Introduction
Osteoporosis is a disease that frequently presents
asymptomatically, and is typically characterized by low bone mass,
microarchitectural deterioration of bone tissue and decreased bone
strength (1,2). Osteoporosis presents with symptoms such
as low bone density and microarchitectural deterioration of bone
tissue with a consequent increase in bone fragility and
susceptibility to fracture (3), and
with a T-score of −2.5 standard deviations (SD) below the bone
mineral density (BMD) of a healthy person of the same gender
(4), which is most widely measured
using dual energy X-ray absorptiometry (DXA) (5). In the United States, osteoporosis
affects 2% of men and 10% of women aged ≥50 years old (6). Furthermore, 49% of older women and 30%
of older men have osteopenia (7).
Estimates indicate that 50% of women and 20% of men aged >50
years old will experience an osteoporosis-associated fracture.
Among these osteoporosis-associated fractures, hip fracture is the
most devastating of these, due to the consequent disability,
mortality and costs (8). Owing to
the aging of the global population, estimates suggest that the
incidence of osteoporosis will double in the next 20 years
(2). Consequently, an exponential
increase in the numbers of fractures is anticipated, with an
inevitable increased clinical and economic burden for healthcare
systems.
Most strategies for treating bone loss have focused
on pharmacological interventions (9); however, drug treatments may have
adverse effects and poor long-term adherence, despite their
effectiveness (10). Chinese herbal
medicine has been used for thousands of years for the treatment of
bone diseases (11). For
postmenopausal women, Epimedium pubescens flavonoids are one
of the most frequently used herbal compounds that is prescribed for
the treatment of osteoporosis (12).
Epimedium-derived phytoestrogenic flavonoids inhibit bone
resorption, stimulate bone formation and prevent
ovariectomy-induced osteoporosis, without resulting in uterine
hyperplasia. It is suggested that these compounds have an anabolic
effect on osteoporotic bone by concomitantly promoting the
osteogenic differentiation of bone marrow stromal cells while
suppressing adipogenic differentiation (13). Icariin
(C33H40O15; molecular weight:
676.65; Fig. 1), one of the primary
active compounds within Epimedium, reportedly has an
anabolic effect on the bone; it stimulates the proliferation of rat
bone marrow stromal cells, increases the number that stain positive
for osteocalcin (BGLAP) secretion, alkaline phosphatase (ALP) and
enhances ALP activity, and calcium deposition levels in a
dose-dependent manner (14,15). In previous work, we reported that
icariin inhibits osteoporosis in vitro, potentially owing to
its role in increasing bone morphogenetic protein-2 (BMP-2) protein
expression (16), and that icariin
promotes bone formation via the BMP-2/Smad4 signal transduction
pathway in the hFOB 1.19 human osteoblastic cell line (17).
The present study investigated whether icariin
promotes bone fracture healing in ovariectomized osteoporotic (OVX)
rats in vivo, with the intention of determining a novel
method to treat osteoporosis-associated fracture.
Materials and methods
Animals and modeling method
For the present study, 30 6-month-old Sprague-Dawley
(SD) female rats were obtained from Hubei University of Medicine
(Shiyan, China). The rats were housed in a temperature-controlled
room (25°C) with constant humidity (40–50%) and received food and
water ad libitum. Rats were left for 1 week to acclimatise
to their environment, which was subject to a 12/12 h light/dark
cycle. These rats were randomly divided into three groups
consisting of 10 rats per group, as follows: i) Sham surgery (SS);
ii) OVX; and iii) OVX and icariin (OVX + ICA) groups.
Bilateral ovariectomy was performed in 20 female
rats through an incision in the back, under general anesthesia with
an intraperitoneal injection of 10% chloral hydrate at a dose of 3
ml/kg (Chemical Reagent Co., Shanghai, China). Approximately 1.5 cm
of the skin, the abdominal cavity and the muscles were incised, and
the ovaries were exposed (Fig. 2A).
The oviduct was ligated with a silk thread and the ovariectomy was
performed bilaterally (Fig. 2B),
while the remaining 10 animals underwent a sham surgery in which
the bilateral ovaries were examined and returned to the original
position under the same protocol.
Three months after the ovariectomy, a unilateral
cross-tibial fracture was made at the proximal right tibia and
fixed with intramedullary nailing (diameter, 1 mm, length, 50 mm;
Wego Medical Systems Co., Ltd, Weihai, China; Fig. 3), performed under anesthesia. All
procedures were approved by the Animal Research Ethics Board at
Hubei University of Medicine (Shiyan, China).
Treatment method
Icariin was obtained from the Institute of
Pharmaceutical Research (Beijing, China) with a purity of 99%,
dissolved with 0.9% sodium chloride at a concentration of 100
mg/ml. The OVX + ICA group was treated with a daily 150 mg/kg
icariin, administered orally following the intramedullary fixation
and right tibial fracture procedure. The SS group and OVX group
received equal amounts of 0.9% sodium chloride orally.
Specimen collection
X-rays (800 mA, 150 kV, R-500; GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA) were taken at 1, 3 and 5 weeks
after oral treatment, and dual energy X-ray absorptiometry
(DSC-3000, Aloka, Tokyo, Japan) was used to measure the BMD
(mg/cm2) prior to sacrifice, 5 weeks after oral
treatment. The rats were sacrificed by cervical dislocation. Blood
was drawn from the inferior vena cava following sacrifice, added to
anticoagulant, and was stirred at a rate of 3,000 rpm for 20 min to
extract the blood plasma. The extracted blood plasma was stored at
−70°C until analysis.
Blood variable analysis
BGLAP and ALP, as bone formation markers, were each
measured with an ELISA kit (BGLAP, cat. no. DSTCN0; ALP, cat. no.
DY725; R&D Systems, Inc., Minneapolis, MN, USA).
Tartrate-resistant acid phosphatase (TRAP), used as a bone
resorption marker, and blood estradiol levels were also measured
using ELISA kit (cat. no. KGE014; R&D Systems, Inc.).
Statistical analysis
Data are expressed as mean ± standard deviation, and
statistical analyses were performed using SPSS software, version
12.0 (SPSS, Inc., Chicago, IL, USA). One-way analysis of variance
was used to assess differences between the groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
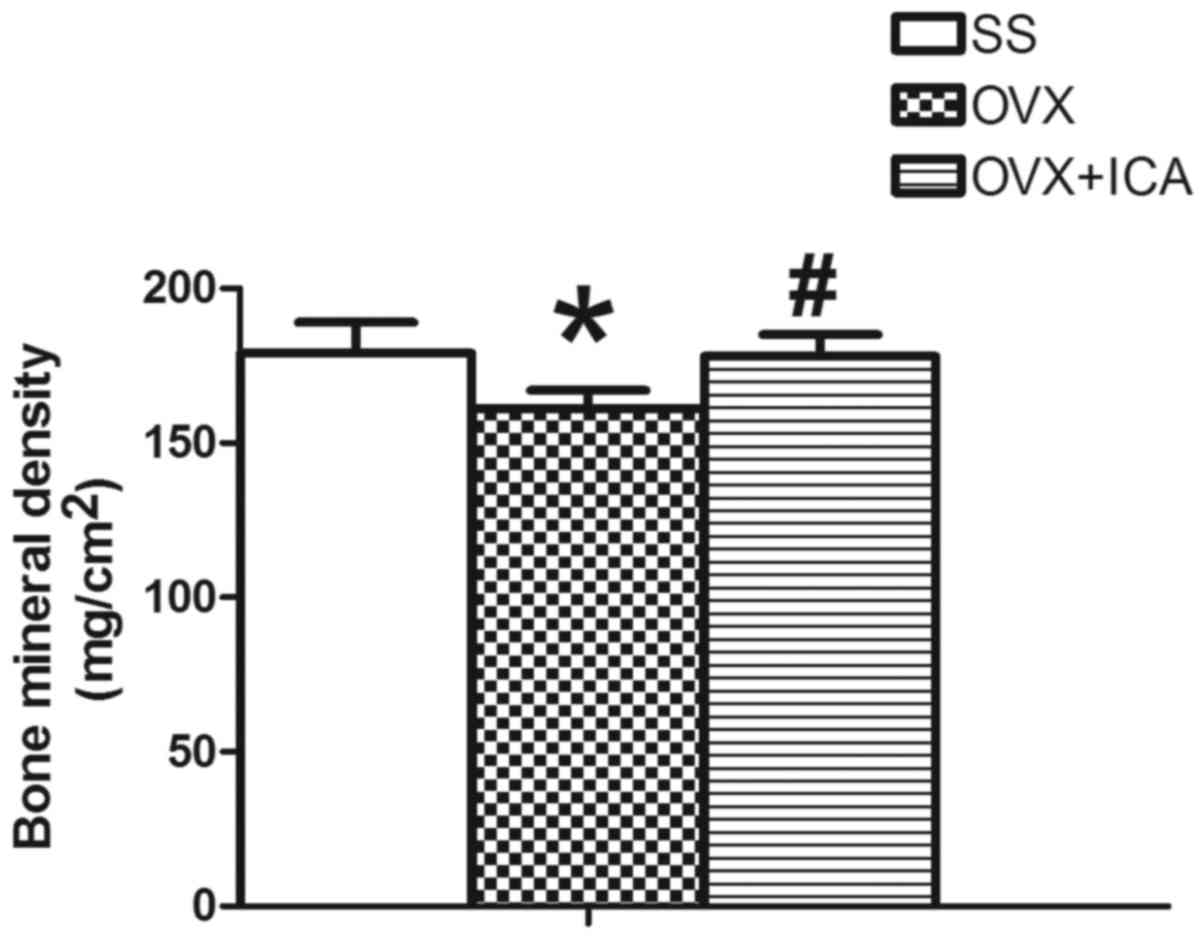
Bone mineral density is altered
following icariin treatment of OVX rats
The BMD of the SS group, measured from the
trabecular bone of the distal femur of the rats, was significantly
higher than that of the OVX group (P<0.05). The BMD of OVX + ICA
group was significantly higher than that of the OVX group
(P<0.05), but was not significantly different from the BMD of
the control group (P>0.05; Fig.
4).
BGLAP, ALP and TRAP levels increase in
OVX rats, and are rescued by icariin treatment
After 5 weeks of oral treatment, the OVX group
demonstrated significantly increased BGLAP, ALP and TRAP levels
compared with the SS group (P<0.05). The OVX + ICA group
demonstrated a reduction of BGLAP, ALP and TRAP levels compared
with the OVX group (P<0.05; Figs.
5 and 6).
Changes are observed in blood
estradiol levels in OVX rats, which are not rescued following
icariin treatment
OVX rats exhibited a significant reduction in their
blood estradiol level compared with that in the SS group
(P<0.05). However, the OVX + ICA group exhibited no significant
increase in the blood estradiol levels compared with those in the
OVX group (P>0.05; Fig. 7).
X-rays of rat tibias reveal incomplete
remodeling in OVX rats, which is improved by icariin treatment
The effects of icariin on callus formation,
remodeling and bone union were observed at 1, 3 and 5 weeks after
treatment. Callus formation and bone union were observed in the OVX
+ ICA group at 5 weeks but, in the OVX group, a small callus was
formed and remodeling remained incomplete, with no union of bones
(Fig. 8).
Discussion
Osteoporosis, which results from a disturbance in
normal bone remodeling by increasing bone resorption relative to
bone formation, is identified by a low bone mass and leads to a
high risk of fractures (18).
Osteoporosis is often an undiagnosed disease prior to the
occurrence of fracture. Bone fragility occurs due to an excess of
resorption and reduced bone formation, resulting in an increased
risk of hip and vertebral fractures (19). It is reported that osteoporosis
affects about 25 million individuals in the United States alone,
and it is estimated that women >50 years old possess an 11–18%
risk of suffering a hip fracture (20). Therefore, to prevent and treat the
osteoporosis, medication, exercise and additional treatments such
as hormone replacement therapy are commonly used (21,22).
Amongst these, hormone replacement therapy is the most widely used;
however, previous evidence indicates that long-term treatments with
these drugs may cause adverse reactions, such as an increased risk
of ovarian and endometrial cancer (23,24).
Thus, an alternative therapeutic strategy with a proven efficacy
and safety is required to prevent and treat osteoporosis. Icariin,
one of the primary active ingredients of Epimedium,
reportedly has an anabolic effect on bones; this may contribute to
its role in the induction of osteoblast proliferation and
differentiation, which results in bone formation (25,26).
The ovariectomy model is a well-established animal
model in osteoporosis studies (27).
The OVX rat model was selected for the present study as it shares
numerous similarities with postmenopausal bone loss and is
recommended by the US Food and Drug Administration as a test
species for evaluating the long-term skeletal safety and efficacy
of osteoporosis therapies (20).
Previous laboratory studies have reported that osteoporosis impairs
fracture healing in early and late stages (28,29). In
the present study, it was investigated whether icariin promotes
bone fracture healing in OVX rats in vivo; this was
determined 3 months after the ovariectomy, the unilateral
cross-tibia fracture was made and the fracture had been fixed with
an intramedullary nailing.
Decreased BMD is one of the major factors
jeopardizing bone strength, resulting in increased susceptibility
to fractures (30). The present
study revealed that OVX reduced BMD in the distal femurs of female
SD rats, which are rich in trabecular bone, whilst treatment with
icariin prevented these decreases in BMD.
BGLAP is closely bonded with hydroxyapatite and
calcium in the bone, and is an established bone formation marker;
this was used to predict the bone loss rate as it is indirectly
involved in the activation of osteoblasts during bone generation
(31). ALP is a hydrolase enzyme
responsible for removing phosphate groups from many types of
molecules, including nucleotides, proteins, and alkaloids (32). ALP increases during active bone
formation, as ALP is a byproduct of osteoblast activity (heightened
levels of which appear in Paget's disease) (33). TRAP is a glycosylated monomeric
metalloprotein enzyme expressed in mammals, which may be used as a
bone resorption marker. Osteopontin and bone sialoprotein, which
are bone matrix phosphoproteins, are highly efficient in
vitro TRAP substrates that bind to osteoclasts when
phosphorylated. Upon partial dephosphorylation, osteopontin and
bone sialoprotein are incapable of binding to osteoclasts (34). From this effect, it has been
hypothesized that TRAP is secreted from the ruffled membrane of
osteoclasts, where it dephosphorylates osteopontin and allows
osteoclast migration and additional resorption to occur (35). The OVX group significantly increased
the blood BGLAP, ALP and TRAP levels, while the OVX + ICA group
rescued these. These results may be due to decreased estrogen
increasing the number of osteoblasts and osteoclasts, or altered
activity of these cells.
The most common type of osteoporosis is the
post-menopausal bone loss associated with ovarian hormone
deficiency (36). Several previous
studies report that estrogen is the most important hormone in
maintaining bone mass and that a deficiency of this hormone is a
major cause of bone loss associated with age in both genders
(37–39). Notably, when the circulating estrogen
level decreases, calcium in the bones rapidly decreases, and
calcium loss occurs via an increase in its urinary excretion
(40). In the current study, rat
blood estradiol level was gauged using an ELISA kit. Those rats
which experienced the menopause induced by ovariectomy demonstrated
a significant decrease in blood estradiol compared with the SS
group. Nian et al (41)
suggested that icariin has an antiosteoporotic effect, similar to
estrogen, and that it may be effective for prevention of bone
fractures induced by estrogen deficiency. In the present study, the
OVX + ICA group that was treated with icariin intragastrically for
5 weeks demonstrated a non-significant increase in blood estradiol
compared with the OVX group. Ye et al (42) previously reported that nonconjugated
forms of icaritin and desmethylicaritin, two derivatives of
icariin, possess estrogen-like activity; however, icariin appeared
to have no estrogenicity in the MCF-7 cell line model in
vitro. Mok et al (43)
revealed that icariin exerts anabolic effects in bone, possibly by
activating the endoplasmic reticulum in a ligand-independent
manner. In previous work, we reported that icariin inhibits
osteoporosis in vitro, potentially owing to its role in
increasing BMP-2 protein expression (16), and that icariin promotes bone
formation via the BMP-2/Smad4 signal transduction pathway in the
hFOB 1.19 human osteoblastic cell line (17).
The present study investigated the effects of
icariin on callus formation, remodeling and bone union at 1, 3 and
5 weeks after treatment by examining X-rays. Callus formation and
bone union increased every 2 weeks in the SS group and the fracture
line was fuzzy at 5 weeks after sham surgery. In the OVX group,
small calluses were formed and the fracture line was evident,
revealing an absence of union. Callus formation and bone union
increased temporally following icariin treatment, the fracture line
was almost absent, and bone union and remodeling were observed 5
weeks after intragastric administration.
In conclusion, the present in vivo study
reported that icariin attenuates the decrease in BMD in rats with
osteopenia and that postfracture administration of icariin
accelerates mineralization and osteogenesis and is associated with
improved fracture healing. The current findings indicate that
icariin has the potential to be developed as an alternative for
fracture healing in postmenopausal osteoporosis. However, it should
be noted that the mechanism by which icariin performs these roles
remains to be examined.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81602867), the Hubei
Provincial Department of Education (grant no. Q20132108), a Hubei
Province Health and Family Planning Scientific Research Project
grant (grant no. WJ2015Q042), Hubei Provincial Science and
Technology Department funding (grant no. 2013CFC031).
References
|
1
|
Gallagher JC and Tella SH: Controversies
in osteoporosis management: Antiresorptive therapy for preventing
bone loss: When to use one or two antiresorptive agents? Clin
Obstet Gynecol. 56:749–756. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coughlan T and Dockery F: Osteoporosis and
fracture risk in older people. Clin Med (Lond). 14:187–191. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Zepetnek JO Totosy, Giangregorio LM and
Craven BC: Whole-body vibration as potential intervention for
people with low bone mineral density and osteoporosis: A review. J
Rehabil Res Dev. 46:529–542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pritchard JM, Giangregorio LM, Atkinson
SA, Beattie KA, Inglis D, Ioannidis G, Gerstein H, Punthakee Z,
Adachi JD and Papaioannou A: Changes in trabecular bone
microarchitecture in postmenopausal women with and without type 2
diabetes: A two year longitudinal study. BMC Musculoskelet Disord.
14:1142013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
MacIntyre NJ, Adachi JD and Webber CE: In
vivo measurement of apparent trabecular bone structure of the
radius in women with low bone density discriminates patients with
recent wrist fracture from those without fracture. J Clin Densitom.
6:35–43. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang X, Good LE, Spinka R and Schnatz PF:
Osteoporosis screening in postmenopausal women aged 50–64 years:
BMI alone compared with current screening tools. Maturitas.
83:59–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Looker AC, Melton LJ III, Harris TB,
Borrud LG and Shepherd JA: Prevalence and trends in low femur bone
density among older US adults: NHANES 2005–2006 compared with
NHANES III. J Bone Miner Res. 25:64–71. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Holvik K, Ahmed LA, Forsmo S, Gjesdal CG,
Grimnes G, Samuelsen SO, Schei B, Blomhoff R, Tell GS and Meyer HE:
No increase in risk of hip fracture at high serum retinol
concentrations in community-dwelling older Norwegians: The
Norwegian Epidemiologic Osteoporosis Studies. Am J Clin Nutr.
102:1289–1296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamdy RC, Baim S, Broy SB, Lewiecki EM,
Morgan SL, Tanner SB and Williamson HF: Algorithm for the
management of osteoporosis. South Med J. 103:1009–1015. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Green J, Czanner G, Reeves G, Watson J,
Wise L and Beral V: Oral bisphosphonates and risk of cancer of
oesophagus, stomach and colorectum: Case-control analysis within a
UK primary care cohort. BMJ. 341:c44442010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang WL, Sheu SY, Chen YS, Kao ST, Fu YT,
Kuo TF, Chen KY and Yao CH: Enhanced bone tissue regeneration by
porous gelatin composites loaded with the Chinese herbal decoction
Danggui Buxue Tang. PLoS One. 10:e01319992015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang G, Qin L and Shi Y:
Epimedium-derived phytoestrogen flavonoids exert beneficial effect
on preventing bone loss in late postmenopausal women: A 24-month
randomized, double-blind and placebo-controlled trial. J Bone Miner
Res. 22:1072–1079. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng S, Zhang G, He Y, Wang X, Leung P,
Leung K and Qin L: Epimedium-derived flavonoids promote
osteoblastogenesis and suppress adipogenesis in bone marrow stromal
cells while exerting an anabolic effect on osteoporotic bone. Bone.
45:534–544. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu JH, Yao M, Ye J, Wang GD, Wang J, Cui
XJ and Mo W: Bone mass improved effect of icariin for
postmenopausal osteoporosis in ovariectomy-induced rats: A
meta-analysis and systematic review. Menopause. 23:1152–1157. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen KM, Ge BF, Ma HP, Liu XY, Bai MH and
Wang Y: Icariin, a flavonoid from the herb epimedium enhances the
osteogenic differentiation of rat primary bone marrow stromal
cells. Pharmazie. 60:939–942. 2005.PubMed/NCBI
|
|
16
|
Cao H, Ke Y, Zhang Y, Zhang CJ, Qian W and
Zhang GL: Icariin stimulates MC3T3-E1 cell proliferation and
differentiation through up-regulation of bone morphogenetic
protein-2. Int J Mol Med. 29:435–439. 2012.PubMed/NCBI
|
|
17
|
Liang W, Lin M, Li X, Li C, Gao B, Gan H,
Yang Z, Lin X, Liao L and Yang M: Icariin promotes bone formation
via the BMP-2/Smad4 signal transduction pathway in the hFOB 1.19
human osteoblastic cell line. Int J Mol Med. 30:889–895.
2012.PubMed/NCBI
|
|
18
|
Zhang Z, Xiang L, Bai D, Fu X, Wang W, Li
Y, Liu H, Pan J, Li Y, Xiao GG and Ju D: Treatment with rhizoma
dioscoreae extract has protective effect on osteopenia in
ovariectomized rats. ScientificWorldJournal.
2014:6459752014.PubMed/NCBI
|
|
19
|
de Laet CE, van der Klift M, Hofman A and
Pols HA: Osteoporosis in men and women: A story about bone mineral
density thresholds and hip fracture risk. J Bone Miner Res.
17:2231–2236. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Choi JS, Kim JW, Kim KY, Cho HR, Choi IS
and Ku SK: Antiosteoporotic effects of polycan in combination with
calcium lactate-gluconate in ovariectomized rats. Exp Ther Med.
8:957–967. 2014.PubMed/NCBI
|
|
21
|
Milat F and Ebeling PR: Osteoporosis
treatment: A missed opportunity. Med J Aust. 205:185–190. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Henriksen K, Byrjalsen I, Andersen JR,
Bihlet AR, Russo LA, Alexandersen P, Valter I, Qvist P, Lau E, Riis
BJ, et al: SMC021 Investigators: A randomized, double-blind,
multicenter, placebo-controlled study to evaluate the efficacy and
safety of oral salmon calcitonin in the treatment of osteoporosis
in postmenopausal women taking calcium and vitamin D. Bone.
91:122–129. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Strom BL, Schinnar R, Weber AL, Bunin G,
Berlin JA, Baumgarten M, DeMichele A, Rubin SC, Berlin M, Troxel AB
and Rebbeck TR: Case-control study of postmenopausal hormone
replacement therapy and endometrial cancer. Am J Epidemiol.
164:775–786. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rossing MA, Cushing-Haugen KL, Wicklund
KG, Doherty JA and Weiss NS: Menopausal hormone therapy and risk of
epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev.
16:2548–2556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kapoor S: Icariin and its emerging role in
the treatment of osteoporosis. Chin Med J (Engl).
126:4002013.PubMed/NCBI
|
|
26
|
Luo Z, Liu M, Sun L and Rui F: Icariin
recovers the osteogenic differentiation and bone formation of bone
marrow stromal cells from a rat model of estrogen
deficiency-induced osteoporosis. Mol Med Rep. 12:382–388.
2015.PubMed/NCBI
|
|
27
|
Li CL, Liu XL, Cai WX, Lu WW, Zwahlen RA
and Zheng LW: Effect of ovariectomy on stimulating intracortical
remodeling in rats. Biomed Res Int. 2014:4214312014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Namkung-Matthai H, Appleyard R, Jansen J,
Hao Lin J, Maastricht S, Swain M, Mason RS, Murrell GA, Diwan AD
and Diamond T: Osteoporosis influences the early period of fracture
healing in a rat osteoporotic model. Bone. 28:80–86. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kubo T, Shiga T, Hashimoto J, Yoshioka M,
Honjo H, Urabe M, Kitajima I, Semba I and Hirasawa Y: Osteoporosis
influences the late period of fracture healing in a rat model
prepared by ovariectomy and low calcium diet. J Steroid Biochem Mol
Biol. 68:197–202. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park JA, Ha SK, Kang TH, Oh MS, Cho MH,
Lee SY, Park JH and Kim SY: Protective effect of apigenin on
ovariectomy-induced bone loss in rats. Life Sci. 82:1217–1223.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Johansen JS, Riis BJ, Delmas PD and
Christiansen C: Plasma BGP: An indicator of spontaneous bone loss
and of the effect of oestrogen treatment in postmenopausal women.
Eur J Clin Invest. 18:191–195. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sarvari BK, Mahadev D Sankara, Rupa S and
Mastan SA: Detection of bone metastases in breast cancer (BC)
patients by serum tartrate-resistant acid phosphatase 5b (TRACP
5b), a bone resorption marker and serum alkaline phosphatase (ALP),
a bone formation marker, in lieu of whole body skeletal
scintigraphy with Technetium99m MDP. Indian J Clin Biochem.
30:66–71. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pruessner HT: Detecting celiac disease in
your patients. Am Fam Physician. 57:1023–1034. 1998.PubMed/NCBI
|
|
34
|
Wu G, Guo JJ, Ma ZY, Wang J, Zhou ZW and
Wang Y: Correlation between calcification and bone sialoprotein and
osteopontin in papillary thyroid carcinoma. Int J Clin Exp Pathol.
8:2010–2017. 2015.PubMed/NCBI
|
|
35
|
Ek-Rylander B, Flores M, Wendel M,
Heinegard D and Andersson G: Dephosphorylation of osteopontin and
bone sialoprotein by osteoclastic tartrate-resistant acid
phosphatase. Modulation of osteoclast adhesion in vitro. J
Biol Chem. 269:14853–14856. 1994.PubMed/NCBI
|
|
36
|
Rugpolmuang L and Waikakul S: Effect of a
short-term treatment with once-a-week medication of alendronate 70
mg on bone turnover markers in postmenopausal women with
osteoporosis. J Med Assoc Thai. 98 Suppl 8:S70–S75. 2015.PubMed/NCBI
|
|
37
|
Xu F, Ding Y, Guo Y, Liu B, Kou Z, Xiao W
and Zhu J: Anti-osteoporosis effect of Epimedium via an
estrogen-like mechanism based on a system-level approach. J
Ethnopharmacol. 177:148–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bordinhon M, Müller SS and Silva MD:
Clinical, biomechanical and histological study on oophorectomy
induced menopause. Acta Ortop Bras. 22:260–263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Du Z, Steck R, Doan N, Woodruff MA,
Ivanovski S and Xiao Y: Estrogen deficiency-associated bone loss in
the maxilla: A methodology to quantify the changes in the maxillary
intra-radicular alveolar bone in an ovariectomized rat osteoporosis
model. Tissue Eng Part C Methods. 21:458–466. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hattori S, Agata U, Park JH, Iimura Y,
Tokuda S, Ezawa I and Omi N: The relationship between salivary
calcium concentration and differences in bone mineral density level
in female rats. J Nutr Sci Vitaminol (Tokyo). 60:152–158. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nian H, Ma MH, Nian SS and Xu LL:
Antiosteoporotic activity of icariin in ovariectomized rats.
Phytomedicine. 16:320–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ye HY and Lou YJ: Estrogenic effects of
two derivatives of icariin on human breast cancer MCF-7 cells.
Phytomedicine. 12:735–741. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mok SK, Chen WF, Lai WP, Leung PC, Wang
XL, Yao XS and Wong MS: Icariin protects against bone loss induced
by oestrogen deficiency and activates oestrogen receptor-dependent
osteoblastic functions in UMR 106 cells. Br J Pharmacol.
159:939–949. 2010. View Article : Google Scholar : PubMed/NCBI
|