Introduction
In an aging population, osteoporosis, which is
defined as bone mineral density reduction and bone
microarchitecture deterioration, is becoming an increasingly common
health problem (1). Even minor
trauma, such as a fall from standing height, is sufficient to cause
severe fractures at multiple sites (principally in the spine, hip,
distal radius and proximal humerus (1). Such fractures are referred to as
fragility fractures and are exclusively related to osteoporosis
(1). Furthermore, once these
fractures occur, measures adopted for osteoporosis prevention and
treatment become difficult and less effective (1). If internal fixation techniques fail
(due to screw loosening or pull-out), aseptic prosthetic loosening
after arthroplasty and periprosthetic fractures become more likely
due to decreased strength and increased fragility of the
osteoporotic bone (1). This
situation may be worsened when reconstructing large bone defects
and non-unions become unavoidable after major trauma or tumor
resections (1). To date, autologous
cancellous bone grafting remains the therapeutic gold standard
method used to reinforce bone mass and strength; however, limited
graft availability, donor site morbidity and decreased bone marrow
osteogenesis limit its use for this type of clinical application
(2–4).
Cell-based bone tissue engineering holds great
promise. Selecting suitable seed cells, which possess favorable
bone formation capacity, is critical for the fabrication of
eligible bioengineering composites. The osteogenic potency of bone
marrow mesenchymal stem cells (BMSCs) and adipose tissue
mesenchymal stem cells (ASCs) has been widely identified and used
to enhance bone tissue formation by combination with bioengineering
scaffolds and/or osteogenic cytokines (1,5,6).
However, widespread application of BMSCs and ASCs
for this purpose is unsuitable in elderly patients. Osteoporosis is
frequently related to obesity (7,8),
although the underlying mechanism for this has not yet been fully
elucidated. Osteoblasts and adipocytes are both derived from BMSCs;
therefore, decreased osteogenesis and increased adipogenesis of
BMSCs increases bone marrow adiposity and also enhances body fat
deposition (9,10). The application of BMSCs with reduced
osteogenic capacity could limit the potential of these cells for
the treatment of bone defects. However, abundant fat tissues could
be a source of alternatives to BMSCs for this purpose. Compared
with BMSCs, ASCs exhibit superior osteogenic capacity, and could be
used to maintain adequate capability in elderly people suffering
from osteoporosis (1,11,12). As
terminally differentiated cells, mature adipocytes can be
transformed into more primitive dedifferentiated fat cells (DFATs),
which regain multilineage differentiation potential (13–15).
After osteogenic differentiation, DFATs combined with biocomposites
have been used to form new bone tissue in ectopic sites and to
repair lacunar bone defects (14,16).
Derived from mature adipocytes, which constitute the major part of
adipose tissue, DFATs are a more abundant cell type compared with
ASCs. Therefore, DFATs should be a key focus of bone tissue
engineering research, especially for the treatment of osteoporosis
and related diseases.
Previous studies have demonstrated that ASCs from
osteoporotic patients (opASCs) possess favorable osteogenic potency
(1). In the present study, DFATs
were derived from the fat cells of osteoporotic patients (opDFATs)
and their osteogenic potential was evaluated both in vitro
and in vivo.
Materials and methods
Isolation and culture of opDFATs and
opASCs
Before cell harvesting, related protocols were
approved by the Medical Ethics Committee of Yantai Yuhuangding
Hospital Affiliated to Qingdao University Medical College (Yantai,
China; approval no. YYYLLS[2014]126) and written informed consent
was provided by all patients. Subcutaneous fat tissues were
obtained from 12 patients between January and December 2014 (8
females and 4 males; average age 65.2±16.8 years) during surgery
for the repair of hip fractures by internal fixation or total hip
arthroplasty. The adipose tissues were washed with sterilized
phosphate buffered saline, finely minced and digested with 0.1%
collagenase I (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at
37°C for 1 h. Collagenase I was neutralized by adding an equal
volume of control medium containing Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc.) and 10% fetal bovine
serum (Hangzhou Sijiqing Biological Engineering Materials Co.,
Ltd., Hangzhou, China), then the tissues were filtered through a
150-µm mesh filter to remove the debris. The filtrate was
centrifuged at 150 × g for 6 min. The top layer, containing
unilocular adipocytes, and the pellet were collected separately.
The pellet was resuspended and processed consecutively to isolate
and culture opASCs in vitro as previously described
(1).
Mature adipocytes were isolated using the ceiling
culture method. A total of ~5×104 mature adipocytes were
placed in each 25 cm2 culture flask (Nalge Nunc
International, Penfield, NY, USA) that were completely filled with
the control medium and incubated at 37°C under 5% CO2.
The cells floated and adhered to the top inner layer of the flasks.
During the first 3–4 days, mature adipocytes adhered loosely to the
ceiling surface and cell movement could be observed with gentle
shaking of flasks. At day 7–8, the cells started to lose their
spherical shapes and adhered more strongly to the ceiling layer; no
cell movement could be detected with shaking. After sufficient
attachment of cells (usually 10–12 days), the flask was reinverted.
The medium was removed and replaced every 3 days with ~5 ml on each
occasion. At confluence, the cells were passaged with 0.25%
trypsin/EDTA and replated at a 1:3 dilution.
In vitro osteogenic
differentiation
OpDFATs and opASCs at passage 2 were induced in
6-well plates (2×104 cells/well) in osteogenic
differentiation medium [control medium supplemented with 0.1 µM
dexamethasone, 50 µg/ml ascorbic acid-2-phosphate and 10 mM
β-glycerophosphate (all Sigma-Aldrich; Merck KGaA)]. The medium was
replaced every 3 days. In order to evaluate the osteogenic potency
of opDFATs, alkaline phosphatase (ALPase) activity and calcium
deposition assays were conducted using the diazo coupling method
and von Kossa staining as previously described (17,18),
respectively, at 14 and 21 days after induction.
The DNA content of opDFAT and opASC wells was
determined by fluorometric assay. At day 1, 7 and 14, cells were
treated with trypsin, collected by centrifugation at 150 × g for 6
min at room temperature and lysed by sonication. The homogenate was
mixed with Hoechst 33258 stain (Dojindo Molecular Technologies,
Inc., Kumamoto, Japan). Emission and excitation spectra were
obtained using a Modulus Microplate Luminometer (Turner BioSystems;
Promega Corporation, Madison, WI, USA) at 458 and 356 nm,
respectively.
ALPase activity and cell
mineralization measurement under osteogenic differentiation in
vitro
At day 7 and 14, opDFATs and opASCs were lysed by
trypsinization, centrifugation and sonication. The ALPase activity
of cell lysates was determined by measuring the release of
p-nitrophenol from p-nitrophenyl phosphate (Sigma-Aldrich; Merck
KGaA). The release of p-nitrophenol was monitored by measuring the
optical density at 405 nm. The optical densities were then compared
with a standard p-nitrophenol solution (Sigma-Aldrich; Merck KGaA).
The protein concentrations of cell lysates were determined
biochemically using the Bicinchoninic Acid Protein Assay kit
(Pierce; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols. Results are expressed as nmol
p-nitrophenol per µg protein per min.
For evaluation of cell mineralization, cell lysates
were obtained using the aforementioned method and incubated
overnight at 4°C with 1 ml 0.5 N HCl with gentle shaking.
Ca2+ ion levels were determined using the
o-cresolphthalein complexone method with a commercial Calcium C kit
(Wako Pure Chemical Industries, Ltd., Osaka, Japan) according to
the manufacturer's protocols.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) of osteogenesis-specific genes
under osteogenic differentiation in vitro
At days 1, 7 and 14, total RNA was extracted from
opDFATs and opASCs using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and was reverse-transcribed to cDNA using a
PrimeScript™ RT reagent kit (Takara Biotechnology Co., Ltd.,
Dalian, China) according to the manufacturer's protocol. Then, qPCR
assays were performed in a total volume of 25 µl containing 1 µl
cDNA, 10 µM gene-specific primers, 2x SYBR Premix Ex Taq™ (Takara
Biotechnology Co., Ltd.), 50x ROX Reference Dye and dH2O, in an ABI
7500 Real-Time Thermocycler (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The reaction conditions were as follows: Initial
denaturation at 94°C for 10 min, followed by 45 cycles of 94°C for
40 sec, 60°C for 30 sec and 72°C for 30 sec. β-actin was used as an
internal control to evaluate total RNA input. The expression levels
of the osteogenesis-specific genes collagen I (COL I), osteocalcin
(OC) and bone sialoprotein (BSP) were calculated using the
comparative threshold-cycle method. The efficiency of each assay
was calculated using the formula E=10-1/slope. The following primer
sequences were used in RT-qPCR assays (sense, antisense): β-actin
(5′-ACAGAGCCTCGCCTTTGCC-3′, 5′-ACATGCCGGAGCCGTTGTC-3′), COL I
(5′-GCAAGGGAGAAAAGGGTGAACC-3′, 5′-GTGGCTCCAGCAGGACCAG-3′), OC
(5′-CTCCAGGCACCCTTCTTTCC-3′, 5′-ATTCCTCTTCTGGAGTTTATTTGGG-3′), BSP
(5′-ATACCATCTCACACCAGTTAGAATG-3′,
5′-AACAGCGTAAAAGTGTTCCTATTTC-3′).
In vivo implantation of opDFATs
Approval was obtained from the Institutional Animal
Review Committee of Yantai Yuhuangding Hospital Affiliated to
Qingdao University Medical College (approval no. YYYDWS[2014]058)
prior to beginning animal research in this study. A total of 6
4-week-old nude mice (sex ratio 1:1; body weight 14–16 g) were
bought from the Animal Center of BinZhou Medical College (Yantai,
China) and housed in specific pathogen free cages, supplied with
autoclaved food, water and bedding ad libitum at 26–28°C
with 40–60% humidity at the animal center of BinZhou Medical
College, (Yantai, China). Before implantation, collagen I gel and
poly (lactide-co-glycolide)/β-tricalcium phosphate (PLGA-β-TCP)
porous scaffold were prepared as previously described (1). Passage 2 opDFATs were suspended in 100
µl collagen I at 2×106 cells/ml and seeded into the
porous PLGA-β-TCP scaffold on ice. They were then incubated at 37°C
for 30 min to allow gel formation to fabricate the
opDFAT-COL/PLGA-β-TCP composite. The constructs were cultured in
osteogenic medium at 37°C in an atmosphere containing 5% CO2 for 14
days. Mice were anesthetized with 1% pentobarbital sodium at 40
mg/kg (Sigma-Aldrich; Merck KGaA) and constructs were implanted
into the space between the subcutaneous tissue and the deep fascia
of nude mice. The mice were sacrificed by cervical dislocation 4
weeks later and the implants were harvested and prepared for
histological analyses by hematoxylin and eosin staining. An
acellular COL/PLGA-β-TCP composite was implanted as a blank
control.
In addition, scanning electron microscopy (SEM) was
used to observe cell adhesion and fabrication structure of the
opDFAT-COL/PLGA-β-TCP composite. Briefly, the osteogenic medium was
removed following 14 days of in vitro culture and the
cell-scaffold composites were washed 3 times with PBS, fixed with
2.5% glutaraldehyde, dehydrated through a graded series of ethanol,
and critical point dried. Samples were fixed onto SEM aluminum
stubs, sputter coated with gold and observed under a scanning
electron microscope (Hitachi Ltd., Tokyo, Japan).
Statistical analysis
All quantitative data are expressed as the mean ±
standard deviation. SPSS 19.0 software (IBM SPSS, Armonk, NY, USA)
was used for data analysis. Student's t-tests were used to analyze
the results of ALPase activity and extracellular mineralization
assays. The relative expression level of osteogenesis-specific
genes was compared by analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
In vitro osteogenic differentiation of
opDFATs
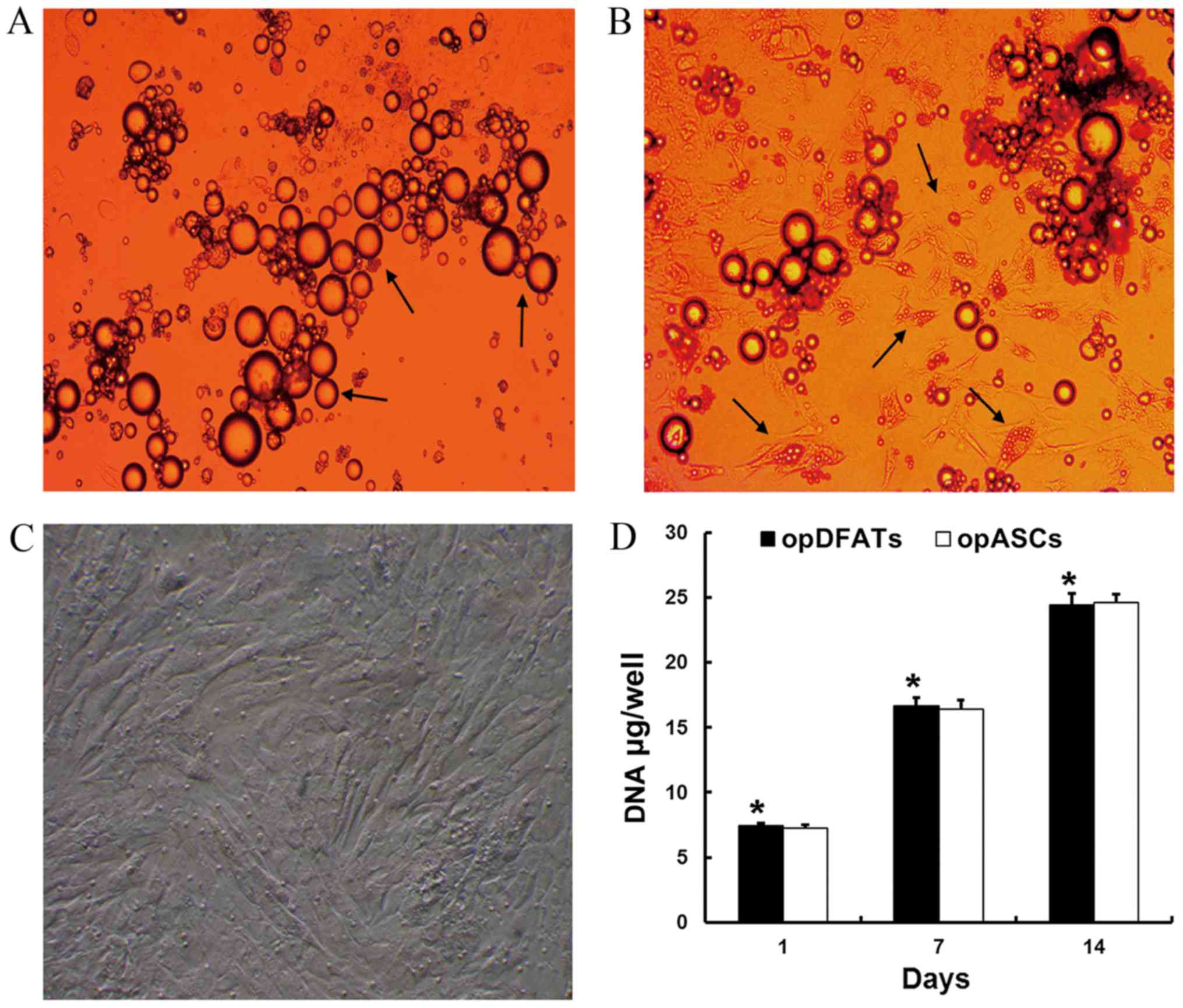
At day 1, the unilocular mature adipocytes floated
on the top layer of the medium and made contact with the inner
ceiling surface of the flasks (Fig.
1A). Small movements of the mature adipocytes were observed
with gentle shaking. At day 6–7, the unilocular adipocytes adhered
tightly to the inner ceiling surface of the flasks. Mature
adipocytes started to lose their spherical shape and exhibit
fibroblast-like morphology. Unilocular lipid droplets in the
cytoplasm gradually split into smaller droplets at day 10–12
(Fig. 1B). The culture medium was
then replaced and the flasks were reinverted so that the cells were
cultured on the bottom surface. Primary culture duration to reach
confluence was usually 15–20 days (Fig.
1C).
Subsequently, the cells were passaged with 0.25%
trypsin/EDTA and replated at a 1:3 dilution. The primary culture of
opASCs was performed as described previously (8). OpDFATs and opASCs at passage 2 were
cultured in osteogenic medium and received further examination. As
shown in Fig. 1D, opDFATs and opASCs
exhibited similar growth profiles. The DNA content of opDFATs
increased continuously, with no statistically significant
differences detected as compared with opASCs.
ALPase activity and extracellular
mineralization of opDFATs
From day 7 to 14, the ALPase activity of opDFATs was
found to be significantly enhanced (P<0.05; Fig. 2A). There was no statistically
significant difference between ALPase activity levels in opDFATs
and opASCs (Fig. 2A). The results of
the diazo coupling method revealed an extensive purple-stained
area, confirming ALPase activity in opDFATs (Fig. 2B).
Extracellular mineralization of opDFATs increased
significantly from day 7 to 14 (P<0.05; Fig. 2C). No statistically significant
difference was detected between extracellular mineralization levels
in opDFATs and opASCs (Fig. 2C). Von
Kossa staining of extracellular matrix mineralization confirmed
that calcium deposition was present in opDFAT cell culture, visible
as black nodules (Fig. 2D).
Osteogenesis-specific gene
expression
COL I expression in opDFATs increased significantly
at days 4, 7 and 14 as compared with day 1 (all P<0.05; Fig. 3A), with no significant differences in
COL I expression levels observed between opDFATs and opASCs.
Compared with day 1, a continuous increase in OC expression was
observed in opDFATs on days 4, 7 and 14, with significant
differences at each time point (all P<0.05; Fig. 3B). No significant differences in OC
expression levels were observed between opDFATs and opASCs.
Similarly, BSP expression in opDFATs increased continuously from
day 1 to days 4, 7 and 14, with significant differences between
each time point (all P<0.05; Fig.
3C). No significant differences in BSP expression levels were
observed between opDFATs and opASCs.
 | Figure 3.Osteogenesis-specific gene expression
of opDFATs and opASCs under osteogenic induction. Reverse
transcription-quantitative polymerase chain reaction was conducted
to evaluate (A) COL I, (B) OC and (C) BSP expression levels.
*P<0.05, statistically significant difference among opDFATs at
different culture time-points (COL I and OC); **P<0.05,
statistically significant difference between opDFATs at days 1, 4,
7 and 14 (BSP). COL I, collagen I; OC, osteocalcin; BSP, bone
sialoprotein; opDFAT, dedifferentiated fat cell obtained from an
osteoporotic patient; opASC, adipose-derived stem cell obtained
from an osteoporotic patient. |
In vivo implantation of the
opDFATs-based composite
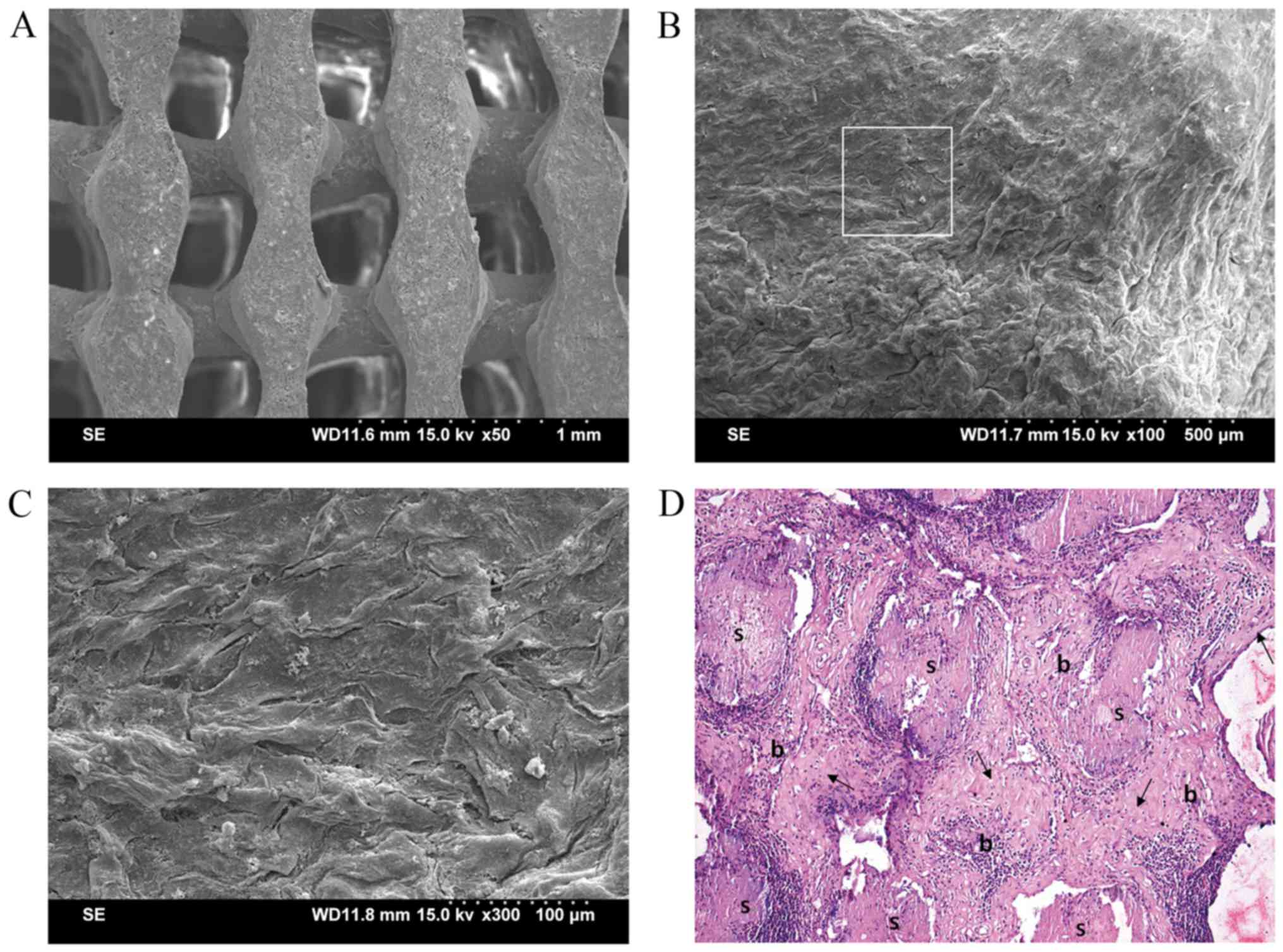
For the opDFAT-COL/PLGA-β-TCP composite, surfaces
and pores of the PLGA-β-TCP scaffold (Fig. 4A) were filled with opDFATs, which
were entrapped by collagen I gel as demonstrated by SEM (Fig. 4B and C). Four weeks after in
vivo implantation, woven bone tissue with visible osteocytes
containing lacunae was formed evenly in the pores of the
opDFAT-COL/PLGA-β-TCP composite (Fig.
4D). In contrast, no bone tissue was observed except connective
tissues in the COL/PLGA-β-TCP composite (data not shown).
Discussion
In the present study, it was demonstrated that
mature adipocyte-derived DFATs isolated from subcutaneous fat
tissues of osteoporotic patients (opDFATs) possess osteogenic
differentiation capacity equivalent to that of opASCs.
Treatment of osteoporotic fractures is highly
demanding because of the inherent changes in bone tissue, including
reduced bone mineral density, unfavorable geometry at cortical bone
sites and deteriorated bone microarchitecture (2,3).
Cell-based tissue engineering can be used to augment bone mass,
strengthen osteointegration around the periprosthetic region and
reconstruct bone defects. BMSCs exhibit a low proliferative
capacity and decreased osteogenic potential among the elderly
population, especially those individuals with osteoporosis
(19,20). Harvesting and in vitro culture
of BMSCs obtained from this population is problematic due to
increased patient distress, donor site morbidity, infection risk
and poor osteogenic capacity (1).
These problems decrease the feasibility of translating the
isolation of BMSCs from osteoporotic patients into a potentially
clinical application (1).
Previous results have suggested that osteoporosis
and obesity share common etiological features. These include
overlapping genetic and environmental predisposition, high
concurrence of osteoporosis and bone marrow adiposity during normal
aging, similar regulation mechanisms via the hypothalamus and
sympathetic nervous system for bone remodeling and adiposity, and
common progenitor mesenchymal stem cells (7). However, the interaction between fat
mass and bone is complex. Previous studies have indicated that
excessive fat mass is associated with beneficial effects on bone
that protect against osteoporosis (21,22),
although conflicting results have also been reported (23,24).
This discrepancy may be due to complicated regulation mechanisms,
which are mediated by adipocyte-derived peptides, pancreatic
hormones and mesenchymal stem cell differentiation (25).
In relation to bone tissue engineering, excessive
fat mass is favorable because it represents an abundant seed cell
source that can be utilized to treat osteoporosis-related fractures
(1). Adipose tissue contains
adipocytes and non-adipose cells (ASCs, fibroblasts, endothelial
cells, blood cells and macrophages) (26). Studies conducted both in vitro
and in vivo have demonstrated that human ASCs can
differentiate towards multiple lineages, such as adipocytes,
osteoblasts, chondrocytes, myocytes, neuronal cells, endothelial
cells and hepatocytes (27). ASCs
have been demonstrated to maintain proliferative and osteogenic
differentiation capacity, particularly in elderly people with
osteoporosis (1,11,12);
however, ASCs account for only a small proportion of the total
cells in adipose tissue. Several studies have also demonstrated
that ASCs are a heterogeneous population containing smooth muscle
cells, endothelial cells, mast cells and lineage-committed
progenitor cells (28,29). These factors could hinder the
osteogenic induction process of ASCs, by making in vitro
culturing more difficult and time-consuming before implantation
in vivo.
Mature adipocytes are functionally the most
important cell type in adipose tissue, with a typical morphology
characterized by the presence of a single, large cytoplasmic lipid
droplet accounting for ~90% of its volume (26). As demonstrated in this study, mature
adipocytes can be isolated using the traditional ceiling culture
method. The unilocular lipid droplets in mature adipocytes cause
the cells to float in the culture medium and they can therefore be
easily isolated from other cell populations. Previously, flow
cytometric analysis has revealed that a highly homogeneous
population of opDFATs cells can be obtained using this technique
(26). As terminally differentiated
cells, mature adipocytes can be dedifferentiated and
transdifferentiated towards multiple lineages, including
chondrocytes, osteoblasts, skeletal myocytes, smooth muscle cells
and cardiomyocytes (26,30–33).
During ceiling culture in vitro, it was observed that the
lipid droplets in mature adipocytes gradually diminished,
accompanied by changes in cell morphology from spherical to
elongated. The cells then dedifferentiated as opDFATs and entered a
proliferation phase similar to opASCs. In vitro
investigations suggested that opDFATs possess favorable osteogenic
potential comparable to that of opASCs. Furthermore, in vivo
studies confirmed ectopic bone tissue formation of opDFAT-based
bioengineering composites. Previous studies have demonstrated the
osteogenic capacity of DFATs and related biocomposites (14,34);
however, there have been few reports of osteogenesis-related
research on DFATs isolated from osteoporotic patients. DFATs
harvested from the ovariectomy-induced osteoporosis model in
rabbits showed osteogenic activity similar to cells from healthy
samples (16). Intra-bone marrow
injection of autologous DFAT cells significantly increased the bone
mineral density at the injected site (16). Hence, opDFATs could become an
alternative to opASCs as a seed cell source for bone defect repair
in osteoporotic patients by bone tissue engineering methods.
Previous results of molecular analysis indicate that
mature adipocytes contain transcripts for embryonic stem cell
genes, which are required for self-renewal and pluripotency
(15). During the dedifferentiation
process in culture, DFATs lose mature adipocyte markers including
lipoprotein lipase, leptin, glucose transporter type 4,
adiponectin, adipocyte protein 2 and preadipocyte factor-1
(15,26). Transcription factors which regulate
adipocytic differentiation (peroxisome proliferator-activated
receptor gamma and CCAAT-enhancer-binding proteins α, β and δ) are
also significantly downregulated, while those that are critical for
osteogenesis and chondrogenesis (runt-related transcription factor
2 and SRY-box 9) are expressed during dedifferentiation (26). Flow cytometric analysis demonstrated
that DFATs exhibit uniform cell surface protein expression similar
to that of BMSCs (26). After
dedifferentiation in culture, DFATs showed the capacity to
transdifferentiate toward the osteogenic lineage under osteogenic
induction in culture (34). Similar
to BMSCs and ASCs, the BMP signaling pathways participate in this
process. Upregulation of BMPR-IB has been shown to promote
osteoblast differentiation of DFATs (34,35).
In addition to the aforementioned confirmation of
opDFATs as a suitable source of seed cells, it has previously been
demonstrated that three-dimensional porous scaffolds act as micro
frames for seed cells to adhere, proliferate and differentiate.
PLGA-β-TCP (7:3 w/w) scaffolds with 90% porosity and 300 to 350 µm
pores were designed and produced using the low-temperature
deposition manufacturing technique (36). Previous experiments by the current
authors have demonstrated that the PLGA-β-TCP scaffold possesses
good biocompatibility, biodegradability, osteoconductivity and
mechanical properties after combination with ASCs in vitro
and in vivo (5). In the
present study, opDFATs were encapsulated in collagen I hydrogel and
then combined with a PLGA-β-TCP porous scaffold. Collagen I
hydrogel can hold large numbers of seed cells in porous scaffolds
and promote their osteogenic differentiation (5,12). SEM
evaluation indicated that opDFATs were in close cell-to-cell
contact, mediated via crosslinking of collagen I fibers and
complete overgrowth around and into the porous scaffold. New bone
tissue was formed after implantation of opDFAT-COL/PLGA-β-TCP
composites in vivo.
Previous results have demonstrated that opASCs
exhibit sustained proliferation and adequate osteogenicity in
contrast to the impaired BMSCs in osteoporotic patients (8). In the present study, mature adipocytes
were harvested and dedifferentiated to generate opDFATs. In
vitro and in vivo osteogenic differentiation analyses
suggested that opDFATs also exhibit favorable osteogenesis
capacity. By using collagen I hydrogel as a cell carrier and
incorporation with a PLGA-β-TCP scaffold, an opDFAT-COL/PLGA-β-TCP
composite was fabricated and demonstrated to form new bone tissue
in vivo. Future studies will focus on the repair of bone
defects in orthotopic sites.
The present study is not without limitations. The
osteogenic potential of opDFATs was primarily evaluated through
in vitro culture and in ectopic sites with COL/PLGA-β-TCP
scaffolds as cell carriers. Future studies should focus on a
combination of opDFATs at different densities with various
biomaterials and their bone formation capacity both in vitro
and in vivo. The opDFATs-based biocomposites should be
applied to repair larger bone defects in animals so as to provide
more convincing data concerning sustained osteogenesis of
opDFATs.
Acknowledgements
The authors acknowledge funding support from the
Shandong Provincial Natural Science Foundation, China (grant no.
ZR2012CQ018).
References
|
1
|
Jiang M, Wang X, Liu H, Zhou L, Jiang T,
Zhou H and Hao W: Bone formation in adipose-derived stem cells
isolated from elderly patients with osteoporosis: A preliminary
study. Cell Biol Int. 38:97–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hao W, Hu YY, Wei YY, Pang L, Lv R, Bai
JP, Xiong Z and Jiang M: Collagen I gel can facilitate homogenous
bone formation of adipose-derived stem cells in PLGA-beta-TCP
scaffold. Cells Tissues Organs. 187:89–102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee JH, Lee JH, Park JW and Shin YH: The
insertional torque of a pedicle screw has a positive correlation
with bone mineral density in posterior lumbar pedicle screw
fixation. J Bone Joint Surg Br. 94:93–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu ZX, Gong FT, Liu L, Ma ZS, Zhang Y,
Zhao X, Yang M, Lei W and Sang HX: A comparative study on screw
loosening in osteoporotic lumbar spine fusion between expandable
and conventional pedicle screws. Arch Orthop Trauma Surg.
132:471–476. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hao W, Pang L, Jiang M, Lv R, Xiong Z and
Hu YY: Skeletal repair in rabbits using a novel biomimetic
composite based on adipose-derived stem cells encapsulated in
collagen I gel with PLGA-beta-TCP scaffold. J Orthop Res.
28:252–257. 2010.PubMed/NCBI
|
|
6
|
Hao W, Dong J, Jiang M, Wu J, Cui F and
Zhou D: Enhanced bone formation in large segmental radial defects
by combining adipose-derived stem cells expressing bone
morphogenetic protein 2 with nHA/RHLC/PLA scaffold. Int Orthop.
34:1341–1349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rosen CJ and Bouxsein ML: Mechanisms of
disease: Is osteoporosis the obesity of bone? Nat Clin Pract
Rheumatol. 2:35–43. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hagey AR and Warren MP: Role of exercise
and nutrition in menopause. Clin Obstet Gynecol. 51:627–641. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carbonare L Dalle, Valenti MT, Zanatta M,
Donatelli L and Lo Cascio V: Circulating mesenchymal stem cells
with abnormal osteogenic differentiation in patients with
osteoporosis. Arthritis Rheum. 60:3356–3365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen JR, Lazarenko OP, Wu X, Tong Y,
Blackburn ML, Shankar K, Badger TM and Ronis MJ: Obesity reduces
bone density associated with activation of PPARγ and suppression of
Wnt/β-catenin in rapidly growing male rats. PLoS One. 5:e137042010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen HT, Lee MJ, Chen CH, Chuang SC, Chang
LF, Ho ML, Hung SH, Fu YC, Wang YH, Wang HI, et al: Proliferation
and differentiation potential of human adipose-derived mesenchymal
stem cells isolated from elderly patients with osteoporotic
fractures. J Cell Mol Med. 16:582–593. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu W, Niklason L and Steinbacher DM: The
effect of age on human adipose-derived stem cells. Plast Reconstr
Surg. 131:27–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park SR, Oreffo RO and Triffitt JT:
Interconversion potential of cloned human marrow adipocytes in
vitro. Bone. 24:549–554. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Justesen J, Pedersen SB, Stenderup K and
Kassem M: Subcutaneous adipocytes can differentiate into
bone-forming cells in vitro and in vivo. Tissue Eng. 10:381–391.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Poloni A, Maurizi G, Leoni P, Serrani F,
Mancini S, Frontini A, Zingaretti MC, Siquini W, Sarzani R and
Cinti S: Human dedifferentiated adipocytes show similar properties
to bone marrow-derived mesenchymal stem cells. Stem Cells.
30:965–974. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kikuta S, Tanaka N, Kazama T, Kazama M,
Kano K, Ryu J, Tokuhashi Y and Matsumoto T: Osteogenic effects of
dedifferentiated fat cell transplantation in rabbit models of bone
defect and ovariectomy-induced osteoporosis. Tissue Eng Part A.
19:1792–1802. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hodson AW and Skillen AW: Comparison of
diazo-coupling, formazan, and silver staining techniques for
visualizing alkaline phosphatase isoenzymes after electrophoresis
in homogeneous-pore and gradient-pore polyacrylamide gels. Anal
Biochem. 169:253–261. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mallory FB: Pathological techniques: A
practical manual for workers in pathological histology including
directions for the performance of autopsies and for
microphotography. WB Saunders, Philadelphia, PA; pp. 143–144.
1983
|
|
19
|
Stolzing A, Jones E, McGonagle D and Scutt
A: Age-related changes in human bone marrow-derived mesenchymal
stem cells: Consequences for cell therapies. Mech Ageing Dev.
129:163–173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou S, Greenberger JS, Epperly MW, Goff
JP, Adler C, Leboff MS and Glowacki J: Age-related intrinsic
changes in human bone-marrow-derived mesenchymal stem cells and
their differentiation to osteoblasts. Aging cell. 7:335–343. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Riis BJ, Rødbro P and Christiansen C: The
role of serum concentrations of sex steroids and bone turnover in
the development and occurrence of postmenopausal osteoporosis.
Calcif Tissue Int. 38:318–322. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lau EM, Chan YH, Chan M, Woo J, Griffith
J, Chan HH and Leung PC: Vertebral deformity in chinese men:
Prevalence, risk factors, bone mineral density, and body
composition measurements. Calcif Tissue Int. 66:47–52. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hsu YH, Venners SA, Terwedow HA, Feng Y,
Niu T, Li Z, Laird N, Brain JD, Cummings SR, Bouxsein ML, et al:
Relation of body composition, fat mass, and serum lipids to
osteoporotic fractures and bone mineral density in Chinese men and
women. Am J Clin Nutr. 83:146–154. 2006.PubMed/NCBI
|
|
24
|
Zhao LJ, Liu YJ, Liu PY, Hamilton J,
Recker RR and Deng HW: Relationship of obesity with osteoporosis. J
Clin Endocrinol Metab. 92:1640–1646. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao LJ, Jiang H, Papasian CJ, Maulik D,
Drees B, Hamilton J and Deng HW: Correlation of obesity and
osteoporosis: Effect of fat mass on the determination of
osteoporosis. J Bone Miner Res. 23:17–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matsumoto T, Kano K, Kondo D, Fukuda N,
Iribe Y, Tanaka N, Matsubara Y, Sakuma T, Satomi A, Otaki M, et al:
Mature adipocyte-derived dedifferentiated fat cells exhibit
multilineage potential. J Cell Physiol. 215:210–222. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schäffler A and Büchler C: Concise review:
Adipose tissue-derived stromal cells-basic and clinical
implications for novel cell-based therapies. Stem cells.
25:818–827. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell
JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH: Multilineage
cells from human adipose tissue: Implications for cell-based
therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoshimura K, Shigeura T, Matsumoto D, Sato
T, Takaki Y, Aiba-Kojima E, Sato K, Inoue K, Nagase T, Koshima I
and Gonda K: Characterization of freshly isolated and cultured
cells derived from the fatty and fluid portions of liposuction
aspirates. J Cell Physiol. 208:64–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kazama T, Fujie M, Endo T and Kano K:
Mature adipocyte-derived dedifferentiated fat cells can
transdifferentiate into skeletal myocytes in vitro. Biochem Biophys
Res Commun. 377:780–785. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oki Y, Watanabe S, Endo T and Kano K:
Mature adipocyte-derived dedifferentiated fat cells can
trans-differentiate into osteoblasts in vitro and in vivo only by
all-trans retinoic acid. Cell Struct Funct. 33:211–222. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jumabay M, Matsumoto T, Yokoyama S, Kano
K, Kusumi Y, Masuko T, Mitsumata M, Saito S, Hirayama A, Mugishima
H and Fukuda N: Dedifferentiated fat cells convert to cardiomyocyte
phenotype and repair infarcted cardiac tissue in rats. J Mol Cell
Cardiol. 47:565–575. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sakuma T, Matsumoto T, Kano K, Fukuda N,
Obinata D, Yamaguchi K, Yoshida T, Takahashi S and Mugishima H:
Mature, adipocyte derived, dedifferentiated fat cells can
differentiate into smooth muscle-like cells and contribute to
bladder tissue regeneration. J Urol. 182:355–365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nakamura T, Shinohara Y, Momozaki S,
Yoshimoto T and Noguchi K: Co-stimulation with bone morphogenetic
protein-9 and FK506 induces remarkable osteoblastic differentiation
in rat dedifferentiated fat cells. Biochem Biophys Res Commun.
440:289–294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu HY, Wu AT, Tsai CY, Chou KR, Zeng R,
Wang MF, Chang WC, Hwang SM, Su CH and Deng WP: The balance between
adipogenesis and osteogenesis in bone regeneration by platelet-rich
plasma for age-related osteoporosis. Biomaterials. 32:6773–6780.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiong Z, Yan Y, Wang S, Zhang R and Zhang
C: Fabrication of porous scaffolds for bone tissue engineering via
low-temperature deposition. Scripta Materialia. 46:771–776. 2002.
View Article : Google Scholar
|