Introduction
Delayed or failed bone union is a common clinical
complication that requires treatment in orthopedics, often
requiring re-admission and surgery (1). Depending on the fracture site, ~5–10%
of fractures may result in delayed union or nonunion (2,3).
Previously, autogenous bone grafts or free vascularized bone grafts
have been widely used for nonunion treatment. However, harvesting
the grafts from the iliac crest is associated with donor site
morbidity and particularly with chronic pain (4).
Cell-based therapies and tissue-engineered
approaches have become potential therapeutic strategies for bone
repair and fracture healing. Producing an optimal cell source for
the generation of functional osteoblasts is critical to achieve
clinical success with these therapeutic strategies. Mesenchymal
stem cells (MSCs) are multipotent somatic stem cells that are able
to differentiate into numerous of cell types, including
chondrocytes, myocytes, osteoblasts and adipocytes (5). It has been demonstrated that MSCs may
provide a source of cells for tissue engineering of bone tissue.
The osteogenic potential of MSCs has already been applied in a
number of clinical situations, including fracture nonunion,
osteogenesis imperfecta, posterior spinal fusion, distraction
osteogenesis and osteoarthritis (6).
The number of human (h)MSCs in tissue and their proliferative
activity has been observed to be reduced with increasing age of
their donor (7), which leads to
difficulties in preparing a sufficient number of hMSCs for cell
therapy in elderly patients. In addition, hMSCs isolated from
patients with osteoporosis exhibit a low proliferative activity and
limited ability to differentiate into the osteogenic lineage
(8,9).
It has been demonstrated that bone morphogenetic
proteins (BMPs) have crucial roles in the process of new bone
formation by inducing the differentiation of hMSCs into
osteoblasts, and promoting osteoblast maturity and endochondral
ossification (10). Among the BMPs
studied (BMP-2, −7 and −9), BMP-2 has the highest osteoinductive
potential (11). Due to the
efficient gene transfer achieved, adenoviral vectors are attractive
vehicles for in vivo gene therapy. It has been demonstrated
that BMP-2-expressing recombinant adenoviral vector gene
(Ad-BMP-2)-modified tissue-engineered bone may efficiently promote
osteogenesis and repair critical-sized bone defects in large
animals (12). However, the effect
of Ad-BMP-2 on the osteogenic ability of human mesenchymal stem
cells has remained elusive.
Therefore, the present study assessed the possible
application of Ad-BMP-2 in order to assess whether it promotes
osteogenic differentiation of hMSCs. In the present study, the
feasibility of using hMSCs in the treatment of delayed and nonunion
complications of fracture repair in vitro was verified to
potentially identify a novel method for treating delayed or failed
bone union.
Materials and methods
hMSC isolation and culture
hMSCs were prepared as described previously
(13) following the standard
protocol by Roseti et al (14). Bone marrow aspirates (50 ml) were
obtained from the iliac crest of 8 healthy volunteer donors (20–35
years of age) at Renmin Hospital, Hubei University of Medicine
(Shiyan, China), and diluted to 1:3 with Iscove's modified
Dulbecco's medium (IMDM; Gibco; Thermo Fisher Scientific Inc.,
Waltham, MA, USA). Following density gradient centrifugation (750 ×
g for 20 min), the mononuclear cell layer was obtained from the
interface. The cells were washed twice with Hanks' balanced saline
solution (Beyotime Institute of Biotechnology, Haimen, China),
suspended in IMDM, supplemented with 10% heat-inactivated fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.),
L-glutamine and Hepes (25 mM), gentamicin (50 µg/ml) and 2%
Ultroser™ G Serum Substitute (Pall Corp., Port Washington, NY,
USA), plated in 75-cm2 flasks at a density of 1.6×105 cells/cm2 and
incubated in a humidified atmosphere containing 95% air and 5%
CO2 at 37°C. After 2 days, when the cells had reached
confluence, adherent cells were harvested by incubation for 10 min
with 0.02% EDTA and 0.05% trypsin at room temperature. Hanks'
balanced saline solution, without calcium and magnesium,
supplemented with 10% FBS was used to wash the cells. Cells were
resuspended in the aforementioned complete IMDM. The resulting cell
population was referred to as primary culture (P0). Cells were
plated at a density of 104 cells/cm2 in 100-mm dishes to propagate
this population (secondary culture; P1). The use of human bone
marrow for this study was approved by the Human Research Ethics
Board at Hubei University of Medicine. All patients provided
informed consent.
hMSC characterization and
phenotype
Following 14 days, the P0 cultures were trypsinized
and passaged. Cell cultures were passaged weekly following P1 and
grew exponentially. To assess the purity of the hMSC cultures,
analysis of these cells was performed using a flow cytometer
(CytoFLEX; Beckman Coulter Inc., Brea, CA, USA). Cells positive for
the Src homology 2 domain (SH2) according to flow cytometric
analysis with SH2-fluorescein isothiocyanate-conjugated antibody
(cat. no. TA504381; Origene Technologies, Inc., Rockville, MD, USA)
were determined to be hMSCs (15).
No detectable contamination with hematopoietic cells was observed,
as indicated by the absence of CD34, a marker of the hematopoietic
lineage (16), as detected through
the use of CD34-FITC-conjugated antibody (cat. no. ZM-0046;
Zhongshan Goldenbridge Biotechnology, Ltd., Beijing, China). These
data indicated that the population of hMSCs was morphologically
homogeneous.
Treatment of cell cultures
Cells at P1 were randomly divided into four groups,
as follows: i) Ad-BMP-2: The concentration of Ad-BMP-2 used in the
culture was 1×1010 optical units/ml, following 24 h of
incubation with Ad-BMP-2 at room temperature, followed by culture
in regular culture medium. ii) Adenoviral vector containing LacZ
(Ad-LacZ): 1×1010 optical units/ml Ad-LacZ replaced
Ad-BMP-2. iii) Control: The positive control group was cultured
with 1 nmol/l dexamethasone, 50 mg/l ascorbic acid and 10 mmol/l
β-sodium phosphate (all from Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) with the medium changed every 3 days. iv) Blank, no
specific treatment. The Ad-BMP-2 and Ad-LacZ vectors were
constructed and donated by Li et al (17) at the Department of Trauma
Orthopedics, Hubei University of Medicine.
Alkaline phosphatase (ALP) activity
assay
To assess osteogenic activity, ALP activity was
measured and scored 12 days following transduction. A 100-µl sample
of the culture supernatant was incubated at 37°C for 30 min with
100 µl p-nitrophenyl phosphate (1 mg/ml; Sigma-Aldrich; Merck KGaA)
in 1 M diethanolamine buffer containing 0.5 mM MgCl2 at pH 9.8. The
addition of 50 µl 0.2 M sodium hydroxide stopped the reaction.
Total protein content was determined using a Bio-Rad Protein Assay
kit II (Bio-Rad Laboratories, Inc., Hercules, CA, USA), the
absorbance was determined at 595 nm and the activity was calculated
according to a series of bovine serum albumin standards. At the end
of the experiment, ALP levels were normalized to the total protein
content. Each sample was repeated in triplicate.
von Kossa staining
Cells were fixed with 4% paraformaldehyde and washed
in phosphate-buffered saline. Cells were then treated with 5%
silver nitrate solution at 37°C in the dark for 30 min. Silver
nitrate solution was then completely washed away with Hanks'
balanced saline solution and the cells were exposed to bright light
for 15 min to develop the color.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Statistical analysis was performed using SPSS software,
version 12.0 (SPSS, Inc., Chicago, IL, USA). One-way analysis of
variance was used to assess the differences between the three
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Characterization and phenotype of
hMSCs
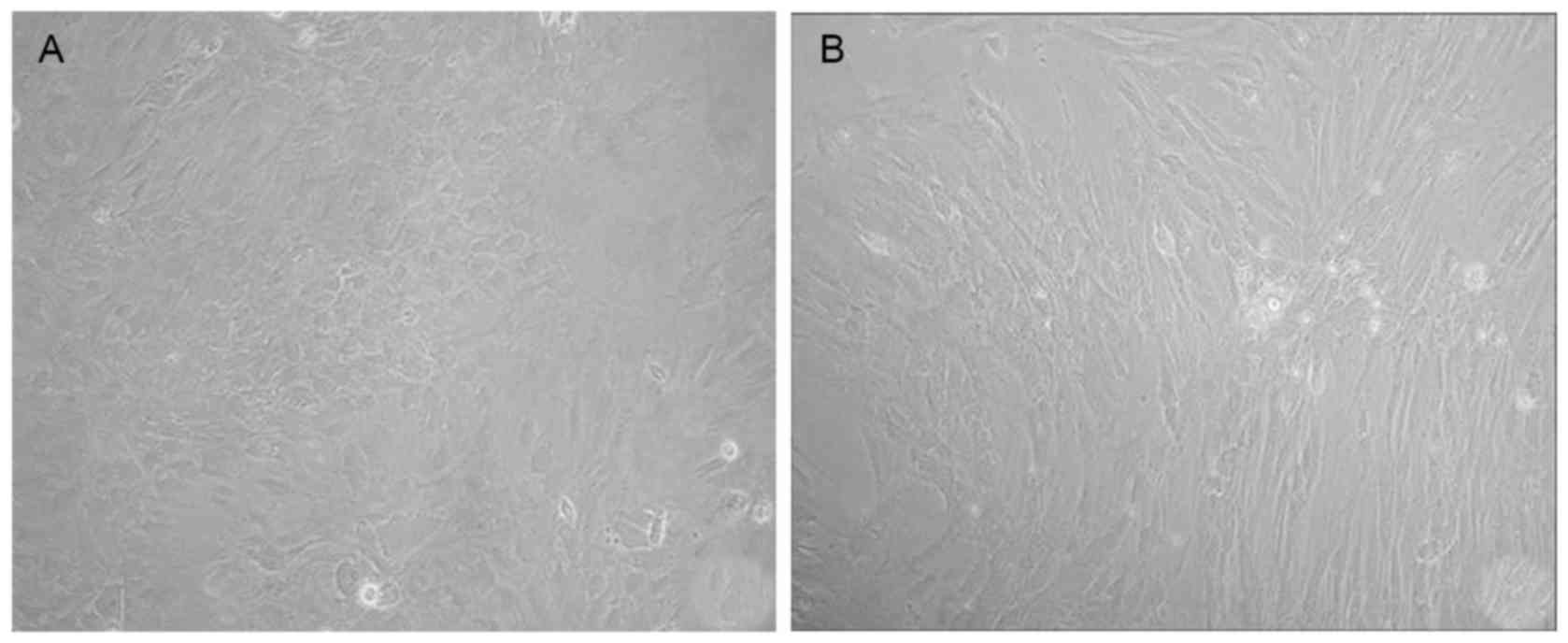
Following four days in culture, freshly harvested
bone marrow cells were adherent (Fig.
1A). On day 14, a morphologically homogeneous population of
fibroblast-like cells was observed to have >90% confluence
(Fig. 1B).
The phenotype of hMSCs was analyzed using flow
cytometry, and cells positive for SH2 were determined to be hMSCs
(Fig. 2A), while CD34 was assessed
to distinguish the cells from hematopoietic lineages (Fig. 2B).
Ad-BMP-2 induces osteoblast-like
morphological changes in hMSCs
Following treatment with Ad-BMP-2, cells gradually
transformed into polygons or irregular shapes, proliferation
decreased and no colony formation was observed (Fig. 3A). The morphology in the positive
control group was similar to that of the hMSCs treated with
Ad-BMP-2. There was no evident change in the morphology of hMSCs in
the Ad-LacZ group (Fig. 3B) and
cells in the blank group, and colony formation was observed.
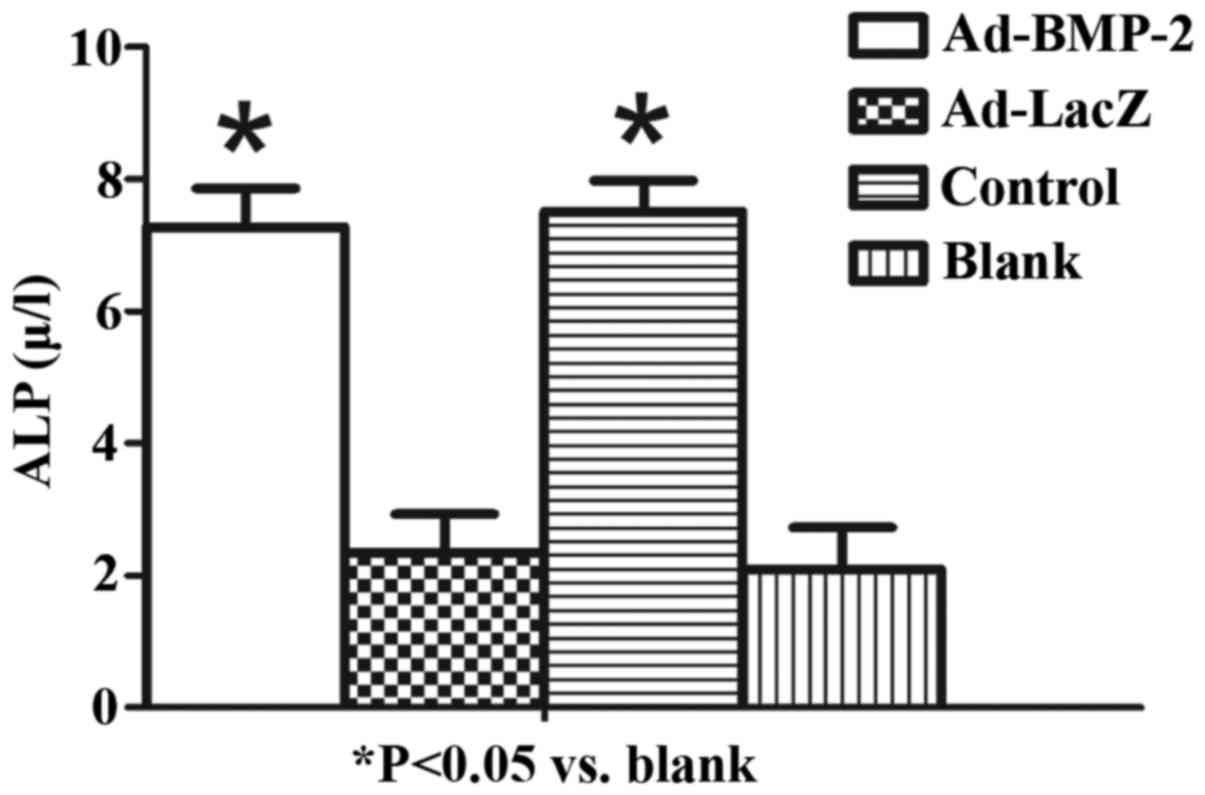
Ad-BMP-2 increases ALP activity in
hMSCs
ALP is an enzyme present in osteoblasts and is
pivotal for bone mineralization (18). The present study evaluated the
osteoinductive effect of BMP-2 on hMSCs. Following treatment, the
Ad-BMP-2 and positive control group demonstrated a significant
increase in the level of ALP (P<0.05; Fig. 4).
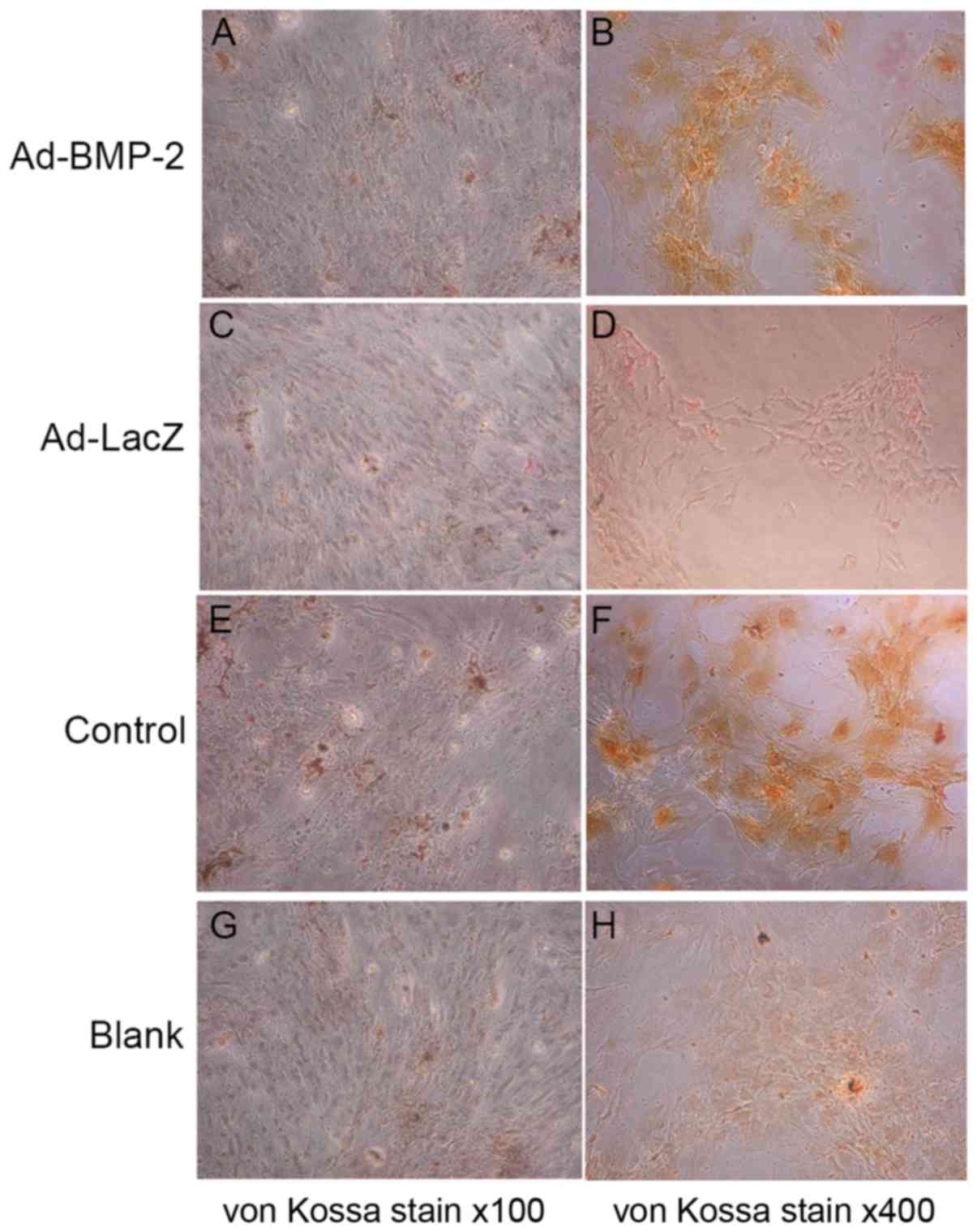
Ad-BMP-2 increases mineralization and
calcification of hMSCs
The mineralization and calcification of the bone
matrix facilitates osteoblast formation and is therefore essential
for the strength and rigidity of the skeletal system (19). To estimate the osteoblastic
mineralization and calcification, von Kossa staining for phosphates
at was performed day 14. Representative images of von Kossa stain
were obtained by microscopy (Fig.
5). Compared with the Ad-LacZ and blank groups, the phosphate
deposition in the Ad-BMP-2 and positive control groups was clearly
increased (Fig. 5).
Discussion
The US Food and Drug Administration (FDA) define a
nonunion as a fracture that does not heal within nine months and
reports nonunion occurring in 1 out of 40 fractures (20). A lack of healing progression within
three consecutive months is the clinical definition of a delayed
union (21). Certain risk factors
may predispose a patient to the development of a nonunion,
including the type and site of fracture, fracture comminution with
bone and soft tissue devascularization, instability, bone loss,
presence of a chronic illness, infection and tobacco use. However,
while it is known that these risk factors may predispose a patient
to develop a nonunion, the underlying physiopathology remains to be
fully elucidated (22).
Human marrow mesenchymal stem cells have become the
primary cell source for bone tissue engineering (23). The theory of osteoblast modulation
suggests that if a pluripotent cell is situated in the proper
milieu it may convey an osteoblast phenotype (24). As described by Chamberlain et
al (25), a several week
incubation procedure that includes a mixed monolayer of hMSCs with
ascorbic acid, dexamethasone and phosphate is the standard approach
for differentiating hMSCs into osteoblasts in vitro
(25), so the present study used
this as a positive control. The methods typically used to incubate
hMSCs include whole bone marrow culture, density gradient
centrifugation and immunomagnetic separation. The primary hMSCs
separated by the final two methods have relatively high purity, but
the cells grow slowly and the culture cycle is long, making it
difficult to meet clinical requirements (26,27). The
primary cells incubated using the whole marrow method are mixed
with hemopoietic stem cells. However, with Ad-BMP-2 and the
extension of the incubation time, the suspension growth hemopoietic
stem cells are removed through the exchange of cell medium. The
present study assessed the use of Ad-BMP-2 with an extended
incubation time using flow cytometry, confirming that the whole
bone marrow culture also reach a high purity, the cells multiply
rapidly and the culture cycle is short, meaning it is suitable for
clinical use.
To improve the osteogenic potential, two strategies
have been developed. The first one is to enhance bone formation by
incorporating bone-favor growth factors into the scaffold, known as
growth factor-based bone tissue engineering. The second is
cell-based bone tissue engineering, building up osteoinductive
capability by growing living osteogenic cells on scaffolds in
vitro (28). Numerous members of
the whole BMP superfamily are associated with bone, cartilage and
joint development. BMP-2 has been approved by the FDA for clinical
practice as the most potent member of the BMP family in promoting
bone and cartilage development. It is therefore a popular choice
for MSCs-based bone tissue engineering. It has been demonstrated
that the BMP-2-modified MSCs increase the ALP activity, cell
proliferation and mineralization in vitro and heal
critical-size bone defects, induce ectopic bone formation, repair
fractures and trigger spinal fusion in vivo (29). BMP-2 serves an important role in
fracture healing: During the process of bone tissue repair, BMP-2
transmits information between cells and intercellular substances
through autocrine and paracrine signaling, regulating the secretion
and proliferation of cells. The adenovirus commonly used as a gene
delivery vector as it has a high transfection efficiency (30). However, the long-term overexpression
of exogenous genes may lead to serious consequences, which are
unpredictable and irreparable. However, the target genes that the
adenovirus is able to mediate do not integrate into the chromosome,
are only expressed in the cytoplasm and typically last for 4–8
weeks (31). Therefore, the
requirement of gene therapy to be delivered quickly is satisfied
while safety is guaranteed.
The flexibility to express the protein focally and
locally, or in a disseminated fashion, as required is the most
relevant advantage of gene therapy. Of note, gene therapy provides
a possibility for intra-cellular production of proteins, thus
facilitating therapeutic pathways to occur (32). Following treatment with Ad-BMP-2,
hMSCs not only adopted osteoblastic features regarding their shape
and growth patterns, but also had an increased expression of ALP.
The present study also used a group transfected with Ad-LacZ to
assess whether the expression of the adenoviral vector, which was
identical to that in Ad-BMP-2, had any effect on osteogenesis. The
results suggested that the stimulation of osteogenesis in the
Ad-BMP-2 group was a result of the expression of BMP-2, not the
adenovirus.
If active bone formation occurs, the level of ALP
increases, as it is a byproduct of osteoblast activity (33). In the present study, the culture
medium was changed to be serum-free prior to ALP detection. This
eliminates any interference with the results due to ALP contained
in serum. Osteoblastic mineralization and calcification are the
most reliable evidence of the osteoblast. In the Ad-BMP-2 and
positive control groups, ALP activity and the level of phosphate
increased, indicating that Ad-BMP-2 has a function in promoting the
osteogenesis of hMSC.
The present study confirmed the feasibility of
transfecting hMSCs with Ad-BMP-2 to treat delayed or nonunion
fractures in vitro. In addition, hMSCs may differentiate
into chondrocytes, myocytes and adipocytes as well as osteoblasts.
Therefore, it is necessary to induce osteoblastic differentiation
prior to transplantation. The traditional method to induce
osteoblast proliferation is to administer dexamethasone, ascorbic
acid and β-sodium phosphate. The present study demonstrated that
the osteogenic differentiation ability of hMSCs in the Ad-BMP-2
group was similar to that in the positive control group. The
induction time was two weeks in the positive control group, while
it was only 24 h in the Ad-BMP-2 group, suggesting that Ad-BMP-2
may reduce osteogenic differentiation time. As for clinical use,
Ad-BMP-2 may significantly reduce the treatment cycle time and the
risk of cell contamination with hematopoietic cells. Therefore, it
has the potential to be a novel therapeutic method for treating
delayed or nonunion fracture healing in the future.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81602867), Hubei
Province Health and Family Planning Scientific Research Project
(no. WJ2015Q042) and projects funded by Hubei Provincial Science
and Technology Department (no. 2013CFC031).
References
|
1
|
Dahabreh Z, Dimitriou R and Giannoudis PV:
Health economics: A cost analysis of treatment of persistent
fracture non-unions using bone morphogenetic protein-7. Injury.
38:371–377. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marsh D: Concepts of fracture union,
delayed union, and nonunion. Clin Orthop Relat Res. Suppl
355:22–30. 1998. View Article : Google Scholar
|
|
3
|
Obermeyer TS, Yonick D, Lauing K, Stock
SR, Nauer R, Strotman P, Shankar R, Gamelli R, Stover M and Callaci
JJ: Mesenchymal stem cells facilitate fracture repair in an
alcohol-induced impaired healing model. J Orthop Trauma.
26:712–718. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qi Y, Zhao T, Yan W, Xu K, Shi Z and Wang
J: Mesenchymal stem cell sheet transplantation combined with
locally released simvastatin enhances bone formation in a rat tibia
osteotomy model. Cytotherapy. 15:44–56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aldahmash A, Zaher W, Al-Nbaheen M and
Kassem M: Human stromal (mesenchymal) stem cells: Basic biology and
current clinical use for tissue regeneration. Ann Saudi Med.
32:68–77. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang ZY, Teoh SH, Hui JH, Fisk NM,
Choolani M and Chan JK: The potential of human fetal mesenchymal
stem cells for off-the-shelf bone tissue engineering application.
Biomaterials. 33:2656–2672. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
D'Ippolito G, Schiller PC, Ricordi C, Roos
BA and Howard GA: Age-related osteogenic potential of mesenchymal
stromal stem cells from human vertebral bone marrow. J Bone Miner
Res. 14:1115–1122. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rodríguez JP, Ríos S, Fernández M and
Santibañez JF: Differential activation of ERK1,2 MAP kinase
signaling pathway in mesenchymal stem cell from control and
osteoporotic postmenopausal women. J Cell Biochem. 92:745–754.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rodríguez JP, Montecinos L, Ríos S, Reyes
P and Martínez J: Mesenchymal stem cells from osteoporotic patients
produce a type I collagen-deficient extracellular matrix favoring
adipogenic differentiation. J Cell Biochem. 79:557–565. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Finkemeier CG: Bone-grafting and
bone-graft substitutes. J Bone Joint Surg Am. 84-A:454–464. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng H, Jiang W, Phillips FM, Haydon RC,
Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, et al:
Osteogenic activity of the fourteen types of human bone
morphogenetic proteins (BMPs). J Bone Joint Surg Am.
85-A:1544–1552. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dai KR, Xu XL, Tang TT, Zhu ZA, Yu CF, Lou
JR and Zhang XL: Repairing of goat tibial bone defects with BMP-2
gene-modified tissue-engineered bone. Calcif Tissue Int. 77:55–61.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun D, Junger WG, Yuan C, Zhang W, Bao Y,
Qin D, Wang C, Tan L, Qi B, Zhu D, et al: Shockwaves induce
osteogenic differentiation of human mesenchymal stem cells through
ATP release and activation of P2X7 receptors. Stem Cells.
31:1170–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roseti L, Serra M and Bassi A: Standard
operating procedure for the good manufacturing practice-compliant
production of human bone marrow mesenchymal stem cells. Methods Mol
Biol. 1283:171–186. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Foster LJ, Zeemann PA, Li C, Mann M,
Jensen ON and Kassem M: Differential expression profiling of
membrane proteins by quantitative proteomics in a human mesenchymal
stem cell line undergoing osteoblast differentiation. Stem Cells.
23:1367–1377. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hammoud M, Vlaski M, Duchez P, Chevaleyre
J, Lafarge X, Boiron JM, Praloran V, De La Grange P Brunet and
Ivanovic Z: Combination of low O(2) concentration and mesenchymal
stromal cells during culture of cord blood CD34(+) cells improves
the maintenance and proliferative capacity of hematopoietic stem
cells. J Cell Physiol. 227:2750–2758. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li WC, Wang DP, Li LJ, Zhu WM and Zeng YJ:
Adenovirus- mediated bone morphogenetic protein-2 gene transfection
of bone marrow mesenchymal stem cells combined with
nano-hydroxyapatite to construct bone graft material in vitro.
Artif Cells Nanomed Biotechnol. 41:103–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mukaiyama K, Kamimura M, Uchiyama S,
Ikegami S, Nakamura Y and Kato H: Elevation of serum alkaline
phosphatase (ALP) level in postmenopausal women is caused by high
bone turnover. Aging Clin Exp Res. 27:413–418. 2013. View Article : Google Scholar
|
|
19
|
Williams DC and Frolik CA: Physiological
and pharmacological regulation of biological calcification. Int Rev
Cytol. 126:195–292. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zura R, Braid-Forbes MJ, Jeray K, Mehta S,
Einhorn TA, Watson JT, Rocca GJ Della, Forbes K and Steen RG: Bone
fracture nonunion rate decreases with increasing age: A prospective
inception cohort study. Bone. 95:26–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liebergall M, Schroeder J, Mosheiff R,
Gazit Z, Yoram Z, Rasooly L, Daskal A, Khoury A, Weil Y and Beyth
S: Stem cell-based therapy for prevention of delayed fracture
union: A randomized and prospective preliminary study. Mol Ther.
21:1631–1638. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mathieu M, Rigutto S, Ingels A, Spruyt D,
Stricwant N, Kharroubi I, Albarani V, Jayankura M, Rasschaert J,
Bastianelli E and Gangji V: Decreased pool of mesenchymal stem
cells is associated with altered chemokines serum levels in
atrophic nonunion fractures. Bone. 53:391–398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang X, Zou S, Ye B, Zhu S, Liu Y and Hu
J: bFGF-Modified BMMSCs enhance bone regeneration following
distraction osteogenesis in rabbits. Bone. 46:1156–1161. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cuomo AV, Virk M, Petrigliano F, Morgan EF
and Lieberman JR: Mesenchymal stem cell concentration and bone
repair: Potential pitfalls from bench to bedside. J Bone Joint Surg
Am. 91:1073–1083. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chamberlain G, Fox J, Ashton B and
Middleton J: Concise review: Mesenchymal stem cells: Their
phenotype, differentiation capacity, immunological features, and
potential for homing. Stem Cells. 25:2739–2749. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Quent VM, Theodoropoulos C, Hutmacher DW
and Reichert JC: Differential osteogenicity of multiple
donor-derived human mesenchymal stem cells and osteoblasts in
monolayer, scaffold-based 3D culture and in vivo. Biomed Tech
(Berl). 61:253–166. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee TH, Kim WT, Ryu CJ and Jang YJ:
Optimization of treatment with recombinant FGF-2 for proliferation
and differentiation of human dental stem cells, mesenchymal stem
cells, and osteoblasts. Biochem Cell Biol. 93:298–305. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meijer GJ, De Bruijn JD, Koole R and van
Blitterswijk CA: Cell-based bone tissue engineering. PLoS Med.
4:e92007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hong D, Chen HX, Ge R and Li JC:
Genetically engineered mesenchymal stem cells: The ongoing research
for bone tissue engineering. Anat Rec (Hoboken). 293:531–537. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rastall DP and Amalfitano A: Recent
advances in gene therapy for lysosomal storage disorders. Appl Clin
Genet. 8:157–169. 2015.PubMed/NCBI
|
|
31
|
Liddle OL, Samuel MI, Sudhanva M, Ellis J
and Taylor C: Adenovirus urethritis and concurrent conjunctivitis:
A case series and review of the literature. Sex Transm Infect.
91:87–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Balmayor ER and van Griensven M: Gene
therapy for bone engineering. Front Bioeng Biotechnol. 3:92015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pruessner HT: Detecting celiac disease in
your patients. Am Fam Physician. 57:1023–1034, 1039-1041.
1998.PubMed/NCBI
|