Introduction
Articular cartilage injury is increasing in
incidence year by year, which is an important healthcare problem.
Cell-based tissue engineering holds promise for restoring cartilage
defects (1). To date, the most
widely used cell sources in cartilage regeneration are mesenchymal
stem cells (MSCs) and mature chondrocytes. Notably, MSCs have
already been used to repair cartilage defects in clinical trials
(2,3). MSCs are easily obtained from various
kinds of tissues, such as bone marrow, synovial tissue and muscle,
and they would not be rejected by the immune system when used in
vivo (4–6). However, the limited proliferation and
differentiation potential has restrained the use of MSCs in
regenerative medicine (7). In
addition, the proliferative capability and differentiation
potential of MSCs has been reported to decline with age (8).
Induced pluripotent stem cells (iPSCs) can be
generated from well-differentiated somatic cells by introducing
defined reprogramming transcription factors using retroviruses
(9). iPSCs possess pluripotency,
proliferation ability and multi-lineage differentiation potential
similar to embryonic stem cells (ESCs) (9–11). In
addition, a variety of new methods have been developed to generate
iPSCs for the purpose of reducing the risk of tumor formation
(12,13). Therefore, iPSCs are regarded as
alternative cell sources in regenerative medicine.
Undifferentiated iPSCs will form teratoma in
vivo (14), which is the main
obstacle to the use of iPSCs for tissue regeneration. The
differentiation of iPSCs into MSCs has the promise to solve this
problem. ESC markers (for example, Nanog and Sox2) were reported to
no longer appear in iPSC-derived mesenchymal stem cells
(iPSCs-MSCs), which may reduce the risk of tumorigenicity when used
in vivo (15,16). Studies have also showed that it is
possible to induce the differentiation of iPSCs-MSCs into
osteogenic, chondrogenic and vascular lineages in vitro
(15–19). However, few studies have used iPSCs
or iPSCs-MSCs to repair cartilage defects in vivo.
In the present study, mesenchymal progenitor cells
were obtained from human iPSCs (hiPSCs) via embryoid body (EB)
formation, a step that mimics embryonic development. The in
vivo ability of hiPSCs-MSCs to repair cartilage defects was
examined using a full-thickness cartilage defect rabbit model.
Materials and methods
hiPSC culture
The hiPSC line (no. 0209-001; Sidan Sai
Biotechnology Co., Ltd., Shanghai, China) was generated previously
by introducing six reprogramming factors (Oct3/4, Sox2, Klf4,
c-Myc, Nanog and Lin 28) into human newborn foreskin fibroblasts
(20). The undifferentiated hiPSCs
were maintained and expanded according to previous reported methods
(20). In brief, chemically
inactivated murine embryonic fibroblasts (MEFs) were used as feeder
cells and were seeded on Matrigel-coated (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) dishes. hiPSCs were cultured on MEF
feeder layers in ES medium (Sidan Sai Biotechnology Co., Ltd.)
supplemented with 4 ng/ml basic fibroblast growth factor (bFGF)
(Peprotech, Inc., Rocky Hill, NJ, USA). The medium was refreshed
every day. Type IV collagenase (Sigma-Aldrich; Merck Millipore) was
used to perform cell passage.
hiPSCs-MSCs preparation
Undifferentiated hiPSCs were detached from culture
dishes using 1 mg/ml type IV collagenase and were then plated onto
low-attachment culture dishes at a density of 1,000–1,200 cell
clusters per 100 mm dish. The cells were allowed to aggregate and
form spheres in a humidified atmosphere at 37°C and 5%
CO2 (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
in a maintenance medium containing Dulbecco's modified Eagle's
medium (DMEM)/F12 and 10% fetal bovine serum (FBS; Invitrogen;
Thermo Fisher Scientific, Inc.). EBs formed after 7 days'
suspension culture and were transferred to gelatin-coated
(Sigma-Aldrich; Merck Millipore) dishes at 800–1,000 EBs/100 mm
dish in expansion medium with DMEM/F12, 10% FBS, 100 U/ml
penicillin, and 100 mg/m2 streptomycin (all from Invitrogen; Thermo
Fisher Scientific, Inc.). The cells sprouted from EBs were
harvested as hiPSC-MSCs and expanded in expansion medium. The
hiPSC-MSCs were purified by removing non-adherent cells.
Flow cytometry
The hiPSCs-MSCs at passage 3 were harvested. One
million cells were suspended in 100 µl buffer that consisted of
0.5% bovine serum albumin (BSA; Sigma-Aldrich; Merck Millipore) and
2 mM EDTA (Sunshine Biotechnology Co., Ltd., Nanjing, China).
Subsequently, 10 µl 1:10 diluted fluorescein isothiocyanate
(FITC)-coupled antibodies recognizing CD11b (130-098-778), CD105
(130-098-778), CD90 (130-097-930), CD45 (130-098-043) and CD34
(130-098-142) (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany)
were added. In addition, 1:10 diluted mouse IgG1 (130-104-562) and
mouse IgG2a (no. 130-098-877) antibodies (MACS; Miltenyi Biotec)
were used as isotype controls. Incubation for 10 min incubation in
the dark at 4°C was performed. The cells were then washed with
buffer containing phosphate-buffered saline, pH 7.2, 0.5% BSA, and
2 mM EDTA by diluting MACS BSA Stock Solution (130-091-376) 1:20
with autoMACS Rinsing Solution (130-091-222) (MACS; Miltenyi
Biotec). Then, the cells were centrifuged at 300 × g for 10 min at
4°C and resuspended in 500 µl of the aforementioned buffer for
analysis by flow cytometry (BD FACSCalibur, BD Biosciences,
Franklin Lakes, NJ, USA). The data was analyzed using Flowjo 7.6
sofrware (BD Biosciences).
Animal model and transplantation
procedure
A total of 36 skeletally mature female New Zealand
white rabbits (age, 12 weeks; weight, 2.0–2.5 kg), purchased from
the Jinling Farm, Nanjing, China were used in this study. Rabbits
were fed a regular diet twice a day and allowed free access to
water. They were housed under controlled conditions (temperature,
25±3°C; humidity, 45±5%; 12-h light/dark cycle). All surgical
procedures were approved by the Institutional Rabbit Care and Use
Committee of Drum Tower Hospital, Medical School of Nanjing
University (Nanjing, China). A full-thickness cartilage defect
model was made in the trochlear grooves of the rabbits as
previously reported (21). In brief,
the rabbits were anesthetized with an intramuscular injection of 20
mg/ml xylazine hydrochloride (Huamu Animal Health Care Co., Ltd.,
Jilin, China) at a dose of 3 ml/kg. The knee articular surface of
the rabbits was exposed through a medial parapatellar approach.
Whether the right or the left knee was used to perform the surgery
was determined randomly. An osteochondral transplantation system
(3.5 mm in diameter, 3.0 mm in depth) was used to create
osteochondral defects. The rabbits were then divided into three
groups according to implantation: Control group, scaffold
implantation group and scaffold/hiPSCs-MSCs (experimental) group
(n=12 per group).
The poly(lactic-co-glycolide) (PLGA) scaffold was
purchased from Shandong Institute of Medical Instruments (Jinan,
China). The average pore diameter of the PLGA scaffold was ~200 µm.
The PLGA scaffold was cut to 3.5×3.5×3 mm dimensions with a razor
blade. The prepared PLGA scaffolds were immersed in Matrigel for 24
h to enhance cell attachment. Then, 5×106 hiPSCs-MSCs
were seeded onto the prepared scaffold. After incubating in
complete medium for 12 h, the PLGA/hiPSCs-MSCs complex was
transplanted into the cartilage defect in the experimental group.
The scaffold implantation group received only PLGA scaffold, and
the control group was untreated. Six rabbits from each group were
sacrificed at 3 and 6 weeks after surgery. The repair quality was
evaluated by gross and histological examination.
Histological analysis
The specimens were cut into 5-µm sections and
stained with hematoxylin and eosin (H&E) and toluidine blue as
previous reported (21). In brief,
the specimens were fixed, decalcified, dehydrated and embedded in
paraffin. The specimens were then cut into 5-µm sections and
stained with H&E (Beyotime Institute of Biotechnology,
Shanghai, China) and toluidine blue (Toyond Biotechnology Co.,
Ltd., Shanghai, China) staining according to the manufacturers'
instructions. The results were assessed independently by 3
different investigators.
Results
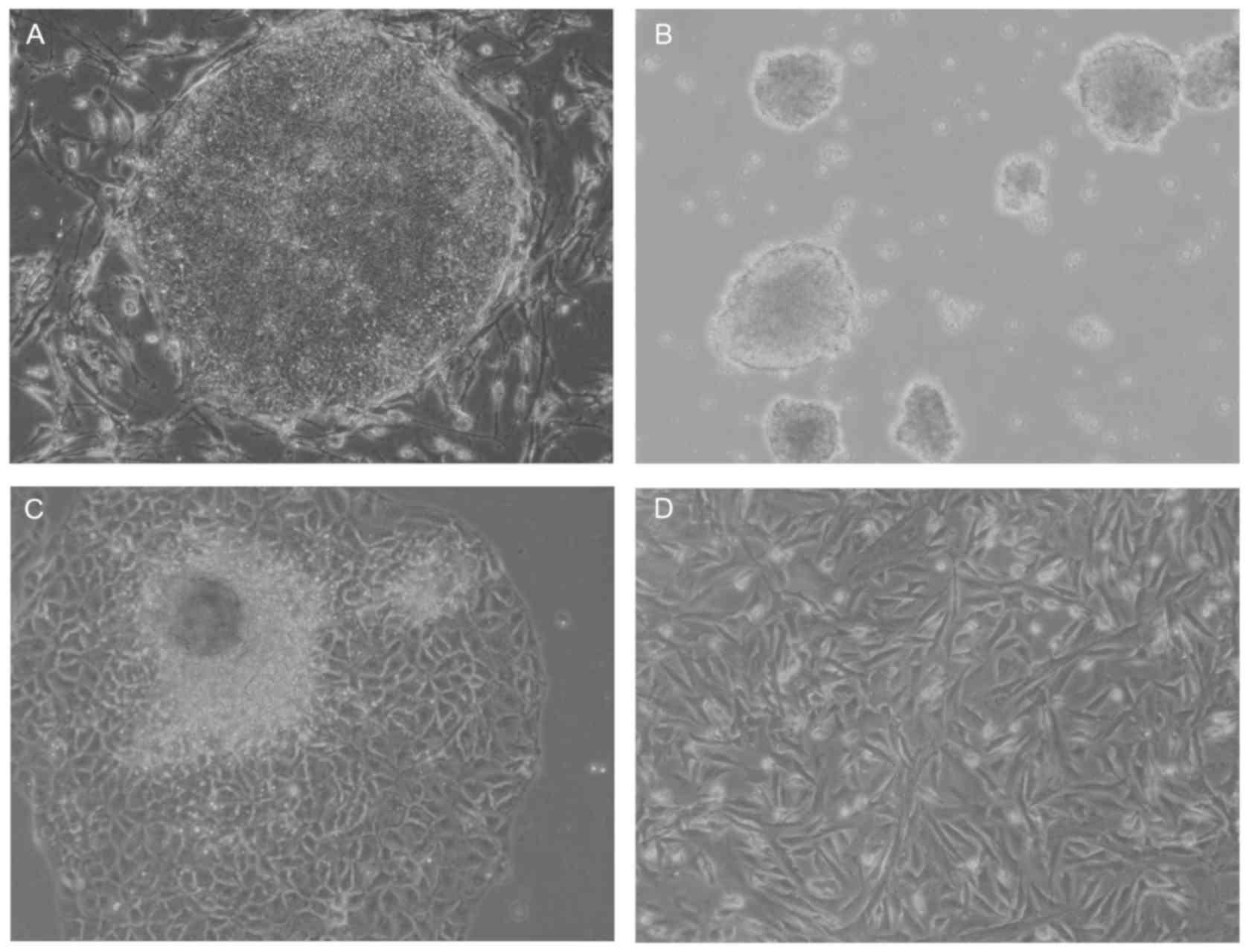
Generation of hiPSCs-MSCs
A multistep culture method consisting of spontaneous
differentiation via a step of EB formation, cell outgrowth from
EBs, and monolayer culture following cell dissociation was used in
the present study to generate hiPSC-MSCs (Fig. 1). hiPSC-MSCs originated from the
mesoderm and neural crest of the EBs and exhibited a spindle-like
shape (Fig. 1D). Flow cytometric
analysis was used to analyze the mesenchymal properties of the
hiPSC-MSCs obtained in this study. The results showed that the
majority of cells expressed CD90, and some expressed CD105, but
most cells did not express CD34, CD11b or CD45 (Fig. 2).
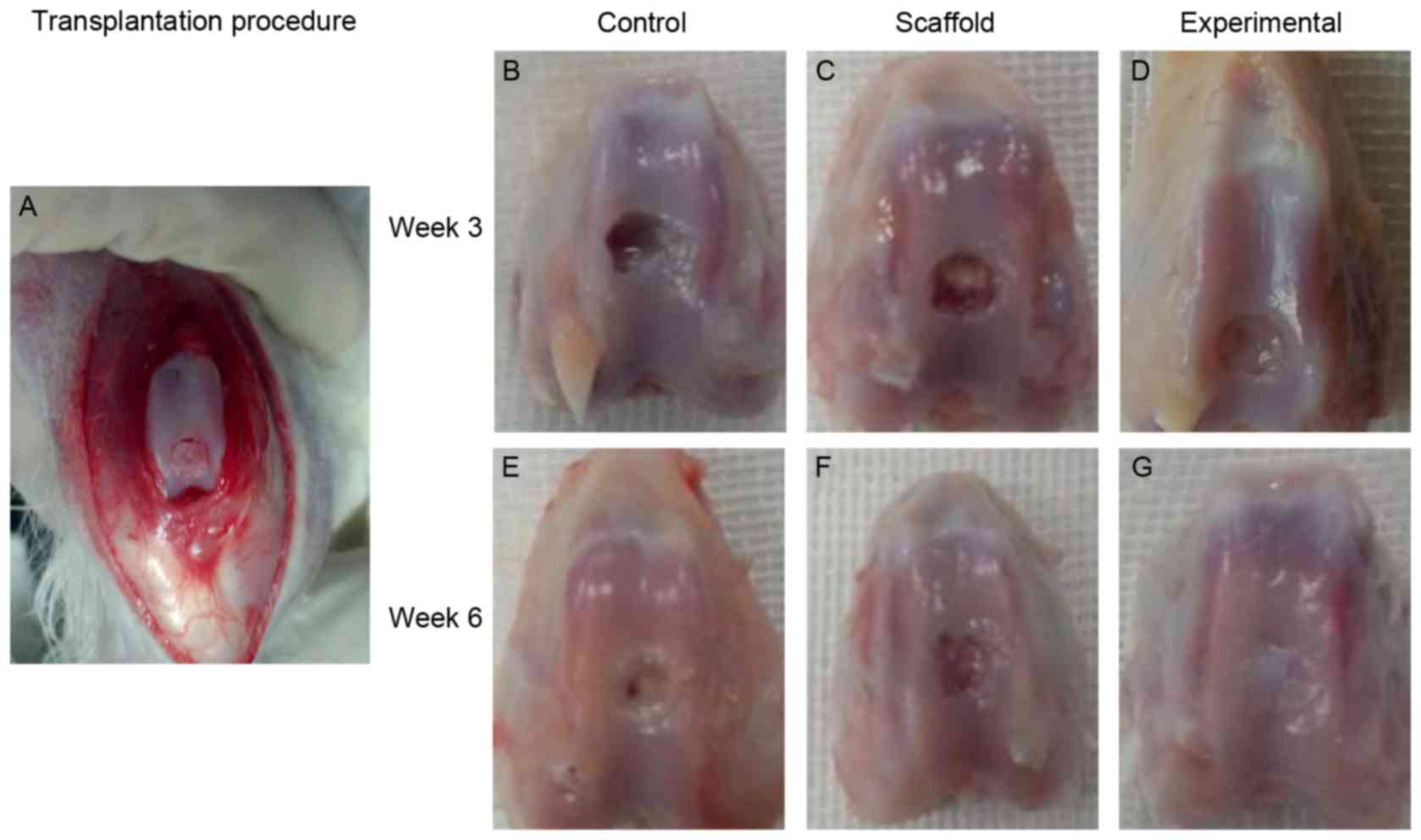
Macroscopic evaluation of repair
quality
Following transplantation (Fig. 3A), in the control and scaffold
implantation groups, little repair tissue was observed in the
cartilage defect 3 weeks after surgery (Fig. 3B and C). However, in the experimental
group, repair tissue covering >50% of the defects was observed
(Fig. 3D). At 6 weeks, the cartilage
defect was only partially covered by fibrous tissue in the control
and scaffold implantation groups (Fig.
3E and F). At 6 weeks, repair tissue almost 100% filled the
cartilage defect in the experimental group (Fig. 3G).
Histological evaluation of the repair
quality
H&E staining showed better repair quality in the
experimental group compared with that in the other two groups
(Fig. 4). Only fibrous tissue was
observed in defects of the control and scaffold implantation groups
at 3 and 6 weeks. In the experimental group, H&E staining
showed cartilage-like tissue in the top layer of the defect at 6
weeks. However, subchondral bone formation was poor in all the
groups. The newly formed tissue was stained slightly in the control
and scaffold implantation groups at 6 weeks by toluidine blue
staining (Fig. 5A-D). The matrix of
regenerated tissue in the top layer of the defect in the
experimental group was stained intensely. However, native cartilage
degeneration was also observed (Fig. 5E
and F).
 | Figure 5.Representative toluidine blue staining
of the sections at 6 weeks. The cartilage defect was poorly
repaired in the control group at (A) low and (B) high magnification
and scaffold only group at (C) low and (D) high magnification. At
high magnification, in the control and scaffold only groups, the
repair tissue was stained slightly and no cartilage-like tissue was
observed. (E) In the experimental group, the repair tissue in the
top layer of the cartilage defect was stained intensely, similar to
native cartilage. Native cartilage degeneration was also observed
(indicated by asterisk). At high magnification (F), cartilage-like
tissue was observed in the top layer of the cartilage defect. (A, C
and E) Magnification, ×20. (B, D and F) Magnification, ×100.
Rabbits transplanted with scaffold/human induced pluripotent stem
cells-mesenchymal stem cells formed the experimental group. R,
repair tissue; C, cartilage. |
Discussion
The results revealed cartilage-like tissue formation
in the top layer of the cartilage defect when hiPSCs-MSCs were
used. An apparently better quality of in vivo cartilage
defect repair in the experimental group compared with the control
and scaffold implantation groups was demonstrated by gross and
histological appearance. Another important finding was that there
was no evidence of teratoma formation in the experimental group.
Although the restoration of full-thickness cartilage defect was not
totally satisfactory, the results of the present study indicated
that iPSCs may be a new cell source for cartilage defect repair
in vivo.
iPSCs have been considered as the optimal cell
source for regenerative medicine because of their self-renewal and
pluripotency capability (22). Few
studies have examined in vivo cartilage defect repair using
iPSCs. Ko et al (19)
reported successful induction of chondrogenesis and repair of
cartilage defect in vivo with hiPSCs in immunosuppressed
rats. Yamashita et al (23)
reported hyaline chondrogenesis from hiPSCs and showed
neo-cartilage survival in joint surface defects following newly
generated cartilage particle transplantation in immunosuppressed
rats and mini pigs. The results of the present study appear to be
inferior to those of the aforementioned studies. This might be the
result of using hiPSCs-MSCs transplantation, rather than newly
generated cartilage transplantation in the present study, as local
environmental inductive effects would be inferior to those of
exogenous growth factors. Similarly, Marquass et al
(24) also demonstrated that
differentiated MSCs showed better histological outcomes compared
with undifferentiated MSCs. However, the rabbit model used in the
present study was more appropriate for the examination of cartilage
defect repair than a rat model, as the cartilage thickness of rats
is much thinner and the endogenous healing potential in rats is
greater (25).
No teratoma formation was observed in the present
study. This suggests that iPSCs-MSCs may be safer than iPSCs when
used in vivo, although the mechanism is not clear. In
previous studies, Ko et al (19) and Yamashita et al (23) did not report teratoma formation in
vivo, consistent with observations in the present study. The
method used to get hiPSCs-MSCs in the present study consisted of
three steps: i) EB formation; ii) cell outgrowth from EBs; and iii)
monolayer cell culture to select cells that can adapt to MSC growth
conditions. Numerous alternative approaches for the preparation of
MSCs from ESCs or iPSCs, such as using co-culture methods (26,27),
gene transfection (28) or
conditioned medium (29) have been
reported. However, the use of other cells or exogenous genetic
material may introduce contamination with animal pathogens or the
risk of tumorigenicity. Thus, the culture protocol used in the
present study, which is simple and reproducible, appears to be
suitable for the generation of MSCs from hiPSCs.
This study also had some limitations. Firstly, no
examination was conducted to confirm whether the newly generated
repair tissue was induced from transplanted hiPSCs-MSCs, or whether
the implanted hiPSCs-MSCs remained in situ. Some unexpected
factors may play a role during cartilage defect repair in
vivo with hiPSCs-MSCs. It is possible that the paracrine effect
of implanted hiPSCs-MSCs contributed to the attraction of host
chondrocytes and MSCs to the cartilage defects. Second, the
follow-up period may have limited the repair quality in this study.
Results were only observed at 3 and 6 weeks, as we were keen to
avoid any rejection reactions in the xenotransplantation model used
in this study. There have been a few studies concerning
xenotransplantation for cartilage defect repair. Pei et al
(30) demonstrated failure of
xenoimplantation using porcine MSCs for rabbit cartilage defects at
a follow up of 6 months. However, Jang et al (31) reported a successful result in
xenoimplantation of human MSCs into rabbit cartilage defects at 4
and 8 weeks. Thus, although there is no consensus for the
appropriate follow-up period in xenoimplantation, the follow-up
period in the present study may have been too short to induce
rejection reactions. Thirdly, the hiPSCs-MSCs were not purified by
cell sorting, which may also limit the cartilage defect repair.
Although this study had some limitations, it
suggested that full-thickness cartilage defects can be repaired
using hiPSCs-MSCs. Further understanding of the differentiation of
iPSCs and a long-term investigation of full-thickness cartilage
defect regeneration with iPSCs are necessary.
Acknowledgements
The present study was supported by the Projects of
International Cooperation and Exchanges Natural Science Foundation
of China (NSFC) (grant no. 81420108021), National Key Technology
Support Program (grant no. 2015BAI08B02), Excellent Young Scholars
NSFC (grant no. 81622033), NSFC (grant no. 81572129), Jiangsu
Provincial Key Medical Center Foundation, Jiangsu Provincial
Medical Talent Foundation and Jiangsu Provincial Medical
Outstanding Talent Foundation, Social Development Project of
Jiangsu Provincial Science and Technology Department (grant no.
BE2016609).
References
|
1
|
Caplan AI: Review: Mesenchymal stem cells:
Cell-based reconstructive therapy in orthopedics. Tissue Eng.
11:1198–1211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wakitani S, Okabe T, Horibe S, Mitsuoka T,
Saito M, Koyama T, Nawata M, Tensho K, Kato H, Uematsu K, et al:
Safety of autologous bone marrow-derived mesenchymal stem cell
transplantation for cartilage repair in 41 patients with 45 joints
followed for up to 11 years and 5 months. J Tissue Eng Regen Med.
5:146–150. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wakitani S, Nawata M, Tensho K, Okabe T,
Machida H and Ohgushi H: Repair of articular cartilage defects in
the patello-femoral joint with autologous bone marrow mesenchymal
cell transplantation: Three case reports involving nine defects in
five knees. J Tissue Eng Regen Med. 1:74–79. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Segawa Y, Muneta T, Makino H, Nimura A,
Mochizuki T, Ju YJ, Ezura Y, Umezawa A and Sekiya I: Mesenchymal
stem cells derived from synovium, meniscus, anterior cruciate
ligament and articular chondrocytes share similar gene expression
profiles. J Orthop Res. 27:435–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zuk PA, Zhu M, Ashjian P, De Ugarte DA,
Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P and Hedrick
MH: Human adipose tissue is a source of multipotent stem cells. Mol
Biol Cell. 13:4279–4295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Im GI, Kim DY, Shin JH, Hyun CW and Cho
WH: Repair of cartilage defect in the rabbit with cultured
mesenchymal stem cells from bone marrow. J Bone Joint Surg Br.
83:289–294. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Payne KA, Didiano DM and Chu CR: Donor sex
and age influence the chondrogenic potential of human femoral bone
marrow stem cells. Osteoarthritis Cartilage. 18:705–713. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takahashi K, Tanabe K, Ohnuki M, Narita M,
Ichisaka T, Tomoda K and Yamanaka S: Induction of pluripotent stem
cells from adult human fibroblasts by defined factors. Cell.
131:861–872. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wernig M, Meissner A, Foreman R, Brambrink
T, Ku M, Hochedlinger K, Bernstein BE and Jaenisch R: In vitro
reprogramming of fibroblasts into a pluripotent ES-cell-like state.
Nature. 448:318–324. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kimura T, Kaga Y, Sekita Y, Fujikawa K,
Nakatani T, Odamoto M, Funaki S, Ikawa M, Abe K and Nakano T:
Pluripotent stem cells induced from mouse somatic cells by
small-molecule compounds. Stem Cells. 33:45–55. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin T and Wu S: Reprogramming with small
molecules instead of exogenous transcription factors. Stem Cells
Int. 2015:7946322015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Imaizumi M, Nomoto Y, Sato Y, Sugino T,
Miyake M, Wada I, Nakamura T and Omori K: Evaluation of the use of
induced pluripotent stem cells (iPSCs) for the regeneration of
tracheal cartilage. Cell Transplant. 22:341–353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Teramura T, Onodera Y, Mihara T, Hosoi Y,
Hamanishi C and Fukuda K: Induction of mesenchymal progenitor cells
with chondrogenic property from mouse-induced pluripotent stem
cells. Cell Reprogram. 12:249–261. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X,
Zhang Y, Lam FF, Kang S, Xia JC, Lai WH, et al: Functional
mesenchymal stem cells derived from human induced pluripotent stem
cells attenuate limb ischemia in mice. Circulation. 121:1113–1123.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Peppo GM, Marcos-Campos I, Kahlter DJ,
Alsalman D, Shang L, Vunjak-Novakovic G and Marolt D: Engineering
bone tissue substitutes from human induced pluripotent stem cells.
Proc Natl Acad Sci USA. 110:pp. 8680–8685. 2013; View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Diekman BO, Christoforou N, Willard VP,
Sun H, Sanchez-Adams J, Leong KW and Guilak F: Cartilage tissue
engineering using differentiated and purified induced pluripotent
stem cells. Proc Natl Acad Sci USA. 109:pp. 19172–19177. 2012;
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ko JY, Kim KI, Park S and Im GI: In vitro
chondrogenesis and in vivo repair of osteochondral defect with
human induced pluripotent stem cells. Biomaterials. 35:3571–3581.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liao J, Wu Z, Wang Y, Cheng L, Cui C, Gao
Y, Chen T, Rao L, Chen S, Jia N, et al: Enhanced efficiency of
generating induced pluripotent stem (iPS) cells from human somatic
cells by a combination of six transcription factors. Cell Res.
18:600–603. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu X, Shi D, Shen Y, Xu Z, Dai J, Chen D,
Teng H and Jiang Q: Full-thickness cartilage defects are repaired
via a microfracture technique and intraarticular injection of the
small-molecule compound kartogenin. Arthritis Res Ther. 17:202015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ng KK, Thatte HS and Spector M:
Chondrogenic differentiation of adult mesenchymal stem cells and
embryonic cells in collagen scaffolds. J Biomed Mater Res A.
99:275–282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamashita A, Morioka M, Yahara Y, Okada M,
Kobayashi T, Kuriyama S, Matsuda S and Tsumaki N: Generation of
scaffoldless hyaline cartilaginous tissue from human iPSCs. Stem
Cell Reports. 4:404–418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marquass B, Schulz R, Hepp P, Zscharnack
M, Aigner T, Schmidt S, Stein F, Richter R, Osterhoff G, Aust G, et
al: Matrix-associated implantation of predifferentiated mesenchymal
stem cells versus articular chondrocytes: In vivo results of
cartilage repair after 1 year. Am J Sports Med. 39:1401–1412. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chu CR, Szczodry M and Bruno S: Animal
models for cartilage regeneration and repair. Tissue Eng Part B
Rev. 16:105–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barberi T, Willis LM, Socci ND and Studer
L: Derivation of multipotent mesenchymal precursors from human
embryonic stem cells. PLoS Med. 2:e1612005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bigdeli N, Karlsson C, Strehl R, Concaro
S, Hyllner J and Lindahl A: Coculture of human embryonic stem cells
and human articular chondrocytes results in significantly altered
phenotype and improved chondrogenic differentiation. Stem Cells.
27:1812–1821. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu C, Jiang J, Sottile V, McWhir J,
Lebkowski J and Carpenter MK: Immortalized fibroblast-like cells
derived from human embryonic stem cells support undifferentiated
cell growth. Stem Cells. 22:972–980. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hwang YS, Polak JM and Mantalaris A: In
vitro direct chondrogenesis of murine embryonic stem cells by
bypassing embryoid body formation. Stem Cells Dev. 17:971–978.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pei M, Yan Z, Shoukry M and Boyce BM:
Failure of xenoimplantation using porcine synovium-derived stem
cell-based cartilage tissue constructs for the repair of rabbit
osteochondral defects. J Orthop Res. 28:1064–1070. 2010.PubMed/NCBI
|
|
31
|
Jang KM, Lee JH, Park CM, Song HR and Wang
JH: Xenotransplantation of human mesenchymal stem cells for repair
of osteochondral defects in rabbits using osteochondral biphasic
composite constructs. Knee Surg Sports Traumatol Arthrosc.
22:1434–1444. 2014. View Article : Google Scholar : PubMed/NCBI
|