of cochlear sensory
cells: Otoprotective strategies. Int J Dev Neurosci. 18:259–270.
2000. View Article : Google Scholar : PubMed/NCBI
|