Introduction
Acute myelomonocytic leukemia, a clonal disorder of
hematopoiesis, is a genetically heterogeneous disease that is
characterized by the uncontrolled proliferation and accumulation of
immature and dysfunctional hematopoietic progenitors (1,2). One
quarter of adults diagnosed with acute myeloid leukemia (AML) are
known to have preceding hematologic disorders (3). Currently, cytotoxic chemotherapy is
widely used for the treatment of AML patients (4). It has been reported that post-remission
therapy is essential for AML patients with complete
hematologically-defined remission. (5). It has also been reported that increased
consumption of fruits and vegetables can decrease the risk of
cancer (6), cardiovascular disease
and atherosclerosis in humans (7).
Previous studies have reported that dietary agents
are able to safely modulate physiological function and enhance
anti-cancer activity (8–10). Numerous studies have demonstrated
that a diet rich in whole grains may decrease the risk of certain
chronic diseases, such as obesity (11), cardiovascular disease (12,13),
diabetes (14) and certain cancer
types (14,15). It has been reported that regions
where rice is a staple food have a low prevalence of certain
chronic diseases due to rice containing antioxidant compounds, such
as anthocyanins (16). It was
reported that Oryza sativa japonica variety Dongjin rice
(the parental plant of the resveratrol-enriched rice DJ-526), is
rich in fiber and polyphenols, and is reported to have an
anti-obesity effect (17). Numerous
reports have indicated that the consumption of refined grains is
associated with a higher risk of cancer (18,19).
However, previous results have also suggested that dietary
anthocyanins may reduce the risk of chronic diseases, including
cancers (20). Furthermore, black
rice anthocyanins have been reported to possess anti-metastasis
potential in human breast cancer cells in vivo and in
vitro (21). Anti-angiogenesis
and anti-cancer invasion activities of anthocyanins have also been
identified in vitro and in vivo (22,23). The
current authors recently demonstrated that anthocyanins from AUPGA
suppressed human oral cancer CAL 27 cell metastasis in vitro
(24).
The effects of anthocyanins on immune responses have
not previously been demonstrated. Thus, in the current study, the
effects of Asia University-selected purple glutinous indica rice
(AUPGA) on the immune responses in leukemia mice were investigated.
BALB/c mice were injected with WEHI-3 cells to generate leukemia
mice, which were then treated orally with AUPGA. The immune
responses were evaluated in vivo. AUPGA promoted immune
responses in leukemia BALB/c mice, including an increase in
macrophage phagocytosis and natural killer (NK) cell
activities.
Materials and methods
Materials and reagents
Crude extract of anthocyanins from the rice bran of
AUPGA was provided by Dr Hui-Yi Lin (China Medical University,
Taichung, Taiwan). The composition of this extract was analyzed and
cyanidin-3-O-glucoside (an anthocyanin) was the only component
found. AUPGA was dissolved in dimethyl sulfoxide at 1% stock
solution and kept at −20°C in a 50-ml tube covered with aluminum
paper to avoid contact with light. RPMI-1640 cell culture medium,
fetal bovine serum (FBS), L-glutamine, penicillin and streptomycin
were obtained from Gibco (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). All antibodies were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA).
Cell culture
Murine acute myelomonocytic leukemia WEHI-3 cell
line was obtained from the Food Industry Research and Development
Institute (Hsinchu, Taiwan). Cells were cultured in
75-cm3 flasks in RPMI-1640 medium supplemented with 10%
FBS, 100 units/ml penicillin, 100 µg/ml streptomycin and 2 mM
L-glutamine, and maintained at 37°C in a humidified atmosphere of
5% CO2 (25,26). Cells were allowed to equilibrate for
24 h before being used.
Male BALB/c mice
A total of 50 eight-week-old male BALB/c mice,
weighing 22–25 g each, were purchased from the National Laboratory
Animal Center (Taipei, Taiwan) and housed in stainless steel,
mesh-bottomed cages. Mice were kept under specified pathogen-free
conditions in the animal center of China Medical University
(Taichung, Taiwan). All experiments were carried out in accordance
with the Institutional Guidelines for Animal Welfare of China
Medical University and were approved by the Institutional Animal
Care and Use Committee of China Medical University, as previously
described (25,26).
Treatment of animals with AUPGA
BALB/c mice were randomly divided into five groups
(n=10 per group). Groups II–V were intraperitoneally injected with
106 WEHI-3 leukemia cells. Group I mice received a
normal diet ad libitum as the control. Group II mice
received a normal diet ad libitum with 100 µl olive oil by
oral gavage. Group III mice received a normal diet ad
libitum and AUPGA (20 mg/kg with 100 µl olive oil by oral
gavage). Group IV mice received a normal diet ad libitum and
AUPGA (50 mg/kg with 100 µl olive oil by oral gavage). Group V mice
received a normal diet ad libitum and AUPGA (100 mg/kg with
100 µl olive oil by oral gavage). AUPGA in olive oil was
administered by oral gavage once every three days for 21 days and
all mice were weighed throughout the oral treatment. At the end of
treatment, all mice were weighed and sacrificed by euthanasia with
CO2, as previously described (25,26).
Immunofluorescence staining for cell
surface markers
The blood and spleen of each mouse was collected.
Splenocytes were isolated from the spleen in order to evaluate NK
cell activity, as previously described (25,26). To
analyze blood samples (0.2 ml each) for cell markers, red blood
cells were lysed using 1X Pharm Lyse™ lysing buffer (BD
Pharmingen, San Diego, CA, USA), according to the manufacturer's
instructions. After centrifugation at 1,500 × g for 15 min at 4°C,
white blood cells were collected and stained with phycoerythrin
(PE)-labeled anti-mouse CD3 (BD553062, BD Biosciences, San Jose,
CA, USA), PE-labeled anti-mouse CD19 (BD553786, BD Biosciences),
fluroscein isothiocyanate (FITC)-labeled anti-mouse CD11b
(BD553310, BD Biosciences) and FITC-labeled anti-mouse Mac-3
(BD553324, BD Biosciences) antibodies (the dilution of each
antibody was 1:12) for 30 min at 4°C. The cells were washed with
PBS and analyzed for cell marker population by flow cytometry, as
previously described (25,26).
Flow cytometric assay for macrophage
phagocytosis
Macrophages were obtained from the peripheral blood
mononuclear cells (PBMCs) and peritoneum of each mouse, as
previously described (21,23). Isolated macrophages were maintained
in the FACS tube (Falcon 352052, BD Biosciences; 100 µl PBMCs/tube,
1 ml peritoneal fluid/tube) and 10 µl Escherichia coli-FITC was
added to each well, based on the PHAGOTEST® kit
manufacturer instructions (BD Biosciences). All samples were
analyzed for phagocytosis by flow cytometry and quantified using
CellQuest software (version 5.2.1; BD Biosciences), as previously
described (25,26).
Flow cytometric assay for NK cell
cytotoxic activity
Splenocytes (2.5–5×105 cells/well) from
each mouse were maintained on a 96-well plate containing 100 µl
RPMI-1640 medium in each well. YAC-1 target cells (1×104
cells) in serum-free RPMI-1640 medium and PKH-67/Dil.C buffer
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) were added to each
well, based on the manufacturer's instructions, to form
target:effector ratios of 25:1 and 50:1 (splenocytes:YAC-1 target
cells). All samples were mixed well for 2 min, then 2 ml PBS was
added to each well for 1 min. Next, 4 ml medium was added to each
well and the cells were incubated for 10 min. All steps were
performed at 25°C. The cells were centrifuged at 25°C at 290 × g
for 2 min. NK cell cytotoxic activity was assayed by flow
cytometry, as previously described (25–27).
Assay for T and B cell
proliferation
Splenocytes (105 cells/well) were
maintained in a 96-well plate with 100 µl RPMI-1640 medium in each
well. For B cell proliferation analysis, 1 µg/ml lipopolysaccharide
(LPS) was added to the well to stimulate cells for three days. For
T cell proliferation analysis, 1 µg/ml concanavalin A was added to
the well to stimulate cells for five days. At the end of
incubation, each sample was assayed for T or B cell proliferation
using a CellTiter 96 Aqueous One Solution Cell Proliferation Assay
kit (Promega Corporation, Madison, WI, USA), as previously
described (25,26).
Statistical analysis
Data are presented as the mean ± standard deviation.
Differences between the control and AUPGA-treated groups were
analyzed using the Student's t-test and SigmaPlot software version
10.0 (Systat Software, Inc., London, UK). P<0.05 was considered
to indicate a statistically significant difference.
Results
Effects of AUPGA treatment on the
body, liver and spleen weights of leukemia BALB/c mice
Groups II–V were treated with WEHI-3 to generate
leukemia and Group I was a normal control. Group II was a WEHI-3
control without AUPGA treatment, while Groups III, IV and V were
treated with AUPGA (20, 50 and 100 mg/kg, respectively) for 22
days. During the experimental period, all mice were weighed every
three days. Representative images of the animals, livers and
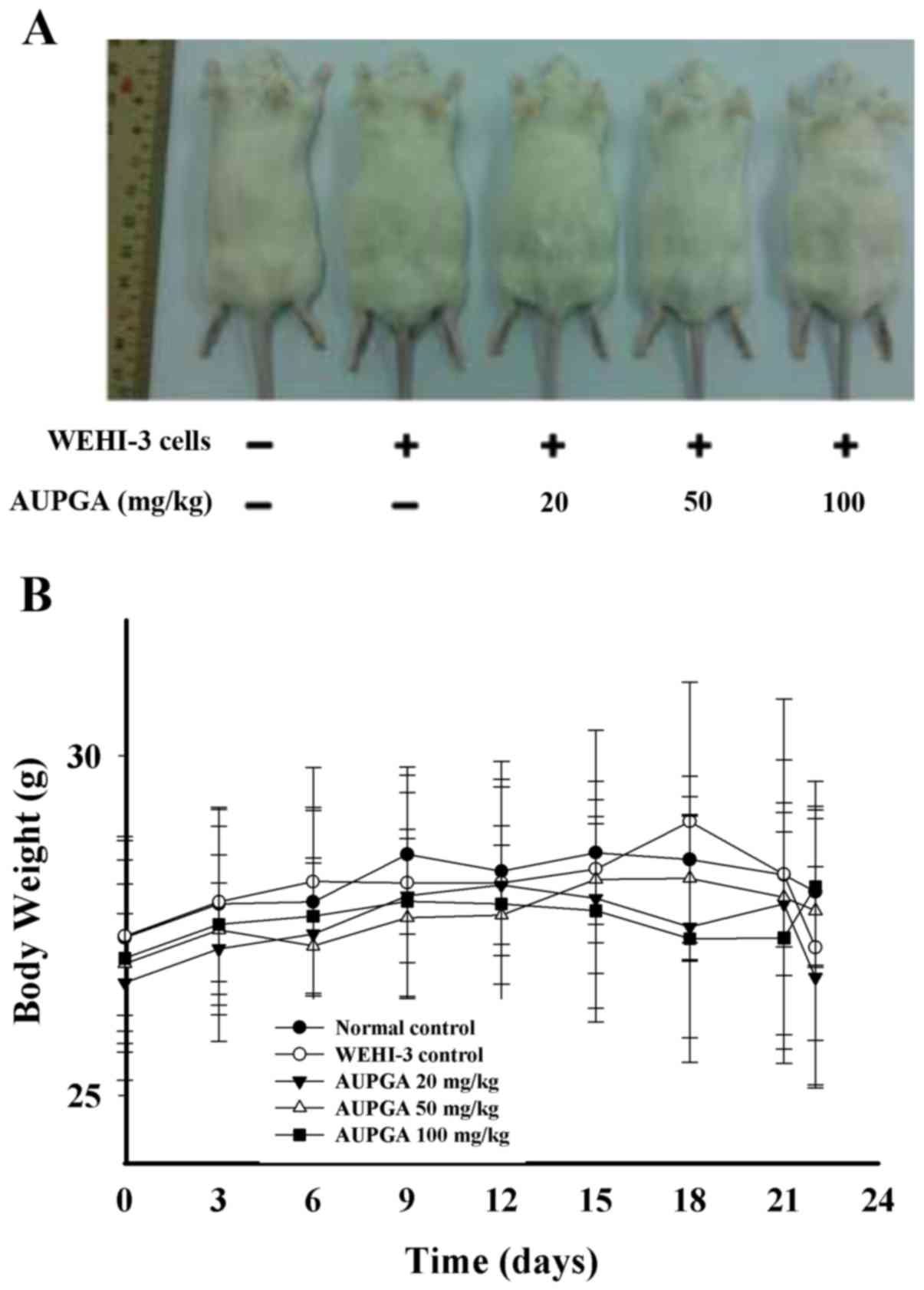
spleens, as well as the weights of each, are presented in Fig. 1A-F. These results demonstrated that
AUPGA did not affect the appearance of the mice (Fig. 1A) or cause any significant change in
body weight (Fig. 1B) in the mice of
each group. However, the liver weight was significantly decreased
(P<0.05; Fig. 1C and D) and the
spleen weight was significantly increased (P<0.05; Fig. 1E and F) as compared with the
AUPGA-untreated leukemia mice.
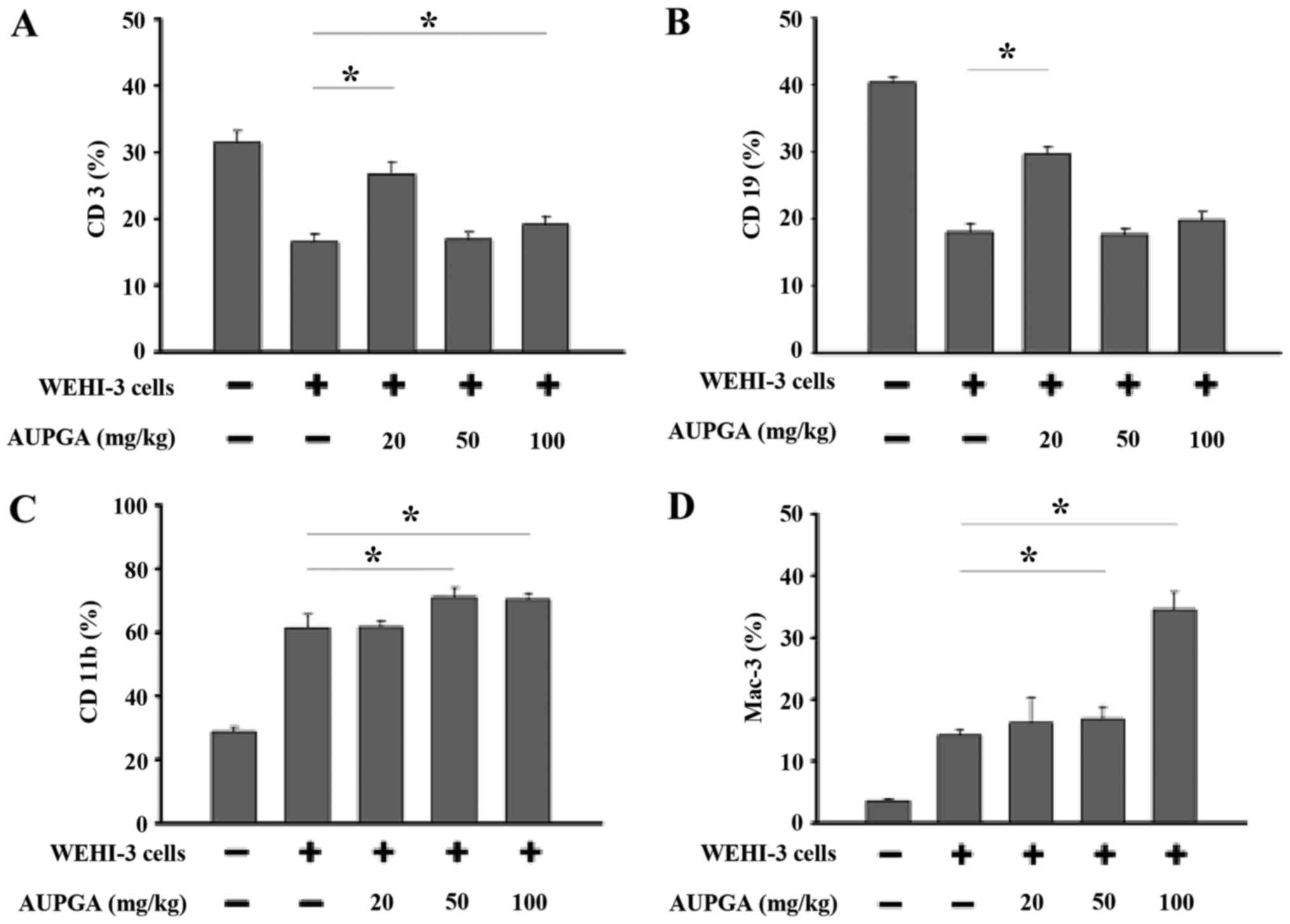
Effects of AUPGA treatment on cell
marker levels in leukemia BALB/c mice
White blood cells were isolated from each group, and
the levels of cell markers CD3, CD19, CD11b and Mac-3 were measured
by flow cytometry. As shown in Fig.
2A, AUPGA treatment at 20 or 100 mg/kg significantly increased
the T cell (CD3) population compared with AUPGA-untreated leukemia
mice (P<0.05). AUPGA treatment at 20 mg/kg also led to a
significant increase in the B cell (CD19) population as compared
with AUPGA-untreated leukemia mice (P<0.05; Fig. 2B). AUPGA treatment at 50 or 100 mg/kg
led to a significant increase in both the monocyte (CD11b)
population (P<0.05; Fig. 2C) and
the levels of macrophage (Mac-3) (P<0.05; Fig. 2D).
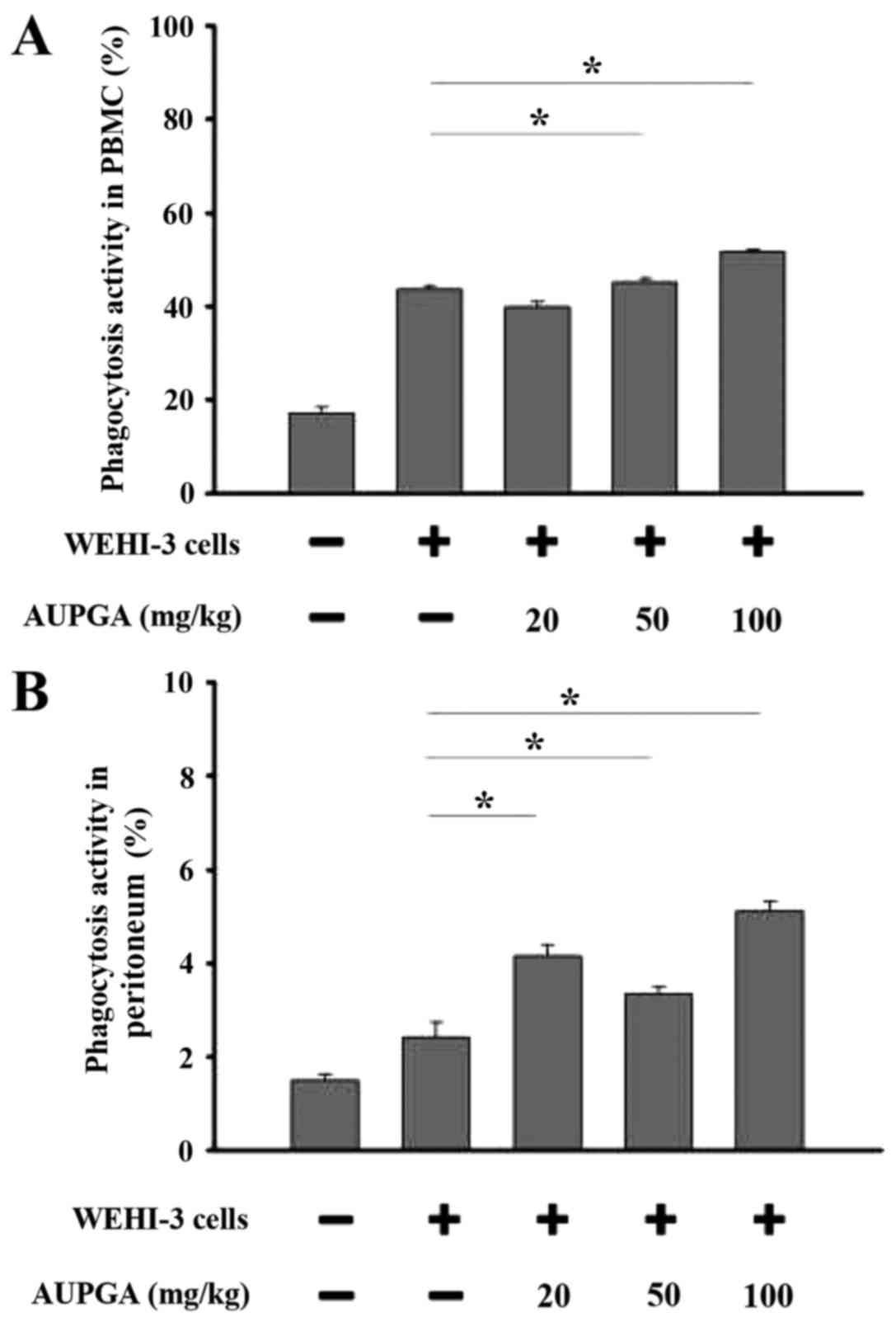
Effect of AUPGA treatment on
macrophage phagocytosis in the PBMCs and peritoneum of leukemia
BALB/c mice
Cells were collected from the PBMCs and peritoneum
of each group. The percentage of macrophage phagocytosis was
analyzed by flow cytometry. As shown in Fig. 3A, AUPGA treatment at 50 and 100 mg/kg
significantly promoted macrophage phagocytosis in the PBMCs as
compared with AUPGA-untreated leukemia mice (P<0.05). All doses
of AUPGA treatment significantly promoted macrophage phagocytic
activity in the peritoneum as compared with AUPGA-untreated
leukemia mice (P<0.05; Fig.
3B).
Effect of AUPGA treatment on the
cytotoxic activity of NK cells from leukemia BALB/c mice
YAC-1 targets cells were added to splenocytes from
mice in each group. NK cell activity was then evaluated using flow
cytometry. As shown in Fig. 4, all
doses of AUPGA treatment significantly decreased NK cell cytotoxic
activity as compared with AUPGA-untreated leukemia mice
(P<0.05).
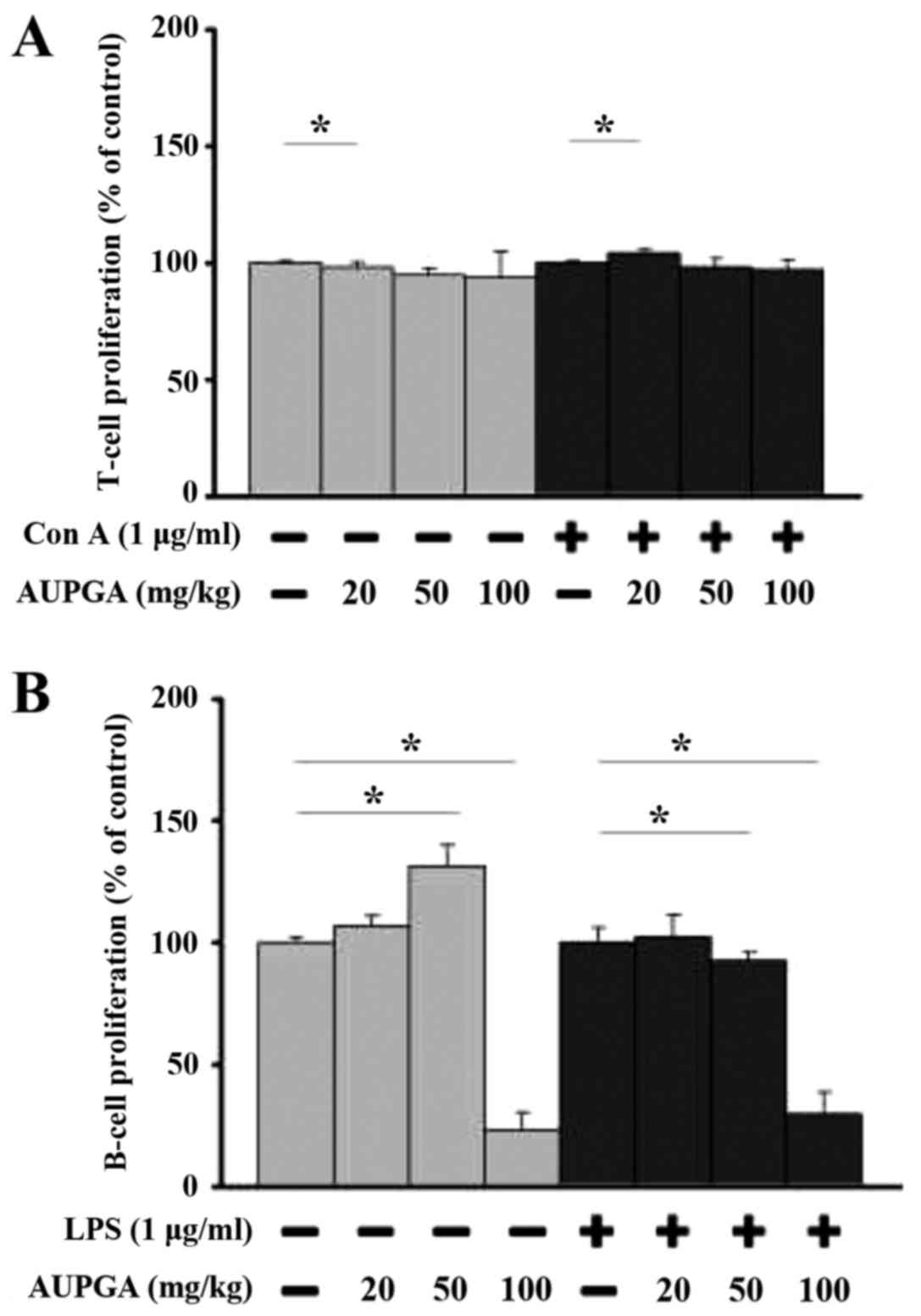
Effect of AUPGA treatment on T and B
cell proliferation from leukemia BALB/c mice
Splenocytes from each group were used for T and B
cell proliferation assays. AUPGA treatment at 20 mg/kg
significantly promoted T cell proliferation with or without
concanavalin A pretreatment (P<0.05; Fig. 5A), and AUPGA treatment at 50 and 100
mg/kg significantly decreased B cell proliferation with or without
LPS pretreatment (P<0.05; Fig.
5B) as compared with AUPGA-untreated leukemia mice.
Discussion
Plant-derived bioactive compounds, such as
paclitaxel from Taxus brevifolia and camptothectin from
Camptotheca acuminate, are commonly used in the clinical
treatment of cancer (28,29). In 2006, the Food and Drug
Administration in the USA approved the marketing of
Veregen® (the first botanical product to be approved as
a drug) for treatment of external genital and perianal warts
(30). However, to date, there have
been few studies regarding the biological activities of AUPGA in
animal models. In particular, the effects of AUPGA on immune
responses in vivo have not been elucidated. Thus, the
current study aimed to investigate how AUPGA treatment affected
immune responses in leukemia BALB/c mice in vivo.
Normal BALB/c mice were intraperitoneally injected
with WEHI-3 cells to induce leukemia. Mice were then divided into
groups and were orally treated with or without AUPGA once every
three days for 22 days. It was found that AUPGA had no significant
effect on body weight in the leukemia mice but decreased the liver
weight and increased the spleen weight as compared with the
AUPGA-untreated leukemia mice. It was also found that AUPGA
promoted the T cell (CD3) population at 20 and 100 mg/kg
treatments, but only 20 mg/kg of AUPGA treatment led to an increase
in the B cell (CD19) population. AUPGA treatment at 50 or 100 mg/kg
led to an increase in the monocyte (CD11b) population and the
levels of macrophage (Mac-3). It has previously been reported that
it is possible for macrophages to kill and eliminate cancer cells
(31–33). Thus, it was proposed by the current
authors that the activation of macrophages by AUPGA may reduce
cancer risk.
Macrophages serve a key function in the response to
antigens in the animal body and are able to destroy target cells
in vivo (24). The current
study demonstrated that AUPGA treatment at 50 or 100 mg/kg
significantly promoted macrophage phagocytosis in samples from the
PBMCs, and all tested doses of AUPGA treatment significantly
promoted macrophage phagocytic activity in samples from the
peritoneum. All tested doses of AUPGA treatment significantly
decreased NK cell activity. However, this observed alteration in NK
cell function is also a decreased immune response. Thus, AUPGA may
have a cell specificity that requires further investigation in the
future. Furthermore, AUPGA treatment at 20 mg/kg significantly
promoted T cell proliferation and treatment at 50 or 100 mg/ml
significantly decreased B cell proliferation, which was stimulated
by LPS. Oral intake of LPS has been reported to be effective in
preventing certain diseases (34).
Further investigations are required in this area, including in
vivo studies for WEHI-3 cell xenograft mice treated with AUPGA
by oral gavage.
In conclusion, the present study indicated that the
crude extract of anthocyanins from the rice bran of AUPGA did not
alter the body weight of leukemia BALB/c mice in vivo, but
did promote CD3 (T cell), CD19 (B cell), CD11b (monocyte) and Mac-3
(macrophage) populations in leukemia mice. Furthermore, AUPGA
treatment promoted macrophage phagocytosis and decreased NK cell
activity in leukemia BALB/c mice in vivo.
Acknowledgements
This study was supported by a grant from China
Medical University, Taichung, Taiwan (grant no.
CMU103-ASIA-01).
References
|
1
|
Cancer Genome Atlas Research Network, .
Genomic and epigenomic landscapes of adult de novo acute myeloid
leukemia. N Engl J Med. 368:2059–2074. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thiede C: Mutant DNMT3A: Teaming up to
transform. Blood. 119:5615–5617. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Juliusson G, Antunovic P, Derolf A,
Lehmann S, Möllgård L, Stockelberg D, Tidefelt U, Wahlin A and
Höglund M: Age and acute myeloid leukemia: Real world data on
decision to treat and outcomes from the Swedish Acute Leukemia
Registry. Blood. 113:4179–4187. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Du Y, Lu F, Li P, Ye J, Ji M, Ma D and Ji
C: SMG1 acts as a novel potential tumor suppressor with epigenetic
inactivation in acute myeloid leukemia. Int J Mol Sci.
15:17065–17076. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gundermann S, Klinker E, Kimmel B, Flierl
U, Wilhelm M, Einsele H and Kunzmann V: A comprehensive analysis of
primary acute myeloid leukemia identifies biomarkers predicting
susceptibility to human allogeneic Vγ9Vδ2 T T cells. J Immunother.
37:321–330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soerjomataram I, Oomen D, Lemmens V,
Oenema A, Benetou V, Trichopoulou A, Coebergh JW, Barendregt J and
de Vries E: Increased consumption of fruit and vegetables and
future cancer incidence in selected European countries. Eur J
Cancer. 46:2563–2580. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pratheeshkumar P, Sreekala C, Zhang Z,
Budhraja A, Ding S, Son YO, Wang X, Hitron A, Hyun-Jung K, Wang L,
et al: Cancer prevention with promising natural products:
Mechanisms of action and molecular targets. Anticancer Agents Med
Chem. 12:1159–1184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hatcher H, Planalp R, Cho J, Torti FM and
Torti SV: Curcumin: From ancient medicine to current clinical
trials. Cell Mol Life Sci. 65:1631–1652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu BL, Zhang X, Zhang W and Zhen HN: New
enlightenment of French Paradox: Resveratrol's potential for cancer
chemoprevention and anti-cancer therapy. Cancer Biol Ther.
6:1833–1836. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Salvioli S, Sikora E, Cooper EL and
Franceschi C: Curcumin in cell death processes: A challenge for CAM
of age-related pathologies. Evid Based Complement Alternat Med.
4:181–190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Misra A, Rastogi K and Joshi SR: Whole
grains and health: Perspective for Asian Indians. J Assoc
Physicians India. 57:155–162. 2009.PubMed/NCBI
|
|
12
|
Flint AJ, Hu FB, Glynn RJ, Jensen MK,
Franz M, Sampson L and Rimm EB: Whole grains and incident
hypertension in men. Am J Clin Nutr. 90:493–498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu FB: Diet and lifestyle influences on
risk of coronary heart disease. Curr Atheroscler Rep. 11:257–263.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maki KC, Beiseigel JM, Jonnalagadda SS,
Gugger CK, Reeves MS, Farmer MV, Kaden VN and Rains TM: Whole-grain
ready-to-eat oat cereal, as part of a dietary program for weight
loss, reduces low-density lipoprotein cholesterol in adults with
overweight and obesity more than a dietary program including
low-fiber control foods. J Am Diet Assoc. 110:205–214. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haas P, Machado MJ, Anton AA, Silva AS and
de Francisco A: Effectiveness of whole grain consumption in the
prevention of colorectal cancer: Meta-analysis of cohort studies.
Int J Food Sci Nutr. 6:1–13. 2009. View Article : Google Scholar
|
|
16
|
Goufo P and Trindade H: Rice antioxidants:
Phenolic acids, flavonoids, anthocyanins, proanthocyanidins,
tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci
Nutr. 2:75–104. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park SY, Lee SM, Yeo Y, Kweon SJ, Cho HS
and Kim JK: Comparison of the nutritional compositions of
insect-resistant and glufosinate-tolerant rice and conventional
rice. J Appl Biol Chem. 56:5–9. 2013. View Article : Google Scholar
|
|
18
|
Chan JM, Wang F and Holly EA: Whole grains
and risk of pancreatic cancer in a large population-based
case-control study in the San Francisco Bay area, California. Am J
Epidemiol. 166:1174–1185. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garavello W, Lucenteforte E, Bosetti C and
La Vecchia C: The role of foods and nutrients on oral and
pharyngeal cancer risk. Minerva Stomatol. 58:25–34. 2009.PubMed/NCBI
|
|
20
|
Okarter N and Liu RH: Health benefits of
whole grain phytochemicals. Crit Rev Food Sci Nutr. 50:193–208.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luo LP, Han B, Yu XP, Chen XY, Zhou J,
Chen W, Zhu YF, Peng XL, Zou Q and Li SY: Anti-metastasis activity
of black rice anthocyanins against breast cancer: Analyses using an
ErbB2 positive breast cancer cell line and tumoral xenograft model.
Asian Pac J Cancer Prev. 15:6219–6225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Favot L, Martin S, Keravis T,
Andriantsitohaina R and Lugnier C: Involvement of cyclin-dependent
pathway in the inhibitory effect of delphinidin on angiogenesis.
Cardiovasc Res. 59:479–487. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Syed DN, Afaq F, Sarfaraz S, Khan N,
Kedlaya R, Setaluri V and Mukhtar H: Delphinidin inhibits cell
proliferation and invasion via modulation of Met receptor
phosphorylation. Toxicol Appl Pharmacol. 231:52–60. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fan MJ, Wang IC, Hsiao YT, Lin HY, Tang
NY, Hung TC, Quan C, Lien JC and Chung JG: Anthocyanins from black
rice (Oryza sativa L.) demonstrate antimetastatic properties by
reducing MMPs and NF-κB expressions in human oral cancer CAL 27
cells. Nutr Cancer. 67:327–338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chueh FS, Lin JJ, Lin JP, Yu FS, Lin JH,
Ma YS, Huang YP, Lien JC and Chung JG: Crude extract of Polygonum
cuspidatum promotes immune responses in leukemic mice through
enhancing phagocytosis of macrophage and natural killer cell
activities in vivo. In Vivo. 29:255–261. 2015.PubMed/NCBI
|
|
26
|
Yu FS, Yang JS, Yu CS, Chiang JH, Lu CC,
Chung HK, Yu CC, Wu CC, Ho HC and Chung JG: Safrole suppresses
murine myelomonocytic leukemia WEHI-3 cells in vivo, and stimulates
macrophage phagocytosis and natural killer cell cytotoxicity in
leukemic mice. Environ Toxicol. 28:601–608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu HF, Tung WL, Yang JS, Huang FM, Lee CS,
Huang YP, Liao WY, Chen YL and Chung JG: In vitro suppression of
growth of murine WEHI-3 leukemia cells and in vivo promotion of
phagocytosis in a leukemia mice model by indole-3-carbinol. J Agric
Food Chem. 60:7634–7643. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oberlies NH and Kroll DJ: Camptothecin and
taxol: Historic achievements in natural products research. J Nat
Prod. 67:129–135. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wall ME: Camptothecin and taxol: Discovery
to clinic. Med Res Rev. 18:299–314. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meltzer SM, Monk BJ and Tewari KS: Green
tea catechins for treatment of external genital warts. Am J Obstet
Gynecol. 200:233.e1–7. 2009. View Article : Google Scholar
|
|
31
|
Inui T, Kubo K, Kuchiike D, Uto Y,
Nishikata T, Sakamoto N and Mette M: Oral colostrum
macrophage-activating factor for serious infection and chronic
fatigue syndrome: Three case reports. Anticancer Res. 35:4545–4549.
2015.PubMed/NCBI
|
|
32
|
Inui T, Kuchiike D, Kubo K, Mette M, Uto
Y, Hori H and Sakamoto N: Clinical experience of integrative cancer
immunotherapy with GcMAF. Anticancer Res. 33:2917–2919.
2013.PubMed/NCBI
|
|
33
|
Mills CD and Ley K: M1 and M2 macrophages:
The chicken and the egg of immunity. J Innate Immun. 6:716–726.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Inagawa H, Kohchi C and Soma G: Usefulness
of oral administration of lipopolysaccharide for disease prevention
through the induction of priming in macrophages. Anticancer Res.
34:4497–4501. 2014.PubMed/NCBI
|