Introduction
Alzheimer's disease (AD), a neurodegenerative
disorder with the clinical characteristics of progressive memory
loss and cognitive function impairment (1), is the most common cause of dementia
worldwide (2). The financial costs
are immense due to the prevalence of AD (3). Hence, it is urgent to develop
appropriate means for the management and prevention of AD.
The pathogenesis of AD is closely associated with
the accumulation of neurofibrillary tangles and senile plaques
(SPs) in affected brain regions (4,5).
β-amyloid peptide (Aβ), the major component of SPs, has been
reported to have a causative role in the progression of AD as has a
toxic effect on neuronal cells (6).
Aβ fragments, including Aβ1–40, Aβ25–35 and
Aβ1–42, have been generated through the split of amyloid
precursor protein (7). The
neurotoxicity of Aβ1–42 was found to be significantly
higher than that of Aβ25–35 and Aβ1–40, and
Aβ1–42 is able to induce an AD model (1,8–10). Oxidative stress may be involved in
the pathogenesis of AD and is the major mechanism underlying
Aβ-induced neurotoxicity (11–13).
Several studies suggested that Aβ1–42 caused
intracellular accumulation of reactive oxygen species (ROS),
leading to lipid and protein oxidation, DNA damage and activation
of cell cycle checkpoint signaling (1,14,15).
Excessive amounts of H2O2 may lead to
oxidative damage and induce apoptosis of PC12 cells (16). Therefore, targeting of oxidative
stress may be a promising approach for the development of
therapeutic strategies for inhibiting Aβ-induced neurotoxicity in
AD.
Herba (H.) Cistanche, a Chinese
herbal medicine commonly used in mainland China for nourishing the
kidneys and replenishing essence and blood, has been used to treat
memory loss and senile constipation (17). Phenylethanoid glycoside (PhG), one of
the major constituents in H. Cistanche, improves the
impairment of neuronal apoptosis caused by Aβ25–35 via
its anti-oxidant effects (18,19). A
previous study identified five major components from total PhGs,
namely acteoside, 2′-acetylacteoside, echinacoside, cistanosides
and isoacteoside (20). Among these
components, acteoside and echinacoside have been reported to have
neuroprotective effects on Aβ25–35- or
H2O2-induced neurotoxicity (21–23). For
instance, Wu et al (24)
suggested that acteoside and echinacoside ameliorated cognitive
dysfunction caused by Aβ1–42. The present study aimed to
investigate the protective effects of PhGs in an in vitro
rat cell model of AD.
Materials and methods
Preparation of PhGs
Total PhGs were extracted from H. Cistanche as
previously described (25). The
air-dried stem of H. Cistanche was powdered and extracted by
percolation with 80% EtOH. The percolate was evaporated under
reduced pressure, followed by re-suspension in an appropriate
amount of H2O2 (100 µmol/l). The mixture was
isolated on an SP-825 macroporous resin column (Mitsubishi
Chemical, Tokyo, Japan) and eluted with 0, 30, 50, 70 and 90% EtOH
in water. To obtain the PhG-rich fraction, the 30–50% EtOH eluents
were concentrated and dried under reduced pressure. Ultraviolet
(UV) spectrophotometry was performed to determine the total PhGs.
The content of echinacoside and acteoside was determined by
high-pressure liquid chromatography according to a previous
protocol (26). A Hypersil ODS-2
column (4.6×250 mm, 5 µm; Dalian Elite Analytical Instruments, Co.,
Ltd., Dalian, China) was used and maintained at room temperature.
The mobile phases were methyl cyanides and water containing 0.4%
phosphoric acid (v/v; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). The flow rate was 0.75 ml/min and the wavelength was set
to 333 nm.
Cell culture and drug treatment
The PC12 rat pheochromocytoma cell line was provided
by Dr He Chunhui, the Medical School, Xinjiang Medical University
(Urumqi, China). Cells were cultured in high-glucose Dulbecco's
modified Eagle's medium (DMEM; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 10% fetal bovine serum (Sangon Biotech
Co. Ltd., Shanghai, China), 100 U/ml penicillin and 100 U/ml
streptomycin in an incubator at 37°C containing 5% CO2
and 95% air. Upon reaching 80% confluence, the cells were treated
with 0.25% trypsin and passaged.
In order to eliminate the interference of the drug
itself on the growth of PC12 cells, a toxicity experiment was
performed. In brief, PC12 cells (3×104 cells/ml) were
seeded in 96-well plates at 100 µl/well and incubated at 37°C
overnight. After discarding the supernatant, 200 µl complete DMEM
was added in the blank group, while cells in the intervention
groups were treated by PhGs at various doses (5, 25, 50, 75, 100,
125, 150, 175 and 200 µg/ml). Following incubation of the cells at
37°C for 48 h, 20 µl MTT solution (Sigma-Aldrich; Merck KGaA) was
added to each well. After incubation for 4 h, the supernatant was
discarded and 150 µl dimethylsulfoxide was added per well, followed
by agitation for 10 min. The optical density (OD) values at 490 nm
were detected using an ELISA plate reader.
Aβ1–42-induced PC12 cell
injury
Aβ1–42 peptide purchased from Bioss
Biotech (Beijing, China) was dissolved in water (100 µg/ml).
Subsequently, the mixture was incubated at 37°C for 4 days and
stored at 4°C prior to use.
PC12 cells were seeded in 96-well plates
(3x104 cells in 100 µl per well). After culture for 24 h
for adherence, 50 µl Aβ1–42 at various final
concentrations (0, 0.25, 0.5, 1, 1.5 or 2 µM) dissolved in
serum-free DMEM was added, followed by incubation for 24, 48, 72 or
96 h. Cell viability was evaluated by an MTT assay. The optimal
Aβ1–42 concentration was 0.5 determined to be µM.
PC12 cells (3x104 cells per well) were
treated with various doses of PhGs (0, 0.5, 5, 25 or 50 µg/ml) in
the presence of 0.5 µM Aβ1–42 for 24 h. Cell viability
was evaluated by an MTT assay.
H2O2-induced
PC12 cell injury
PC12 cells were plated seeded in 96-well plates
(3x104 cells in 100 µl per well). After culture for 24 h
for adherence, 100 µl H2O2 at various final
concentrations (0, 25, 50, 100, 200, 300, 400 and 500 µM) dissolved
in DMEM with or without PBS (0.01 mol/l) was added, followed by
incubation for 24 h. Cell viability was evaluated by an MTT assay.
The optimal H2O2 concentration and solvent
was determined to establish the in vitro model of AD.
PC12 cells (3x104 cells per well) were
treated with various doses of PhGs (0, 0.5, 5, 25 and 50 µM). After
culture for 24 h for adherence, PC12 cells were treated with 100 µl
H2O2 dissolved in DMEM with PBS in the
presence of PhGs for 24 h. The cell viability was evaluated by an
MTT assay.
Lactate dehydrogenase (LDH) release
assay
Cell injury was assessed through measuring the LDH
activity in the supernatant of PC12 cells using an LDH kit
according to the manufacturer's protocol (cat. no. 20150604;
Nanjing Jiancheng Bioengineering Institute, Nanjing, China). In
brief, double-distilled H2O, 0.2 µmol/ml pyruvic acid,
matrix buffer and coenzyme I buffer were added in sequence at 48 h
after drug treatment. After incubation at 37°C for 15 min,
2,4-dinitro-phenylhydrazine was added. Subsequently, 250 µl of a
0.4 M NaOH solution was added to each well. The supernatant was
collected after incubation for 30 min at room temperature. The
absorbance at 450 nm was then measured with a microplate
reader.
Measurement of malondialdehyde
(MDA)
MDA was measured in the supernatant of PC12 cells
using commercial kit (cat. no. 20150604; Nanjing Jiancheng
Bioengineering Institute) according to the manufacturer's protocol
In brief, dehydrated alcohol and other reagents were added in
order, followed by incubation in a water bath at 95°C for 40 min.
The mixture was centrifuged at 1,006 × g and 25°C for 10 min after
cooling. The supernatant was then used to determine the MDA
content. Absorbance was subsequently measured with a microplate
reader at 532 nm.
Assessment of protective effects of
echinacoside and acteoside against AD in vitro
PC12 cells were seeded at a density of
3x104 cells/well in 96-well plates (100 µl/well). Cells
were incubated with drugs including echinacoside (cat. no.
111670-200503; National Institutes for Food and Drug Control,
Beijing, China) and acteoside (cat. no. 111530-200505; National
Institutes for Food and Drug Control) at various concentrations
(0.5, 25 and 50 µg/ml). Subsequently, the cells were treated with
Aβ1–42 or H2O2 for 24 h and the
cell viability was measured by an MTT assay.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Student's t-test was used for inter-group comparisons.
Statistical analyses were performed using SPSS 18.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Quantification of PhGs from H.
Cistanches
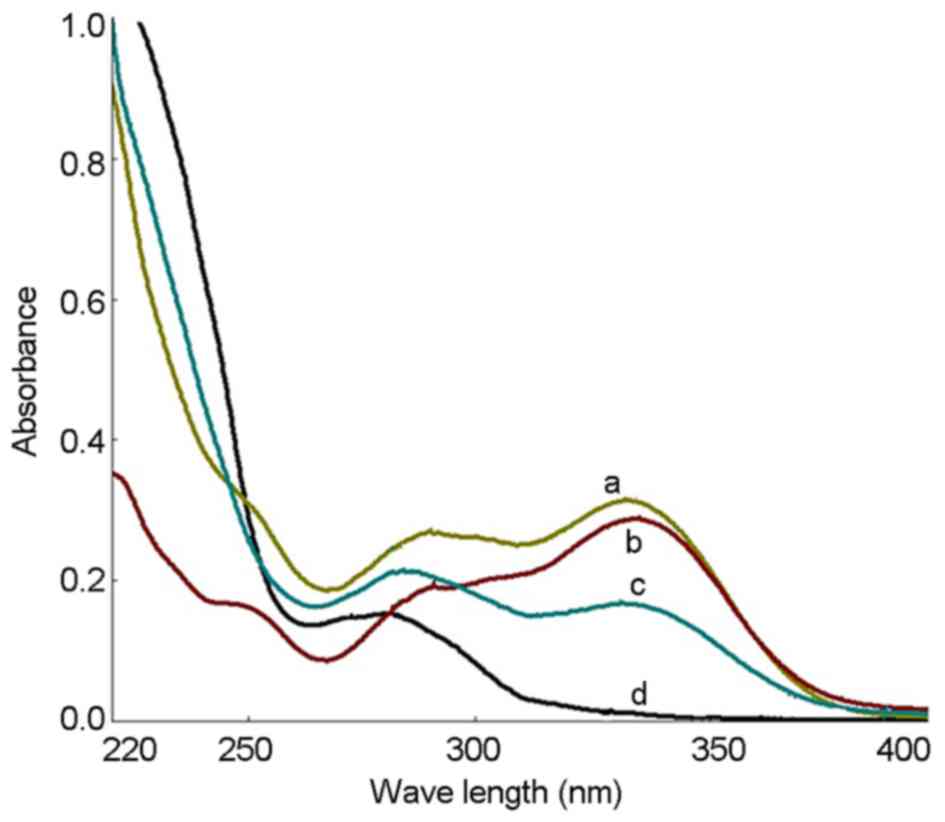
The UV spectra of the PhGs extracted as well as
standard solutions of echinacoside and acteoside were recorded, and
the results showed that the UV spectra were consistent (Fig. 1). UV spectrophotometry showed that
the PhG content was 87.6%. The HPLC results showed that the
contents of echinacoside and acteoside were 37.7 and 17.8%,
respectively (Fig. 2).
Determination of the ideal PhG
concentration
Compared with the blank group, PhG at 75, 100, 125,
150, 175 and 200 µg/ml had a significant inhibitory effect on PC12
cells (P<0.05), while PhG at 5, 25 and 50 µg/ml showed low
toxicity on PC12 cells, and the cell viability was >80%
(Fig. 3). Thus, PhGs at the
concentration of 5, 25 and 50 µg/ml was used for treating PC12
cells in subsequent experiments due to not affecting the cell
viability.
Aβ1–42-induced PC12 cell
injury
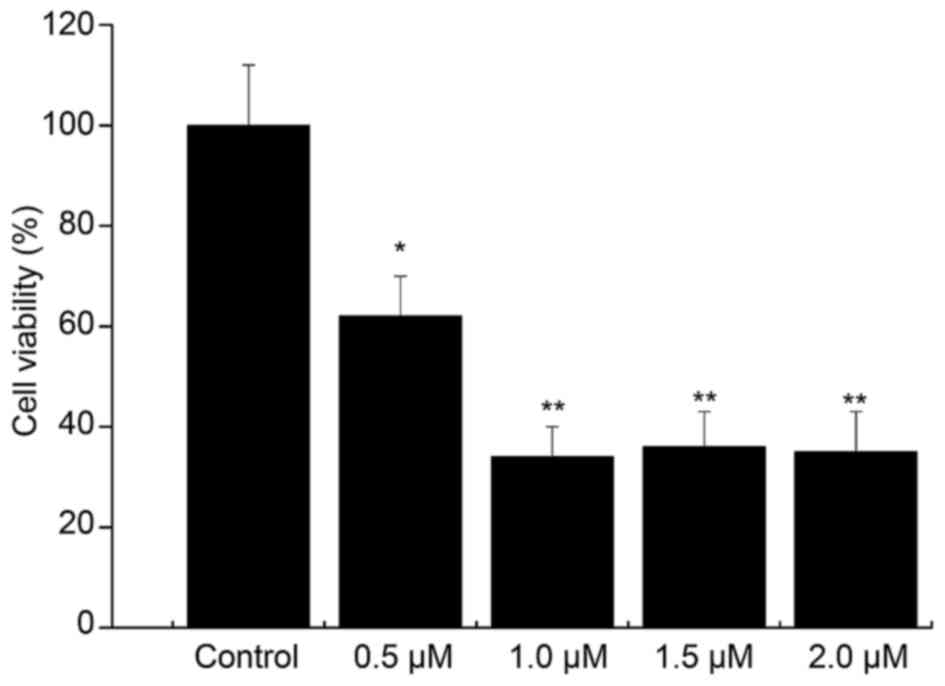
Compared with that in the control group, the cell
viability in the 0.5 µM Aβ1–42 injury group was 63%
(P<0.05). The cell viability was decreased by Aβ1–42
in a concentration-dependent manner, and the viability was <50%
in the 1, 1.5 and 2 µM Aβ1–42 injury groups (Fig. 4). Thus, treatment with 0.5 µM
Aβ1–42 for 48 h was determined to be the optimal
condition for establishing the in vitro AD model.
The activity of PC12 cells treated with 0.5 µM
Aβ1–42 in the presence of safe doses of PhGs (5, 25 and
50 µg/ml) for 24 h was also determined. Compared with the model
group (P<0.01), PhGs showed a significant neuroprotective effect
on PC12 cells. The cell viability was rescued by PhGs in a
dose-dependent manner (Fig. 5).
H2O2-induced
PC12 cell injury
The viability of PC12 cells treated with 25 µM
H2O2 dissolved in DMEM with PBS was 56.43%.
The viability of PC12 cells treated with 200 µM
H2O2 dissolved in DMEM without PBS was 71.64%
(Table I). Thus, PC12 cells treated
with 25 µM H2O2 dissolved in DMEM with PBS
was the selected as the optimal condition for establishing the AD
model.
 | Table I.Cell viability (%) after
H2O2 treatment. |
Table I.
Cell viability (%) after
H2O2 treatment.
|
| Solvent |
|---|
|
|
|
|---|
|
H2O2 concentration
(µM) | DMEM+PBS | DMEM |
|---|
|
0 | 100 | 100 |
| 25 | 56.43a | 107.61 |
| 50 | 54.81a | 108.13 |
| 100 | 52.79a | 105.01 |
| 200 | 46.30a | 71.64a |
| 300 | 53.90a | 60.06a |
| 400 | 44.28a | 61.00a |
| 500 | 43.68 | 60.17a |
Compared with the control group, the cell viability
in the model group was 48.8% (P<0.05). Compared with the model
group, PhGs had a significant neuroprotective effect on PC12 cells.
The cell viability was dose-dependently increased by PhGs, and the
viability of PC12 cells treated with PhGs at concentrations of 5,
25 and 50 µg/ml was 54, 57 and 64%, respectively (Table II).
 | Table II.Cell viability after drug
interference. |
Table II.
Cell viability after drug
interference.
| Group | Cell viability
(%) |
|---|
| Control | 100 |
| Model | 48.83a |
| PhG 5 µg/ml | 53.94b |
| PhG 25 µg/ml | 57.39b |
| PhG 50 µg/ml | 64.00c |
PhGs inhibit injury-induced LDH
release by PC12 cells
Compared with the control group, the LDH content of
the supernatant of injured PC12 cells was increased, which was
inhibited by PhGs in a concentration-dependent manner. This result
indicated that PhGs have a significant neuroprotective effect on
PC12 cells (Fig. 6).
PhGs inhibit injury-induced MDA
production by PC12 cells
Compared with the control group, the MDA content in
the supernatant of injured PC12 cells was increased, which was
inhibited by PhGs in a concentration-dependent manner. This result
indicated that PhGs have a significant neuroprotective effect on
PC12 cells (Fig. 7).
PhG and its components echinacoside
and acteoside rescue the viability of injured PC12 cells
Compared with the model group, treatment with
acteoside significantly increased the viability of
Aβ1–42-injured PC12 cells in a dose dependent manner.
PhGs and echinacoside also significantly increased the viability of
Aβ1–42-injured PC12 cells at all concentrations tested
(Fig. 8A).
Compared with the model group, acteoside
significantly increased the viability of PC12 cells treated with
H2O2. PhGs also increased the viability of
PC12 cells treated with H2O2, while the
effect was not significant at concentrations of 5 and 25 µg/ml
(Fig. 8B). In addition, echinacoside
increased the cell viability at 25 µg/ml.
In conclusion, acteoside, PhGs and echinacoside
exerted significant neuroprotective effects on PC12 cells subjected
to injury with Aβ1–42 or H2O2.
Discussion
Oxidative stress is the major mechanism underlying
Aβ-mediated neurotoxicity in AD (11–13).
Therefore, targeting oxidative stress may represent an approach for
the treatment of AD. In the present study, an in vitro model
of AD comprising Aβ1–42- and
H2O2-induced PC12 cell injury was
successfully established. Results of the MTT, LDH and MDA assays
showed that PhGs increased the cell viability, and decreased LDH
and MDA release by PC12 cells subjected to injury. It can be
concluded that PhGs have significant neuroprotective effects on
PC12 cells.
In order to reduce the effects of PhGs themselves on
PC12 cell growth and prevent abnormal proliferation, the safe dose
of PhGs was determined in a screening assay. The results showed
that PhGs at 75, 100, 125, 150, 175 and 200 µg/ml had a significant
inhibitory effect on PC12 cells (P<0.05, P<0.01), while cell
viability remained >80% at concentrations of 5, 25 and 50 µg/ml.
Thus, PhGs at the concentration of 5, 25 and 50 µg/ml were safe for
PC12 cells.
The injury by Aβ1–42 was affected by
certain factors, including the solvent, incubation time and product
quality. In the present study, Aβ1–42 peptide was
dissolved in water (100 µg/ml) and incubated at 37°C for 4 days in
a CO2 incubator prior to use. PC12 cells were treated
with Aβ1–42 at concentrations of 0.5, 1, 1.5 and 2 µM.
The results showed that the cell viability was decreased with the
increase of Aβ1-42, and the viability was <50% in the
1, 1.5 and 2 µM Aβ1–42 injury groups. Thus, treatment of
PC12 cells with 0.5 µM Aβ1–42 for 48 h was determined to
be the optimal condition for establishing the AD model.
Aβ25–35 has been commonly used to establish AD models
due to low cost and simple operation (27–29). The
neurotoxicity of Aβ1–42 is significantly higher than
that of Aβ25–35, and Aβ1–42 is therefore the
optimal Aβ fragment for establishing an AD model (1,8–10).
H2O2 is an oxidizer and
excessive H2O2 may cause oxidative damage and
induce cell apoptosis (30). In the
present study, PC12 cells were treated with 25–500 µM
H2O2 dissolved in DMEM with or without PBS.
The results showed that H2O2 dissolved in
DMEM without PBS caused abnormal proliferation of PC12 cells. Thus,
treatment of PC12 cells with 25 µM H2O2
dissolved in DMEM with PBS was the optimal condition for
establishing the AD model. Aβ1–42-induced injury was
greater than H2O2-induced injury due to poor
stability of H2O2 and solvent effects.
When the cell is damaged, LDH leakage into the
culture medium is significantly increased. ROS is known to cause
the production of MDA. The content of MDA and LDH therefore reflect
the amount of oxidative damage. In the present study,
damage-induced LDH and MDA activity was decreased with increasing
doses of PhGs. These results indicated that PhGs have a significant
neuroprotective effect on PC12 cells. The MTT assay showed that
PhGs exhibited a dose-dependent neuroprotective effect on PC12
cells.
In conclusion, an in vitro model of AD
comprising Aβ1–42- and
H2O2-induced PC12 cell injury was
successfully established. Treatment with PhGs increased the cell
viability, and decreased LDH and MDA release by PC12 cells treated
with Aβ1–42 or H2O2. PhGs had a
significant neuroprotective effect on Aβ1–42- or
H2O2-induced cell injury.
References
|
1
|
Qu M, Zhou Z, Xu S, Chen C, Yu Z and Wang
D: Mortalin overexpression attenuates beta-amyloid-induced
neurotoxicity in SH-SY5Y cells. Brain Res. 1368:336–345. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uzun S, Kozumplik O and Folnegović-Smalc
V: Alzheimer's dementia: current data review. Collegium
antropologicum. 35:1333–1337. 2011.PubMed/NCBI
|
|
3
|
Brookmeyer R, Johnson E, Ziegler-Graham K
and Arrighi HM: Forecasting the global burden of Alzheimer's
disease. Alzheimer's & dementia. 3:186–191. 2007. View Article : Google Scholar
|
|
4
|
Castellani RJ, Rolston RK and Smith MA:
Alzheimer disease. Disease-a-month. 56:484–546. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mattson MP: Pathways towards and away from
Alzheimer's disease. Nature. 430:631–639. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gouras GK, Tsai J, Naslund J, et al:
Intraneuronal Aβ42 accumulation in human brain. The American
journal of pathology. 156:15–20. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen C, Chen Y, Liu H, et al: Hydrogen
peroxide promotes Aβ production through JNK-dependent activation of
γ-secretase. Journal of Biological Chemistry. 283:17721–17730.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shih P-H, Wu C-H, Yeh C-T and Yen G-C:
Protective effects of anthocyanins against amyloid
β-peptide-induced damage in neuro-2A cells. Journal of agricultural
and food chemistry. 59:1683–1689. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Figueiredo CP, Bicca MA, Latini A,
Prediger R, Medeiros R and Calixto JB: Folic acid plus α-tocopherol
mitigates amyloid-β-induced neurotoxicity through modulation of
mitochondrial complexes activity. Journal of Alzheimer's disease:
JAD. 24:61–75. 2010.
|
|
10
|
Dumont M, Lin MT and Beal MF: Mitochondria
and antioxidant targeted therapeutic strategies for Alzheimer's
disease. Journal of Alzheimer's disease: JAD. 20:S6332010.
View Article : Google Scholar
|
|
11
|
Sonnen JA, Breitner JC, Lovell MA,
Markesbery WR, Quinn JF and Montine TJ: Free radical-mediated
damage to brain in Alzheimer's disease and its transgenic mouse
models. Free Radical Biology and Medicine. 45:219–230. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin MT and Beal MF: Mitochondrial
dysfunction and oxidative stress in neurodegenerative diseases.
Nature. 443:787–795. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trushina E and McMurray C: Oxidative
stress and mitochondrial dysfunction in neurodegenerative diseases.
Neuroscience. 145:1233–1248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang S-H, Lin C-M and Chiang B-H:
Protective effects of Angelica sinensis extract on amyloid
β-peptide-induced neurotoxicity. Phytomedicine. 15:710–721. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burhans WC and Heintz NH: The cell cycle
is a redox cycle: linking phase-specific targets to cell fate. Free
Radical Biology and Medicine. 47:1282–1293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xue HY, Gao GZ, Lin QY, Jin LJ and Xu YP:
Protective effects of aucubin on H2O2-induced apoptosis in PC12
cells. Phytotherapy Research. 26:369–374. 2012.PubMed/NCBI
|
|
17
|
Li X, Gou C, Yang H, Qiu J, Gu T and Wen
T: Echinacoside ameliorates D-galactosamine plus
lipopolysaccharide-induced acute liver injury in mice via
inhibition of apoptosis and inflammation. Scandinavian journal of
gastroenterology. 49:993–1000. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu, F-X, Wang X-w, Luo L, Xin H, Na B and
Wang X-F: The effects of glycosides of cistanche on learning and
memory in beta-amyloid peptide induced Alzheimers disease in mice
and its possible mechanism. Chinese Pharmacological Bulletin.
22:5952006.
|
|
19
|
Bao B, Tang X, Tian H, Tong Y, Wu W and
Hong Y: Antioxidant activity of extracts from desert living
Cistanche tubulosa (Schrenk) R. Wright Shanghai J Tradit Chin Med.
44:68–71. 2010.
|
|
20
|
Jiang Y, Li S, Wang Y, Chen X and Tu P:
Differentiation of Herba Cistanches by fingerprint with
high-performance liquid chromatography-diode array detection-mass
spectrometry. Journal of Chromatography A. 1216:2156–2162. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang H, Xu Y, Yan J, et al: Acteoside
protects human neuroblastoma SH-SY5Y cells against
β-amyloid-induced cell injury. Brain research. 1283:139–147. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao Q, Gao J, Li W and Cai D:
Neurotrophic and neurorescue effects of Echinacoside in the
subacute MPTP mouse model of Parkinson's disease. Brain research.
1346:224–236. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kuang R, Sun Y, Yuan W, Lei L, Zheng X and
Food Z: Protective effects of echinacoside, one of the
phenylethanoid glycosides, on H2 O2-induced cytotoxicity in PC12
cells. neurodegenerative diseases. 8:92009.
|
|
24
|
Wu C-R, Lin H-C and Su M-H: Reversal by
aqueous extracts of Cistanche tubulosa from behavioral deficits in
Alzheimer's disease-like rat model: relevance for amyloid
deposition and central neurotransmitter function. BMC complementary
and alternative medicine. 14:2022014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cai RL, Yang MH, Shi Y, Chen J, Li YC and
Qi Y: Antifatigue activity of phenylethanoid-rich extract from
Cistanche deserticola. Phytotherapy research. 24:313–315. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao Y, Zong C, Liu F, et al: Evaluation of
the Intestinal Transport of a Phenylethanoid Glycoside-Rich Extract
from Cistanche deserticola across the Caco-2 Cell Monolayer Model.
PloS one. 10:e01164902015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoon J-H, Youn K, Ho C-T, Karwe MV, Jeong
W-S and Jun M: p-Coumaric Acid and Ursolic Acid from Corni fructus
Attenuated β-Amyloid 25–35-induced Toxicity through Regulation of
the NF-κB Signaling Pathway in PC12 cells. Journal of agricultural
and food chemistry. 62:4911–4916. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu Y, Sun X, Gong T, He Q and Zhang Z:
Antioxidant and Antiapoptotic Effects of 1,
1′-(Biphenyl-4,4′-diyl)-bis (3- (dimethylamino)-propan-1-one) on
protecting PC12 cells from Aβ-induced injury. Molecular
pharmaceutics. 11:428–435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Y, Su Y, Run X, et al: Pretreatment
of PC12 cells with 17β-estradiol prevents Aβ-induced
down-regulation of CREB phosphorylation and prolongs inhibition of
GSK-3β. Journal of Molecular Neuroscience. 50:394–401. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang B, Liu J, Bao Y and An L: Catalpol
inhibits apoptosis in hydrogen peroxide-induced PC12 cells by
preventing cytochrome c release and inactivating of caspase
cascade. Toxicon. 43:53–59. 2004. View Article : Google Scholar : PubMed/NCBI
|