Introduction
Idiopathic inflammatory myopathy (IIM) defines a
group of non-suppurative inflammatory diseases comprising an
immune-mediated attack on skeletal muscle, leading to muscle damage
and weakness in the patient. The adjusted annual incidence of IIM
in the USA ranges from 5.8–7.9 per 100,000 individuals (1).
Dermatomyositis (DM), polymyositis (PM) and
inclusion body myositis (IBM) are the most common IIM subtypes in
clinical practice. However, the mechanisms of IIM have remained to
be fully elucidated. IIM is accompanied by impaired function of
multiple organs, particularly the heart, resulting in a poor
prognosis. Cardiac manifestations constitute a certain percentage
of the causes of myositis-associated death (2,3). Cardiac
manifestations of IIM have been observed on electrocardiography
(ECG) in up to 72% of cases and include tachycardia, arrhythmias,
blocks, abnormal Q-waves and non-specific ST-T wave changes.
Pericardial effusion, atrial and ventricular enlargement as well as
hypokinesis are found on echocardiography. However, most of the
cardiac involvement is nonspecific and subclinical (4–8). Cardiac
involvement is encountered with a high incidence in IIM patients,
but rarely manifests as acute myocardial infarction (AMI) at
initial presentation. The present study described a case with AMI
at initial presentation of PM. A systematic literature review on
AMI in IIM patients was also included.
Materials and methods
Case presentation
A 39-year-old woman was referred to the Department
of Rheumatology and Immunology of Xiangya Hospital (Changsha,
China) on March 20, 2013 with a history of edema, feebleness,
post-exercise tachypnea persisting for half a month and dysphagia
for 4 days. She complained of sudden mild precordial discomfort
without chest tightness or pain lasting for several hours during
the first night of hospitalization. She did not have any history of
chest trauma or long-term use of any medication.
Initial physical examination revealed that her
pulse, blood pressure and respiration were 100/min, 98/64 mmHg and
20/min, respectively. Neck vein distention and examination of the
lung and heart yielded normal findings. The lower limbs contained
pitting edema, the muscle strength of the upper extremities was
grade 4 and that of the lower extremities was grade 3, most
commonly proximal.
Laboratory examination showed anemia [hemoglobin, 86
g/l (normal reference value, 110–150 g/l)] and low complement level
supported by a decrease of C4 [105 mg/l (normal reference value,
120–360 mg/l)] and C3 [580 mg/l (normal reference value, 850–1390
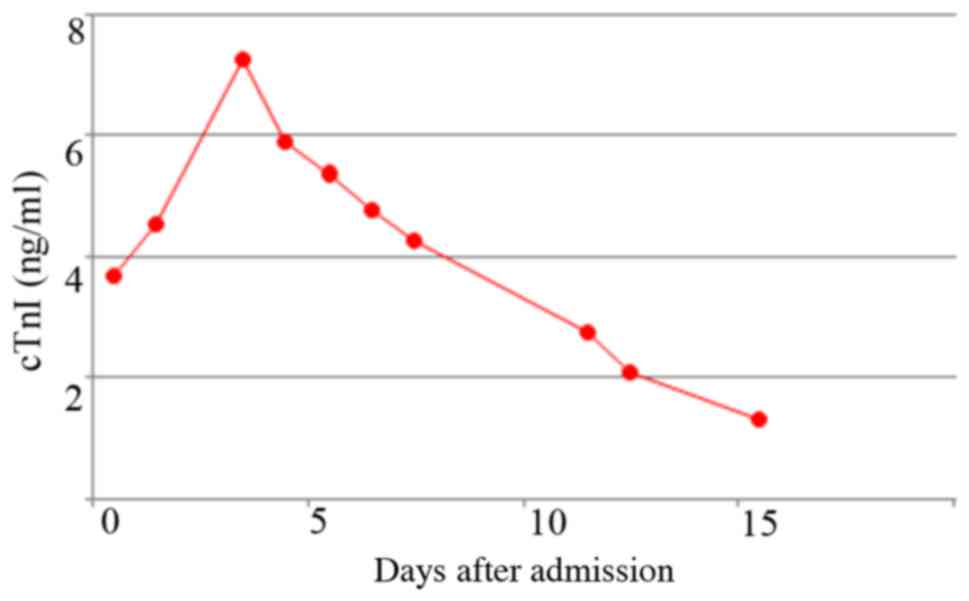
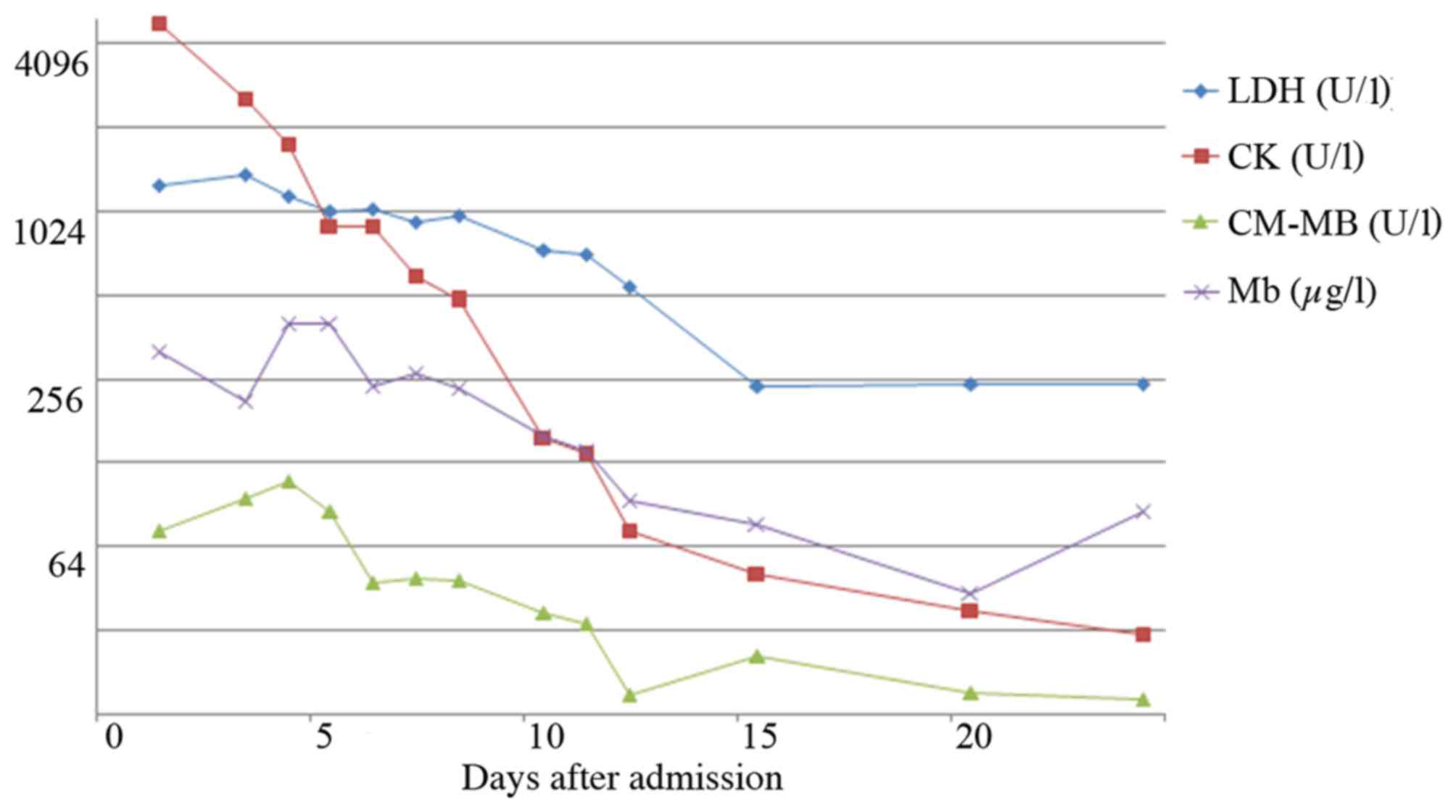
mg/l)]. Cardiac troponin I (cTnI) levels (normal reference value,
<0.16 pg/ml) and myocardial enzymology values (normal reference
value: Lactate dehydrogenase, LDH: 109–245 U/l; creatine kinase,
CK: 24–190 U/l; CK MB isoenzyme, CK-MB: <24 U/l; myoglobin, Mb:
<70 µg/l) were raised (Figs. 1
and 2). The level of N-terminal
pro-brain natriuretic peptide was 1,947 pg/m; (normal reference
value <250 pg/ml) indicating a poor cardiac function. The
patient was negative for detectable autoantibodies, including
antinuclear antibodies, anti-double-stranded DNA, anti-Smith,
anti-Sjögren's syndrome (SS)A, anti-SSB, anti-Jo-1, anti-Scl-70 and
anti-nuclear ribonucleoprotein. The white blood cell count,
platelet count and erythrocyte sedimentation rate were all
normal.
Muscle biopsy on the left arm conformed to PM. The
18-lead ECG on admission revealed sinus rhythm and ST-segment sloop
down in leads V1-V3 (Fig. 3). The
cardiac form, structure, functionality and valve activities were
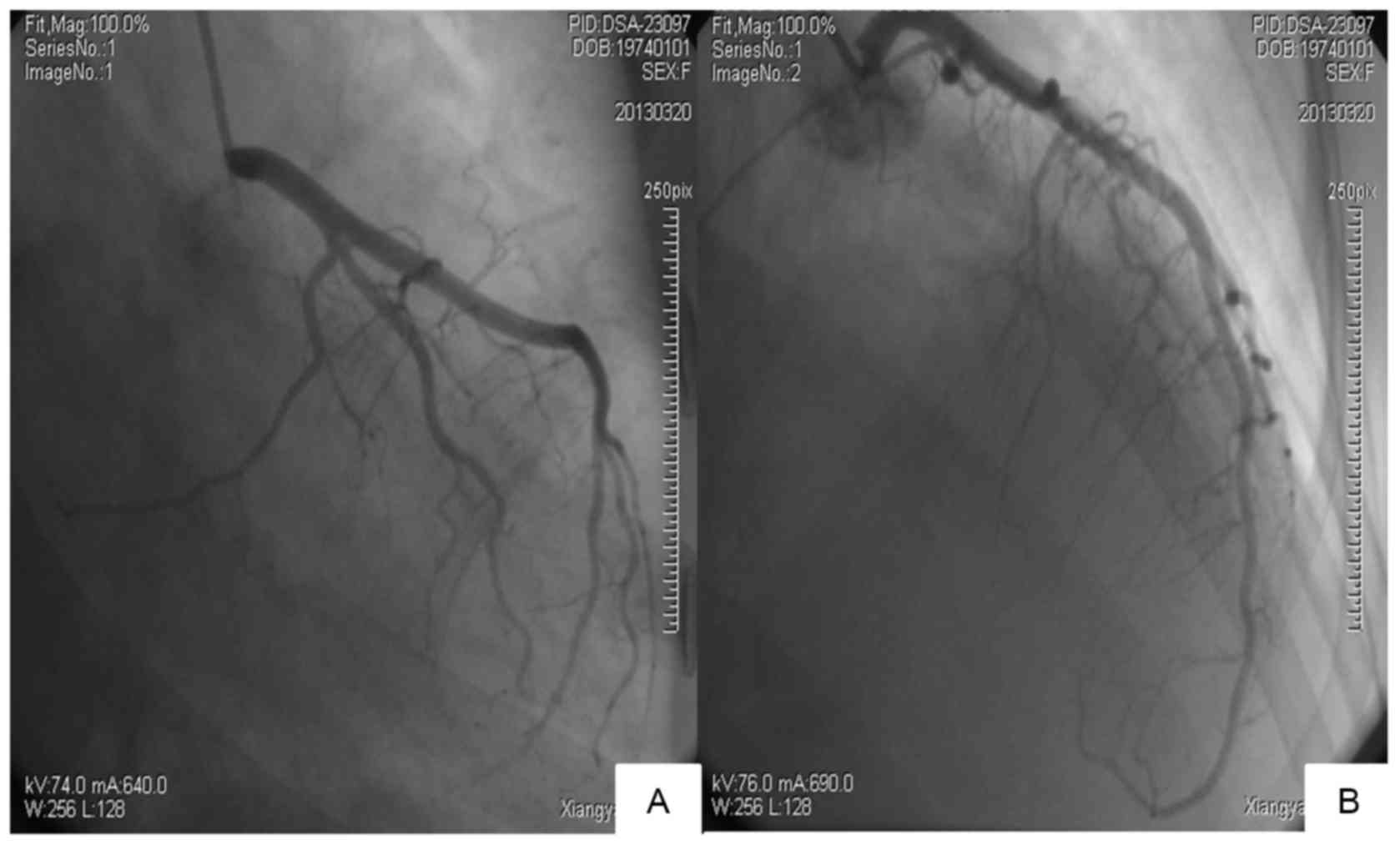
normal on cardiac Doppler ultrasonography. Coronary arteriography
was performed on admission, March 20, 2013 and showed
irregularities in the left anterior descending and right coronary
artery (25% diameter reduction in the middle segment). However, no
culprit lesion was found (Fig.
4).
Based on of all the above data, a diagnosis of PM
with cardiac involvement was made. The patient was given
methylprednisolone (500 mg/day), aspirin (0.1 g per night),
together with sodium nitroprusside and cedilanid to treat cardiac
failure. During the treatment, the patient showed recurrent dyspnea
and chest discomfort, and succumbed to respiratory and circulatory
failure 20 days later, although the myocardial enzymology and cTnI
levels had markedly decreased.
Literature review
In addition to the case reported in the present
study, all available previous studies were retrieved through a
systematic review on AMI in IIM. Studies published in indexed
international journals included in the PubMed database from January
1970 to January 2017 were analyzed. Cases of IIM patients who were
diagnosed with AMI and with sufficient information provided were
included. Studies published in the English language were selected
and additional cross-checks of references cited in them were
performed. As the search strategy, a combination of the following
terms was used: ‘Idiopathic inflammatory myopathy’, ‘polymyositis’
or ‘dermatomyositis’ and ‘acute myocardial infarction’, and 6
studies were retrieved. A total of 6 cases of AMI in IIM were
reported (9–14) (Table
I).
 | Table I.Data on patients with AMI in
polymyositis/dermatomyositis. |
Table I.
Data on patients with AMI in
polymyositis/dermatomyositis.
| Author (ref) | Date | Sex, (age, years | Underlying disease,
duration | Treatment before AMI
onset | Symptoms | Lab findings | Normal ranges | ECG | Cardiac
ultrasound | Coronary
angiography | Treatment at AMI | Outcome | Diagnosis |
|---|
| Ong et al
(13) | 2011 | F (51) | PM, hypertension,
hypercholes-terolemia, NA | Prednisolone (2.5
mg/day), cyclosporine, metoprolol, simvastatin | NA | cTnI, 0.52 µg/l | NA | Non ST-segment
elevation; myocardial infarction | NA | Inferior wall
hypokinesia; no atheromatous coronary obstructions;
acetylcholine-testing: Occlusive spasm in the mid-LAD and distal
RCA | NA | NA | Coronary artery
spasm |
| Riemekasten et
al (14) | 1999 | F (42) | DM, 6 years | Prednisolone (100
mg/day) | Raynaud's phenomenon,
chest pain, acute lung oedema | Elevated, (data not
reported) | NA | Variant angina
pectoris | Apicoseptal and
postero-lateral hypokinesia | Spontaneous coronary
constriction of the circumflex artery; acetylcholine-testing:
Vasospastic constriction | Aspirin,
methylprednisone (250 mg/day), then tapered, cyclosporin A,
diltiazem, amlodipine | Remission | Inflam-matory
processes and vasocon-striction |
| Cohn and Lynfield
(10) | 1979 | M (29) | DM, NA | None | Rash, fever,
weakness, pain in muscles and joints | LDH, 401 IU; CK,
2,295 IU | NA | Inferior and
anteriolateral wall infarction | NA | Normal | Prednisolone (40
mg/day) | Remission | DM with cardiac
muscle involvement |
| Odabasi et al
(12) | 2010 | F (41) | PM, 1 month | None | Proximal weakness,
vomiting syncope; edema | CK, 5,420 IU; AST,
155 IU | CK, <250 IU/l
AST, <37 IU/l | Inferoseptal
myocardial infarction | Mitral valve
prolapse, minimal tricuspid regurgitation | Normal | Prednisolone (60
mg/day), methotrexate | Left hemi-spheric
infarction after 9 years | PM with cardiac
muscle involvement |
| Jajoria et
al (11) | 2009 | F (53) | PM, 20 years | Prednisolone (10
mg/day) | Chest
discomfort | NA | NA | ST-elevation in
leads V3-4, ST depression in leads V5-6, I, II and aVF | NA | LAD dissection from
the mid -to-distal part with 95% stenosis; reduced flow through the
mid-LAD | PCI and stent
placement | Remission | Spontaneous
coronary artery dissection |
| Badui et al
(9) | 1996 | M (40) | DM, 12 years | Prednisolone (5-50
mg/day) | Sore, swelling
muscles, chest pain, nausea palpitation, shortness of breath | LDH, 743 U/l; CK,
938 U/l; CK-MB, 54 U/l | LDH, <230 U/l;
CK, <2000 U/l; CK-MB, <10 U/l; | Acute anterior wall
myocardial infarction | Disclosed anterior
wall akinesis | Normal | Nitroglycerin,
heparin, aspirin, furosemide | Remission | Coronary
arteritis |
| Present study | 2017 | F (39) | PM, half a
month | None | Edema, feebleness,
post-exercise tachypnea, dysphagia, mild precordial discomfort | cTnI, 7.26 µg/l;
LDH, 1,372 U/l; CK, 2,599 U/l; CK-MB, 94 U/l; Mb, 213 µg/l | cTnI, <0.16
pg/ml; LDH, 109-245 U/l; CK, 24-190 U/l; CK-MB, <24 U/l; Mb,
<70 µg/l | Anteroseptal
myocardial infarction | Normal | 25% diameter
reduction in RCA and the middle segment of LAD | Methyl-prednisolone
(500 mg/day), then tapered, aspirin (0.1 g per night), sodium
nitroprusside, cedilanid | Died | Cardiac muscle
involvement mimicking acute myocardial infarction in PM |
The cases comprised 3 PM and 3 DM patients,
including 4 females and 2 males (each of which had DM), and no IBM
was reported (9–14). In cases 2 and 4–6, the course of IIM
was long and they were administered prednisolone at various doses,
while case 3 had an acute onset. Case 1 received metoprolol (95
mg/day) and simvastatin (20 mg/day) for hypertension and
hypercholesterolemia, respectively, prior to admission (13). Case 3 had a history of blunt chest
trauma due to an automobile accident (10). Case 5 had received thrombolysis as a
myocardial infarction therapy 2 weeks prior to admission (11).
Case 2, 5 and 6 had complaints of chest discomfort
or chest pain (9,11,14),
while case 4 mainly presented with vomiting followed by syncope
(12). Case 1 and 3 did not show any
symptoms in the heart (10,13). Only case 1 was subjected to cTnI
examination and showed a mild increase (13). All cases were negative for
antinuclear antibodies. Except for case 5, coronary angiography
showed small changes in all cases, which may not have been
associated with the severe chest symptoms (9–14). Cases
1 and 2 showed occlusive spasm after intracoronary acetylcholine
provocation for vasospasm on coronary angiography (13,14).
Coronary angiography of case 5 showed a left anterior descending
coronary artery (LAD) dissection from the mid-to-distal part with
95% stenosis (11). The diagnosis of
spontaneous coronary artery dissection causing AMI was made and the
condition was successfully managed with percutaneous coronary
intervention and stent placement.
Cases 2–6 responded well to treatment (9–12,14).
Case 2 suffered recurrent severe chest pain and only added calcium
antagonism with amlodipine 5 mg/day markedly improved anginal
symptoms requiring no further hospital admissions for angina
(14). Most studies considered the
cause of the symptoms to be inflammatory processes due to IIM and
vasoconstriction due to impaired regulation of abnormal vasomotion
(9,10,12–14).
Discussion
IIMs are a group of rare systemic diseases, which
frequently show cardiac manifestations, which is, however,
subclinical in most cases. In 1979, Cohn and Lynfield (10) first reported on an IIM patient who
presented with AMI, who was included in the present literature
analysis as case 3. Case 2, 5 and 6 had a long history of IIM
before the onset of AMI and suffered an exacerbation (9,11,14). In
2009, Tisseverasinghe et al (15) reported a high incidence of arterial
events in IIM patients. A longitudinal follow-up study by Lai et
al (16) demonstrated that DM is
associated with an increased risk of cardiovascular events,
particularly AMI. Other studies confirmed this finding (17–19);
however, the specific risk factors of AMI in IIM patients have
remained to be investigated. Flow-mediated dilatation of the
brachial artery, arterial stiffness and carotid artery thickness on
ultrasonography may be predictors of cardiovascular disease in IIM
patients (18). Case 4, who suffered
a left hemispheric infarction 9 years after AMI, indicated that not
only cardiovascular but also cerebrovascular disease poses a high
risk (12,16).
Furthermore, case 1, 2, 5 and 6 received
prednisolone treatment prior to the onset of AMI. Administration of
glucocorticoids at high doses or for a long time is a risk factor
for diabetes mellitus, hypertension and hyperlipidemia, resulting
in atherosclerosis or cardiovascular disease, particularly coronary
heart disease (20). This may in
part explain for the high infarction risk in IIM patients (16–19).
Glucocorticoid dosages should be gradually reduced when the disease
can be controlled.
As shown in Table I,
IIM was in the active state in numerous patients with AMI, which
possibly presented secondarily to the cardiac involvement of IIM
(10,12,13). In
1990, Emslie-Smith and Engel (21)
reported that small arterioles, capillaries and venules are early
and specific targets of the pathological process in DM.
Inflammatory cells such as macrophages, activated-T lymphocytes and
dendritic cells, located around blood vessels in the perimysium
have been reported to cause endothelial dysfunction (22,23). In
addition, chronic inflammation contributes to coagulation by
upregulating pro-coagulants, downregulating anticoagulants and
suppressing fibrinolysis (23,24).
Vasculopathy and a high coagulation state may result in small
artery stenosis and lead to infarction, which has been observed in
numerous organs, including the brain, retina, kidney and spleen
(25,26). Chronic inflammation has been
demonstrated to be associated with other autoimmune diseases.
Furthermore, changes in progesterone may weaken vessels in
peripartum females with IIM (11,27). All
of these factors affect the arterioles in the perimysium of cardiac
muscle.
Acetylcholine testing by coronary angiography was
performed in case 1 and 2 (13,14). An
interesting phenomenon was that while occlusive spasm is at times
found after acetylcholine testing, coronary angiography showed only
mild diameter reduction (13,14). The
abnormalities on angiography returned to normal after intracoronary
nitroglycerine administration. Certain inflammatory pathways, as
well as high expression of adhesion molecules due to elevated
C-reactive protein concentrations, and expansion of CD4+
CD28null T cells, result in damage of endothelial cells
and early atherosclerosis, and lead to abnormal vasomotion by
increasing endothelin-1 and reducing NO production. This may
provide an explanation for the occurrence of coronary artery spasm
(13). Furthermore, inflammatory
cells such as T cells, macrophages and mast cells are all important
effector cells that participate in the pathogenesis of inflammatory
hypersensitivity disease and coronary artery spasm (28).
IIM has a feature of muscle enzyme elevation with
regard to creatine kinase (CK), CK MB isoform, lactate
dehydrogenase and myoglobin. It is difficult to distinguish between
simple IIM and IIM with AMI when the clinical manifestations are
untypical. cTn, composed of the three subunits cTnT, cTnI and cTnC,
is a sensitive laboratory parameter associated with heart
involvement. cTnT but not cTnI, along with CK, was reported to be
significantly higher in IIM with heart involvement (2,29–31).
Only in a small percentage of IIM patients, mild elevation of cTnI
is observed, although it is a sensitive laboratory parameter
associated with cardiac muscle damage. cTnI drops rapidly along
with myocardial enzymes and the ST-segment is depressed on ECG
after administration of aggressive glucocorticoid and
anti-heart-failure treatment, demonstrating that cTnI varies with
the activity of AMI. The present case showed the marked elevation
of cTnI on admission and a continuous high level of cTnI, which
cannot explained by specific drugs, therefore considered to be
indicative of AMI.
The case of the present study was a middle-aged
woman with complaints of persistent chest discomfort, who had no
risk factors for CAD and had no history of corticosteroid intake
for prolonged periods. She was unique in that not only her ECG but
also the dynamic changes of cTnI revealed an anterior wall
myocardial infarction, while coronary angiography revealed no
severe stenosis. Except for case 5, where spontaneous coronary
artery dissection led to stenosis, case 1–4 and 6 showed a similar
phenomenon: Coronary angiography did not conform to the findings of
clinical manifestation, and cTnI and ECG revealed AMI. It remains
elusive whether the case of the present study suffered real AMI or
any other cardiac event mimicking AMI of unknown cause.
Furthermore, it is unknown why AMI only affected the anterior wall
of the patient's heart. Coronary artery spasm may be one of the
causes of the manifestation of AMI (13,14).
However, based on the following facts, vasospasm does not fully
explain the changes in cTnI, myocardial enzymes and ECG findings,
particularly in the case of the present study: Coronary angiography
revealed no severe stenosis, and muscle enzyme and troponin showed
a marked decline after pulse therapy with methylprednisolone.
Therefore, the present case is more likely to have presented with
myocardial involvement mimicking AMI during an exacerbation of
IIM.
To the best of our knowledge, the present study
provided the first literature on myocardial involvement mimicking
AMI in IIM. However, it has limitations mainly due to the rarity of
the condition and the number of patients reviewed. While a few
cases of AMI were reported between 1970 and 1998, they lack certain
sensitive laboratory examinations of heart involvement such as Tn
(23). Furthermore, most of the case
reports retrieved described patients with a favorable response,
while those with poor outcomes were rarely reported. In addition,
no autopsy was performed on the patient of the present study to
confirm the conjecture made.
According to various studies, IIM patients are at
risk of coronary heart disease and myocardial involvement mimicking
AMI may be a rare phenomenon in IIM patients. Myocardial
involvement mimicking AMI may be the chief manifestation of IIM at
initial presentation in the active stage; however, the exact
mechanism remains elusive and requires further study. cTnI may be a
sensitive laboratory marker and the acetylcholine test in coronary
angiography is recommended. It is necessary to highlight the
importance of vigilance regarding cardiovascular diseases in the
management of patients with IIM. Therefore, medical staff is
required to pay more attention to cardiovascular diseases in
clinical practice.
References
|
1
|
Furst DE, Amato AA, Iorga ŞR, Gajria K and
Fernandes AW: Epidemiology of adult idiopathic inflammatory
myopathies in a U.S. Managed care plan. Muscle Nerve. 45:676–683.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Danko K, Ponyi A, Constantin T, Borgulya G
and Szegedi G: Long-term survival of patients with idiopathic
inflammatory myopathies according to clinical features: A
longitudinal study of 162 cases. Medicine. 83:35–42. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sultan SM, Ioannou Y, Moss K and Isenberg
DA: Outcome in patients with idiopathic inflammatory myositis:
Morbidity and mortality. Rheumatology (Oxford). 41:22–26. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Agrawal CS, Behari M, Shrivastava S, Ahuja
GK, Bhandari S and Kothari SS: The heart in
polymyositis-dermatomyositis. J Neurol. 236:249–250. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gottdiener JS, Sherber HS, Hawley RJ and
Engel WK: Cardiac manifestations in polymyositis. Am J Cardiol.
41:1141–1149. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lundberg IE: The heart in dermatomyositis
and polymyositis. Rheumatology (Oxford). 45 Suppl 4:iv18–iv21.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taylor AJ, Wortham DC, Burge JR and Rogan
KM: The heart in polymyositis: A prospective evaluation of 26
patients. Clin Cardiol. 16:802–808. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yazici Y and Kagen LJ: Cardiac involvement
in myositis. Curr Opin Rheumatol. 14:663–665. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Badui E, Valdespino A, Lepe L, Rangel A,
Campos A and Leon F: Acute myocardial infarction with normal
coronary arteries in a patient with dermatomyositis. Case report.
Angiology. 47:815–818. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cohn H and Lynfield YL: Myocardial
infarction in dermatomyositis. Cutis. 23:672–675. 1979.PubMed/NCBI
|
|
11
|
Jajoria P, Tuero EI and Lui CY:
Spontaneous coronary artery dissection causing acute myocardial
infarction in a post-menopausal woman with rheumatological disorder
(polymyositis): Treatment dilemma. J Invasive Cardiol.
21:E132–E133. 2009.PubMed/NCBI
|
|
12
|
Odabasi Z, Yapundich R and Oh SJ:
Polymyositis presenting with cardiac manifestations: Report of two
cases and review of the literature. Clin Neurol Neurosurg.
112:160–163. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ong P, Athanasiadis A, Alscher MD, Fritz
P, Mahrholdt H, Sechtem U and Kaski JC: Coronary artery spasm as a
cause for myocardial infarction in patients with systemic
inflammatory disease. Int J Cardiol. 151:e32–e34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Riemekasten G, Opitz C, Audring H,
Barthelmes H, Meyer R, Hiepe F and Burmester GR: Beware of the
heart: The multiple picture of cardiac involvement in myositis.
Rheumatology (Oxford). 38:1153–1157. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tisseverasinghe A, Bernatsky S and Pineau
CA: Arterial events in persons with dermatomyositis and
polymyositis. J Rheumatol. 36:1943–1946. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lai YT, Dai YS, Yen MF, Chen LS, Chen HH,
Cooper RG and Pan SL: Dermatomyositis is associated with an
increased risk of cardiovascular and cerebrovascular events: A
Taiwanese population-based longitudinal follow-up study. Br J
Dermatol. 168:1054–1059. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Linos E, Fiorentino D, Lingala B, Krishnan
E and Chung L: Atherosclerotic cardiovascular disease and
dermatomyositis: An analysis of the nationwide inpatient sample
survey. Arthritis Res Ther. 15:R72013. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vincze M, Dér H, Kerekes G, Szodoray P,
Zeher M, Dankó K and Soltész P: Decreased flow-mediated dilatation
with increased arterial stiffness and thickness as early signs of
atherosclerosis in polymyositis and dermatomyositis patients. Clin
Rheumatol. 33:1635–1641. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ungprasert P, Suksaranjit P, Spanuchart I,
Leeaphorn N and Permpalung N: Risk of coronary artery disease in
patients with idiopathic inflammatory myopathies: A systematic
review and meta-analysis of observational studies. Semin Arthritis
Rheum. 44:63–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sholter DE and Armstrong PW: Adverse
effects of corticosteroids on the cardiovascular system. Can J
Cardiol. 16:505–511. 2000.PubMed/NCBI
|
|
21
|
Emslie-Smith AM and Engel AG:
Microvascular changes in early and advanced dermatomyositis: A
quantitative study. Ann Neurol. 27:343–356. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Amato AA and Greenberg SA: Inflammatory
myopathies. Continuum (Minneap Minn). 19:1615–1633. 2013.PubMed/NCBI
|
|
23
|
Esmon CT: The interactions between
inflammation and coagulation. Br J Haematol. 131:417–430. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu J, Lupu F and Esmon CT: Inflammation,
innate immunity and blood coagulation. Hamostaseologie. 30:5–6,
8-9. 2010.PubMed/NCBI
|
|
25
|
De Vries S: Retinopathy in
dermatomyositis. AMA Arch Ophthalmol. 46:432–435. 1951. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matsuda Y, Harigai M, Nakajima H, Terajima
H, Yamada T, Fukasawa C, Takeuchi M, Hara M and Kamatani N:
Dermatomyositis with splenic and renal infarctions during
corticosteroid therapy. Intern Med. 39:512–516. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barrett JM, Van Hooydonk JE and Boehm FH:
Pregnancy-related rupture of arterial aneurysms. Obstet Gynecol
Surv. 37:557–566. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Almpanis GC, Kounis GN, Mazarakis A and
Kounis NG: Coronary artery spasm progressing to acute myocardial
infarction in patients with systemic inflammatory disease: A
potential association with kounis syndrome. Int J Cardiol. 151:1–2.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aggarwal R, Lebiedz-Odrobina D, Sinha A,
Manadan A and Case JP: Serum cardiac troponin T, but not troponin
I, is elevated in idiopathic inflammatory myopathies. J Rheumatol.
36:2711–2714. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fisher C, Agrawal S, Wong WM, Fahie-Wilson
M and Dasgupta B: Clinical observations on the significance of
raised cardiac troponin-T in patients with myositis of varying
etiologies seen in rheumatology practice. Clin Rheumatol.
29:1107–1111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gerhardt W and Ljungdahl L: Troponin T: A
sensitive and specific diagnostic and prognostic marker of
myocardial damage. Clin Chim Acta. 272:47–57. 1998. View Article : Google Scholar : PubMed/NCBI
|