Introduction
Diffuse panbronchiolitis (DPB) is an idiopathic
inflammatory disease characterized by chronic respiratory
bronchioles of the bilateral lung that is effectively treated using
macrolides (1), and has historically
been associated with a very poor prognosis. Clinically, DPB is
characterized by persistent cough, sputum and progressive
exertional dyspnea. DPB is a progressive suppurative and
obstructive airway disease, resulting in bronchiectasis,
respiratory failure and mortality, if left untreated (2). This disease has been observed in the
Japanese population, and the incidence of DPB is high among Asian
individuals, the incidence was calculated at ~1 per 10,000
individuals per year. The average age of onset is 40 years,
however, it is rare in children (3).
The exact pathogenesis of this disease is still unknown but it may
be related to infection and genetic factors. The diagnostic
criteria of DPB was as follows: i) A persistent cough, sputum and
exertional dyspnea, ii) history or current symptoms of chronic
sinusitis, iii) bilateral, diffuse, small nodular shadows on a
plain chest radiograph or centrilobular micro nodules on chest
computed tomography (CT) images, iv) coarse crackles, v) the ratio
of forced expiratory volume in 1 sec (FEV1) to forced vital
capacity (FVC) (FEV1/FVC) <70% and partial pressure of oxygen
(PaO2) <80 mmHg (10.64 kPa) and vi) cold agglutinin
CHA titer of ≥64 (4). Long-term low
dose therapy with macrolide antibiotics has been demonstrated to
significantly improve the survival of DPB patients (2). To the best of our knowledge, DPB has
not been reported in a child from Western China. A 13-year-old
Chinese girl was described as the first child in the Chinese
literature with diffuse panbronchiolitis from northern China
(5). The present study describes a
case of DPB in a Chinese boy that was initially misdiagnosed as
bronchial asthma.
Case report
A 13-year-old Chinese boy was admitted to the
respiratory outpatient department of The Third Affiliated Hospital
of Third Military Medical University (Chongqing, China) in November
2014 with the chief complaint of recurrent cough and progressive
exertional dyspnea that had persisted for 1 year. Ethical approval
was obtained from the Institutional Review Board of the Institute
of Surgery Research, Daping Hospital, Third Military Medical
University and signed informed consent was obtained from the
parents of the patient.
The patient had a history of a slight cold over the
previous 2 weeks. He had no family history of asthma and was a
non-smoker. A few moist rales and expiratory rhonchi were audible
bilaterally on basilar areas of the back on chest auscultation. No
clubbing of the fingers was observed. Lung function testing
revealed a forced expiratory volume 1/forced vital capacity ratio
of <70% (normal ratio is ≥70%), and arterial blood gas testing
revealed an arterial partial pressure of oxygen (PaO2)
of <80 mmHg (normal PaO2 ≥80%). The patient was
diagnosed with bronchial asthma, and was prescribed inhaled
fluticasone combined with salmeterol (50/250 µg, twice daily;
GlaxoSmithKline plc. London, UK), and montelukast (4 mg daily, oral
administration, Merck & Co., Inc., Whitehouse Station, NJ,
USA). Regular outpatient department follow-up was recorded.
Following 2 months of treatment, no clinical
improvement was observed and the patient's physiology worsened. The
boy had a cough productive of purulent sputum, particularly in the
morning, and had an extremely nasal voice. Thus, Water's view X-ray
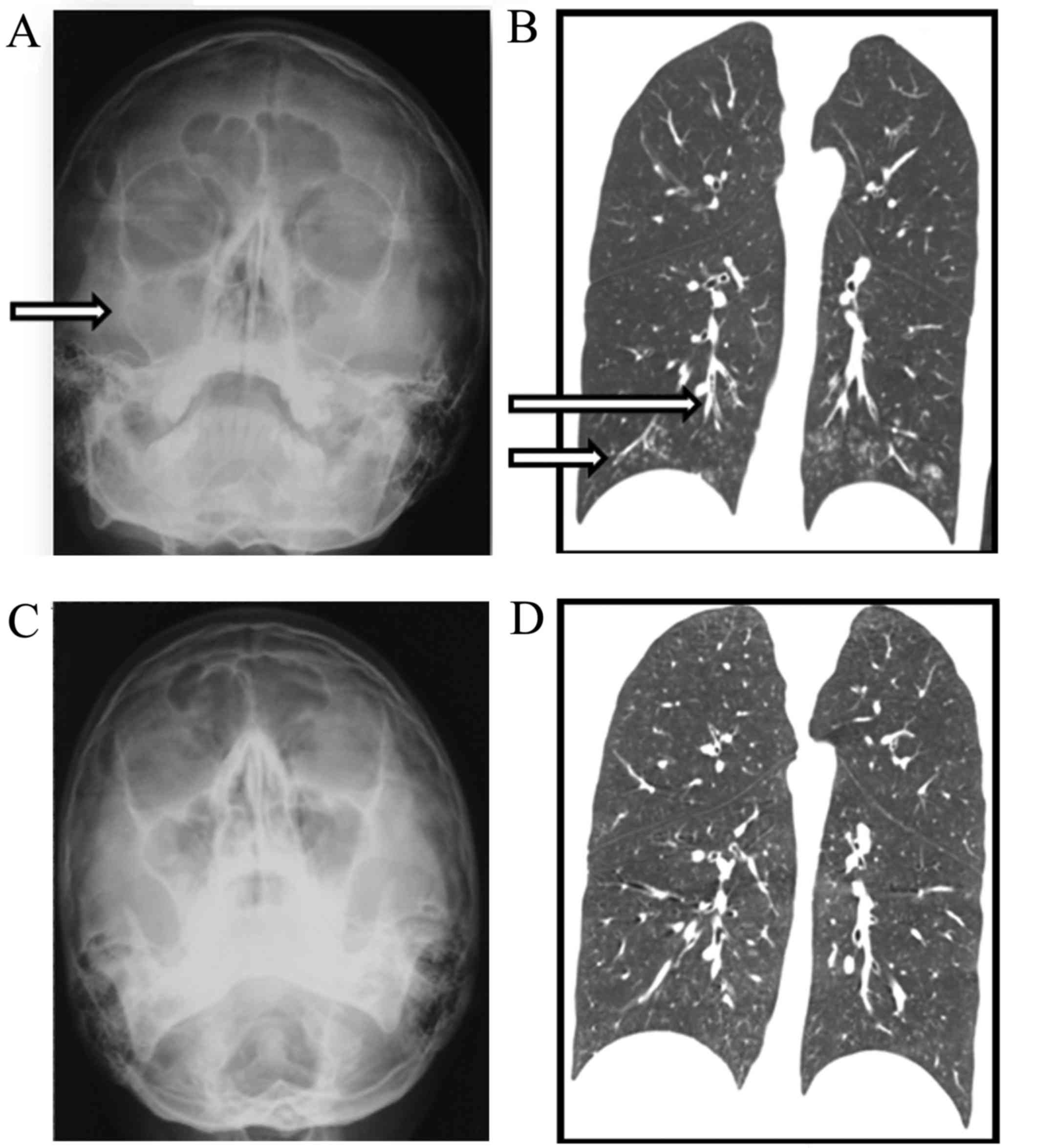
scanning was performed. Bilateral maxillary sinusitis was indicated
(Fig. 1A). X-ray imaging revealed
proliferation of the annular mucosa, but no fluid pooling. Also,
the sinus cavity wall exhibited no bone destruction or absorption
phenomenon, which prompted chronic inflammation of the two
maxillary sinuses. The clinical features of this case were as
follows: Persistent cough, sputum and exertional dyspnea,
expiratory rhonchi and moist rales audible bilaterally on basilar
areas of the back, chronic sinusitis, and spirometrically detected
airway obstruction that did not improve with the use of a
bronchodilator. Therefore, a preliminary diagnosis of DPB was made.
Chest high-resolution computed tomography (HRCT) revealed
centrilobular small nodular opacities, a ‘tree-in-bud appearance’
and thickening of the bronchial walls (Fig. 1B).
On the basis of this clinical profile, the child was
diagnosed with DPB. The initial treatment protocol was combination
therapy with erythromycin (0.5 g, oral administration, once a day;
Wanhe Pharmaceutical Co., Ltd., Tianchang, China) and inhaled
fluticasone combined with salmeterol (50/250 µg, 1 inhalation twice
a day). The adjuvant therapy with inhaled salmeterol/fluticasone
was administered in order to relieve the cough and dyspnea. After 4
weeks of treatment, the presenting symptoms of dyspnea on exertion,
cough and nasal congestion had improved or stabilized. The
fluticasone/salmeterol therapy was then stopped. A subsequent
maintenance treatment with erythromycin at a dose of 0.5 g every
other day was continued for 1 year. During that time, the symptoms
and results of imaging and pulmonary function tests progressively
improved, and remained normal for 1 year following cessation of the
erythromycin therapy (Fig. 1C and
D).
Discussion
The diagnosis of the present case was made using
previously reported diagnostic criteria for DPB (6). DPB was first described in China in
1996, and the average age of onset is 40 years (7). We found by only 3 previous reports of
DPB cases in children. In 2007, a 13-year-old Chinese girl was the
first child to be described with DPB in the Chinese literature,
indicating that DPB morbidity may affect children. The girl who was
diagnosed with diffuse panbronchiolitis following a 12-year history
of progressive course of cough with purulent sputum and chronic
sinusitis (5). A 12-year-old
Caucasian Turkish girl of first-degree consanguineous parentage was
described as the first child in the English language literature
with diffuse panbronchiolitis. She was diagnosed with diffuse
panbronchiolitis after a 5-year history of productive cough with
purulent sputum and chronic sinusitis (8). A 10-year-old South Korean boy who was
adopted by non-smoking professional American parents as a
4-month-old infant was described as the youngest child with diffuse
panbronchiolitis and uniquely provides the results of a
bronchoalveolar lavage. He had a slowly progressive course over a
6-month period (3). Each of the 3
patients in these reports was initially misdiagnosed, with
nasosinusitis, bronchiectasis or even pulmonary tuberculosis as
incorrect diagnoses. The duration of misdiagnosis ranged from 6
months to 12 years. None of these children were diagnosed correctly
at the first instance.
The delay time interval between onset and diagnosis
in the present case was the shortest in a child case of DPB
reported to date. Additionally, to the best of our knowledge, this
case is the first child with DPB reported in Western China. In the
present patient, through early diagnosis and intervention with
erythromycin, complete reversal of the signs of the disease without
evidence of persistent respiratory compromise was achieved. This
was in contrast to the 3 previously reported cases in children, who
had presented with substantial, irreversible lung disease, which
continued to deteriorate, despite treatment with macrolide. A
prolonged period of diffuse panbronchiolitis is associated with
progressive lung damage, so this case illustrates the importance of
early diagnosis and treatment.
In conclusion, DPB may be able to affect children
anywhere in Mainland China; therefore, it is recommended that
respiratory physicians and pediatricians are familiar with the
clinical and radioactive features of DPB. In children who have
chronic cough, expectoration, gasping, decreased pulmonary
ventilation function and PaO2, and nasosinusitis, either
at presentation or in their medical history, this disease should be
considered and HRCT used to obtain an early diagnosis so that
treatment with macrolide is initiated as early as possible.
References
|
1
|
Sugiyama Y: Diffuse panbronchiolitis. Clin
Chest Med. 14:765–772. 1993.PubMed/NCBI
|
|
2
|
Sugimoto S, Miyoshi K, Yamane M and Oto T:
Lung transplantation for diffuse panbronchiolitis: 5 cases from a
single centre. Interact Cardiovasc Thorac Surg. 22:679–681. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weinberger M, Fischer A and Kao S: Diffuse
panbronchiolitis in a 10-year-old boy. Pediatr Pulmonol.
50:E32–E34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chuang MC, Chou YT, Lin YC, Hsieh MJ and
Tsai YH: Diffuse panbronchiolitis-The response and recurrence after
erythromycin therapy. J Formos Med Assoc. 115:876–882. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao SY, Peng Y, Zhou CJ, Jiao Ax and
Jiang ZF: Diffuse panbronchiolitis in a child: Case report and
literature review. Zhonghua Er Ke Za Zhi. 45:504–507. 2007.(In
Chinese). PubMed/NCBI
|
|
6
|
Kudoh S and Keicho N: Diffuse
panbronchiolitis. Clin Chest Med. 33:297–305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu YN, Hu H and Cai ZL: A case of diffuse
panbronchiolitis. Chin J Tuberc Respir Dis. 19:118–119. 1996.(In
Chinese).
|
|
8
|
Asian AT, Ozcelik U, Talim B, Haliloglu M,
Dogru D, Dalgic F and Kiper N: Childhood diffusepanbronchiolitis: A
case report. Pediatr Pulmonol. 40:354–357. 2005. View Article : Google Scholar : PubMed/NCBI
|