Introduction
Chronic renal disease, resulting in chronic renal
failure (CRF), is associated with a number of systemic effects,
including malnutrition and muscle wasting (cachexia) (1,2). CRF is
becoming a major medical issue not only due to an increasing
incidence of tumor malignancy, but also regarding the human and
economic cost for health systems. CRF affects about 10% of the
general adult population worldwide, and is complicated by sepsis
and cardiovascular disease, mostly in parallel (2–5).
Treatment strategies for CRF include low protein
diets and the use of ketoacid analogs, however their associated
side effects, such as a probable increase in proteinuria and
functional impairment (6), may lead
to further renal tubular damage occurring, particularly in pregnant
patients with severe eGFR reduction at baseline (7). In traditional Chinese medicine,
Huang qi (Radix Astragali seu Hedysari) is used to
treat CRF (8) and it has been
previously demonstrated in a rat model of diabetic nephropathy that
the primary active ingredient of Huang qi, Astragalus
polysaccharide (APS), may improve renal function (9). However, the underlying mechanisms
regarding the effects of APS in CRF remain unknown.
The ubiquitin-proteasome pathway (UPP) is the
principal mechanism for protein catabolism within mammalian cells
(10). The UPP pathway consists of
three enzymatic components, E1, E2 and E3 ubiquitin-protein
ligases, of which E3 ubiquitin-protein ligase is considered to be
the key enzyme that is recruited to catalyze ubiquitin transfer to
a substrate protein (10). In
C57/BL6 mice with radiation-induced cell damage, it has been
demonstrated that the UPP is activated in cachexia and that the
nuclear factor (NF)-κB pathway is activated in the kidneys and
cachexic muscle tissue (10,11).
It has been demonstrated that expression of
atrogin-1, a key muscle-specific ligase, increase during muscle
atrophy and in the early stages of CRF when renal cell atrophy
occurs (12). In addition, previous
results in mice have indicated that reduced levels of atrogin-1 may
confer resistance to muscle atrophy following muscle denervation
(13). However, the roles of
atrogin-1 and its regulatory pathways in cachexia remain
unknown.
The present study aimed to evaluate the effects of
APS on muscle cell atrophy in a rat model of CRF in vivo and
in vitro, principally by reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and western blot analysis. The
current study also investigated the potential corresponding roles
of atroglin-1 and the UPP.
Materials and methods
Reagents
The ketoacid analog ketosteril (KT; as compound
α-ketoacid tablets) was purchased from Fresenius Kabi Asia-Pacific,
Ltd. (Hong Kong SAR, China). APS, tumor necrosis factor (TNF)-α and
the NF-κB inhibitor pyrrolidine dithiocarbamate (PDTC) were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Other common laboratory reagents were purchased as reagent-grade
from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Animal experiments
A total of 32 male Sprague-Dawley rats (7–8 weeks
old, 250–300 g) were obtained from the Shanghai SLAC Laboratory
Animal Co., Ltd. (Shanghai, China). The animal experiments in the
current study were approved by the Shanghai Animal Care and Use
Committee, Shanghai Institute of Materia Medica, Chinese Academy of
Sciences [Certificate number, SCXK (Shanghai) 2002–0010; Shanghai,
China]. Rats were housed at a temperature of 22±1°C and humidity of
55±5%. They were fed once a day and housed under a 12 h light/dark
cycle with free access to water. Animals fasted for 12 h before
sacrifice.
After a 3-day adaptation period, rats were divided
into four groups (n=8 rats in each group). Three groups under-went
5/6 subtotal nephrectomy to establish a rat model of CRF-induced
cachexia, as previously described (14). Of the three CRF rat groups, one group
received treatment with APS (intraperitoneally, 3 g/kg/day) for 6
weeks and one group received treatment with KT (intravenously, 0.14
g/ml suspension, l ml/200 g/day) for 4 weeks. The third group
received treatment with saline solution (intraperitoneally, 3
g/kg/day). Rats in the fourth group acted as a negative control
without any renal damage but receiving saline solution
(intraperitoneally, 3 g/kg/day). Following treatments for 6 weeks,
femur skeletal muscle tissues from mice under sodium pentobarbital
anesthesia (intraperitoneal, 40 mg/kg) were collected and stored at
−80°C until use for further biochemistry analysis.
Malnutrition (cachexia) model in rat
L6 myoblasts
Rat L6 myoblasts were purchased from the American
Type Culture Collection (Manassas, VA, USA) and maintained in
high-glucose Dulbecco's modified Eagle's medium supplemented with
10% heat-inactivated fetal bovine serum and 1%
antibiotic-antimycotic solution (cat. no. 15240096; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a
humidified atmosphere of 95% air and 5% CO2 for 24 h. An
L6 muscle cell malnutrition model was induced by pretreatment with
TNF-α (10 ng/ml) for 24 h at 37°C, as described previously
(15).
After washing twice with PBS solution (0.01 M) twice
for 2 min, 1.2×106 L6 cells were treated with APS (15
mg/l) or PDTC (50 µmol/l) or PBS as a control for 48 h following
induction of malnutrition at 37°C. Ten randomly selected fields
were observed using an inverted microscope (Olympus Corporation,
Tokyo, Japan) and the transaction diameter values of all four
groups were measured prior to cell harvesting, as previously
described (16).
Small interfering (si)-RNA knockdown
experiments in vitro
L6 myoblasts treated with APS or PDTC, or PBS as a
control, as described above, were seeded into 6-well plates in
high-glucose Dulbecco's modified Eagle's medium supplemented with
10% heat-inactivated fetal bovine serum and 1%
antibiotic-antimycotic solution (cat. no. 15240096; Invitrogen;
Thermo Fisher Scientific, Inc.) at a density of 3×105
cells/well and incubated for 1 day at 37°C. Cells in each well were
then transfected with a full-length atrogin-1-siRNA
(5′-CTACGTAGTAAGGCTGTTG-3′) transfection mix (100 µl Lipofectamine
2000 in a final volume of 1 ml cell medium; Shanghai GeneChem Co.,
Ltd., Shanghai, China) for 72 h at 37°C, according to the
manufacturer's protocol, and incubated for 72 h at 37°C. All
knockdown experiments were performed in triplicate.
RNA isolation and RT-qPCR
Total RNA from tissues was isolated using a UNIQ-10
column and a TRIzol total RNA isolation kit (both from Sangon
Biotech Co., Ltd., Shanghai, China) after fully grinding on ice for
10 min. Total RNA from cells was also isolated using a UNIQ-10
column and a Trizol total RNA isolation kit. Reverse transcription
was performed using 1 µg total RNA in a reaction volume of 20 µl
with cloned AMV reverse transcriptase (Invitrogen; Thermo Fisher
Scientific, Inc.). A total of 2 µl cDNA was used for qPCR using a
Takara Ex Taq RT-PCR Version 2.1 kit (Takara Bio, Inc., Otsu,
Japan). The following gene-specific PCR primers (Sangon Biotech
Co., Ltd.) were used: atrogin-1 were forward,
5′-AGCTTGTGCGATGTTACCA-3′ and reverse, 5′-GGTGAAAGTGAGACGGAGCA-3′;
ubiquitin were forward, 5′-TCAGATGTGGAGAAAGGAGGG-3′ and reverse,
5′-GTTGAGCCGGCTGAGTTGAT-3′; β-actin were forward,
5′-CATTGCTGACAGGATGCAG-3′ and reverse, 5′-CTGCTGGAAGGTGGACAGTGA-3′.
PCR signals were detected with a DNA Engine Opticon® 2
Continuous Fluorescence Detection system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). PCR conditions were as follows: An
annealing temperature of 60°C, followed by 40 cycles of 94°C for 20
sec, 58°C for 20 sec and 72°C for 20 sec. Melt curve analysis and
electrophoresis in 2% agar were performed in three replicates to
evaluate the purity of PCR products. Negative control reactions (no
template DNA) were included to monitor potential contamination of
reagents. Relative amounts of atrogin-1 and ubiquitin mRNA were
normalized to that of β-actin using the 2−ΔΔCq method
(17).
Protein isolation and western blot
analysis
The concentrations of the protein extracts obtained
from rat skeletal muscle tissue and L6 cells were determined using
a bicinchoninic acid kit (Pierce; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocols. Protein lysates (30 µg)
were then separated on a 10% SDS-PAGE gel followed by transfer to
nitrocellulose membranes (Bio-Rad Laboratories, Inc.). Western blot
analysis was performed according to a standard protocol (18) using primary antibodies against
atrogin-1 (cat. no. ab74023, 1:10,000; Abcam, Cambridge, UK),
ubiquitin (cat. no. sc-4316, 1:1,000) and NF-κB subunit p65 (cat.
no. c-20, 1:2,000; both from Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), and GAPDH (cat. no. A3853, 1:10,000;
Sigma-Aldrich; Merck KGaA). Horseradish peroxidase conjugated mouse
anti-rabbit IgG (sc-2357, 1:5,000; Santa Cruz Biotechnology, Inc.)
was used as a secondary antibody. Resulting protein signals were
detected using an enhanced chemiluminescence system (EMD Millipore,
Billerica, MA, USA) and data was analyzed using the Stata 7.0
software package (StataCorp LLC, College Station, TX, USA). Three
replicates were performed for each experiment.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Differences between groups were assessed using the Student's t-test
and one-way analysis of variance followed by the Tukey's post hoc
test in SPSS 19.0 (IBM SPSS, Armonk, NY, USA). Differences were
considered to be statistically significant when P<0.05.
Results
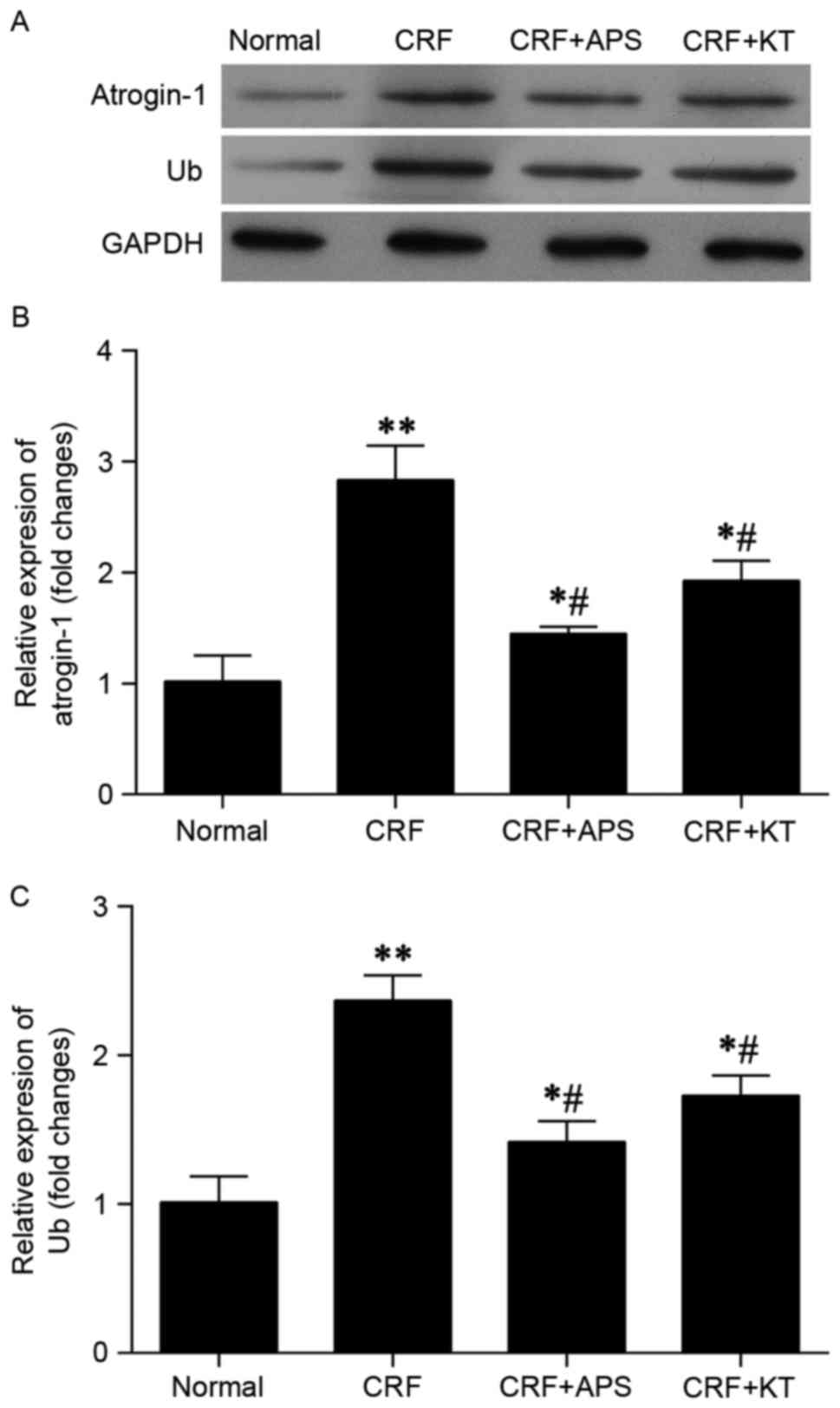
APS reduces atrogin-1 and ubiquitin
expression in vivo
Western blot analysis indicated that expression of
atrogin-1 and ubiquitin protein was markedly decreased in CRF rats
treated with KT (intravenously, 0.14 g/ml suspension; l ml/200
g/day) or APS (intraperitoneally, 3 g/kg/day), compared with
untreated CRF rats (Fig. 1A).
Results from RT-qPCR indicated that levels of atrogin-1 (Fig. 1B) and ubiquitin (Fig. 1C) mRNA in CRF rats treated with KT
were significantly decreased relative to untreated CRF rats
(P<0.05). Treatment with APS also reversed the rise in
astrogin-1 and ubiquitin protein expression (Fig. 1A), and significantly reversed the
elevated levels of atrogin-1 and ubiquitin (both P<0.05;
Fig. 1B and C) in CRF rats. In
comparison to the normal control group, CRF rats treated with KT or
APS, exhibited significantly increased expression of atrogin-1 and
ubiquitin mRNA (P<0.05; Fig. 1B and
C).
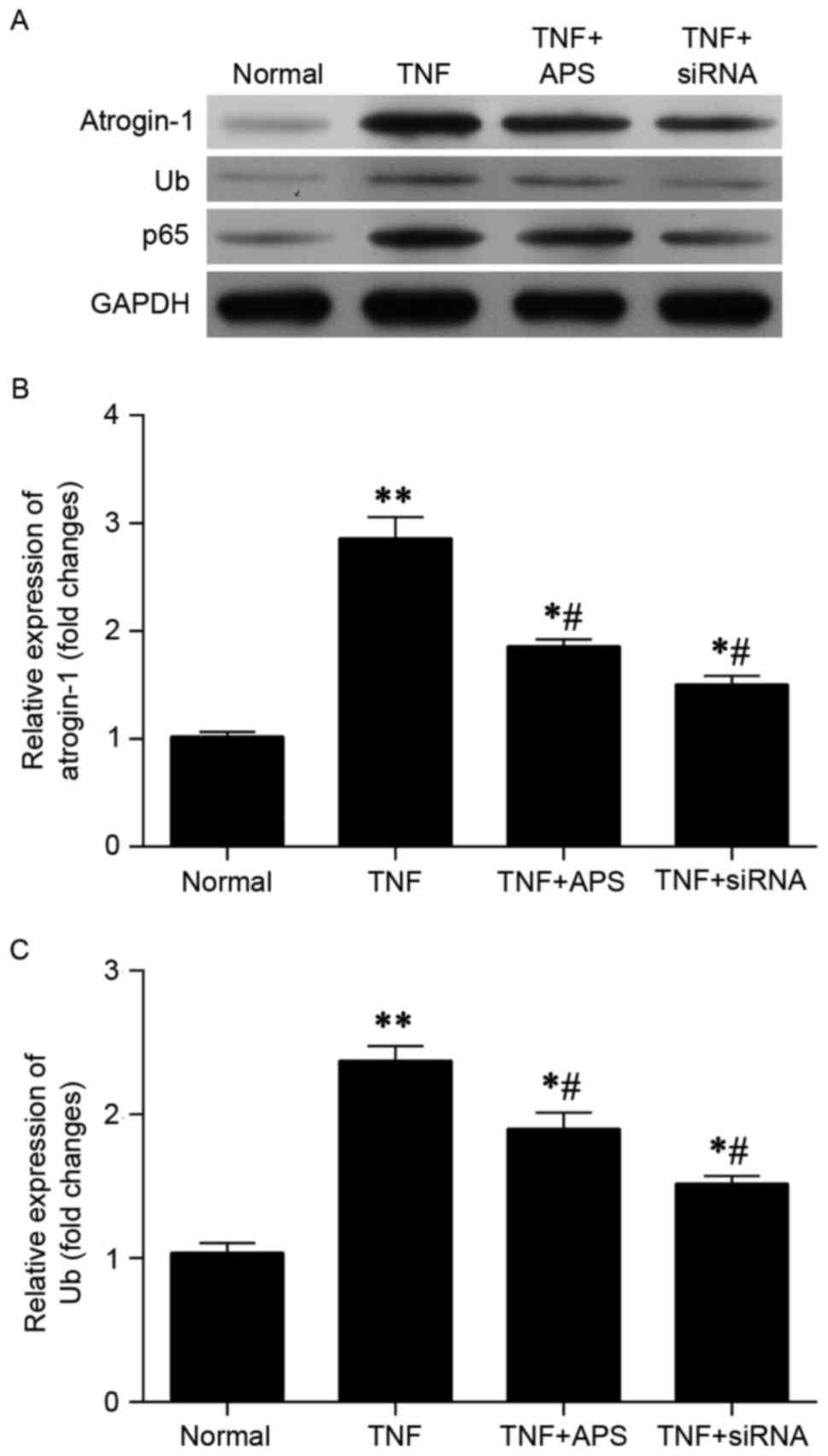
APS reduces atrogin-1 and ubiquitin
expression in vitro
A state of cell malnutrition (cachexia) was
established in vitro by pretreating rat L6 myoblasts with
TNF-α (10 ng/ml). Atrogin-1-siRNA was also used to inhibit
atrogin-1 expression. Efficiency of atrogin-1-siRNA transfection
has been confirmed by western blot analysis and RT-qPCR (data not
shown). Results indicated that the elevated levels of atrogin-1 and
ubiquitin observed in TNF-α treated L6 myoblasts were reversed
following administration of APS (Fig.
2A). NF-κB subunit p65 was also measured, and a lower level of
protein expression was observed in L6 myoblasts following TNF-α +
APS and TNF-α + atrogin-1-siRNA treatment compared with TNF-α
treatment alone (Fig. 2A).
Furthermore, RT-qPCR demonstrated that the elevated levels of
atrogin-1 (Fig. 2B) and ubiquitin
(Fig. 2C) mRNA induced by TNF-α were
significantly reversed 48 h following administration of APS
(P<0.05). In comparison to the normal control group, TNF-α + APS
treated L6 myoblasts and TNF-α + atrogin-1-siRNA-treated L6
myoblasts exhibited significantly increased expression of atrogin-1
and ubiquitin mRNA (P<0.05; Fig. 2B
and C).
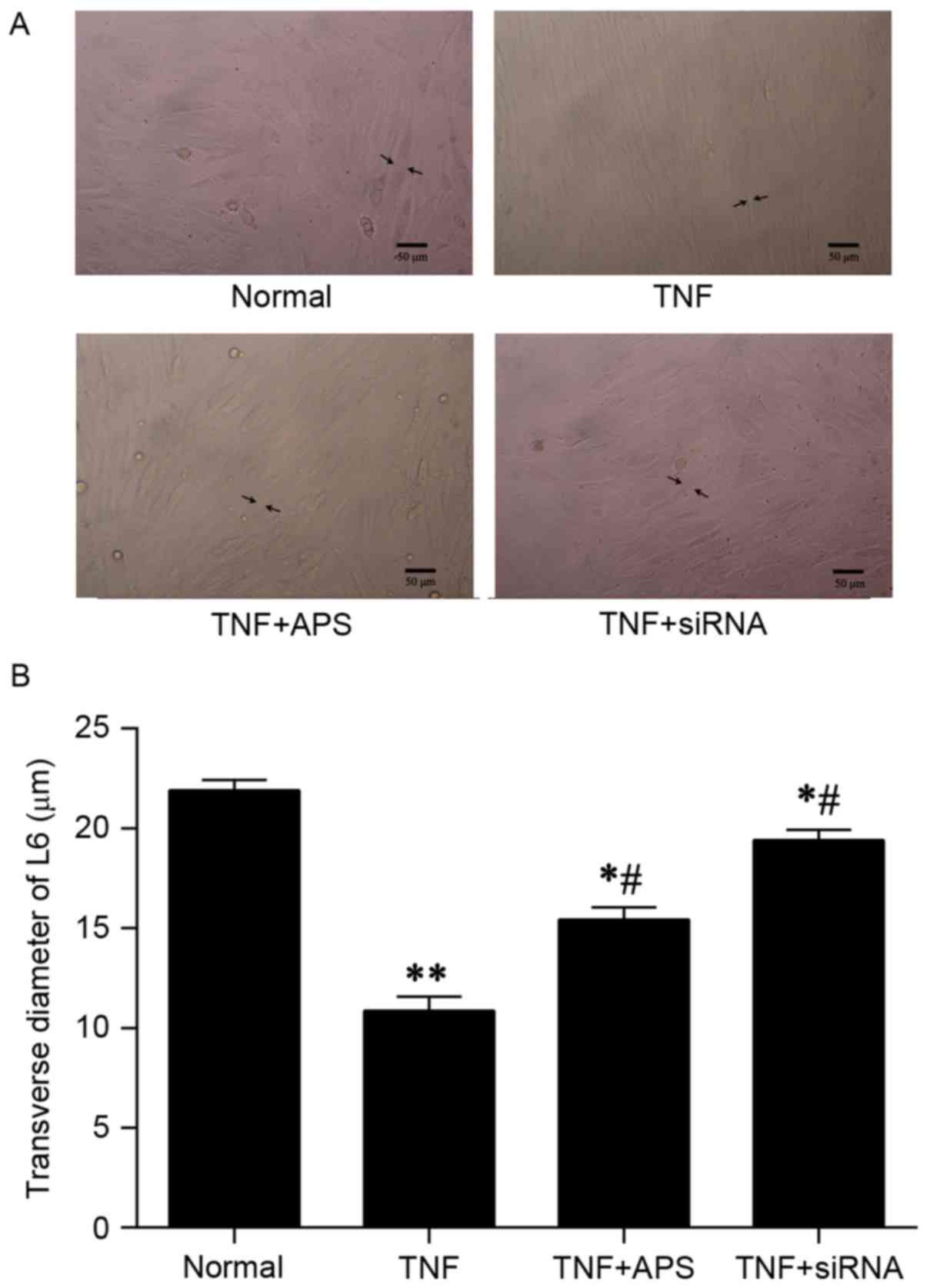
APS inhibits cell atrophy in
vitro
It was observed by fluorescence microscopy that L6
myoblasts treated with TNF-α alone were atrophic, while cell sizes
in the TNF-α + APS treatment group and TNF-α + atrogin-1-siRNA
appeared unaffected, compared with normal control cells (Fig. 3A). In addition, relative to the TNF-α
treatment group, the transverse diameters of the L6 myoblasts in
the APS + TNF-α and atrogin-1-siRNA + TNF-α groups were
significantly increased (P<0.05; Fig.
3B). TNF-α + APS treated L6 myoblasts and atrogin-1-siRNA +
TNF-α treated L6 myoblasts had significantly decreased transverse
diameters compared with the control group, ~70 and 90% of the
control transverse diameter, respectively (both P<0.05; Fig. 3B).
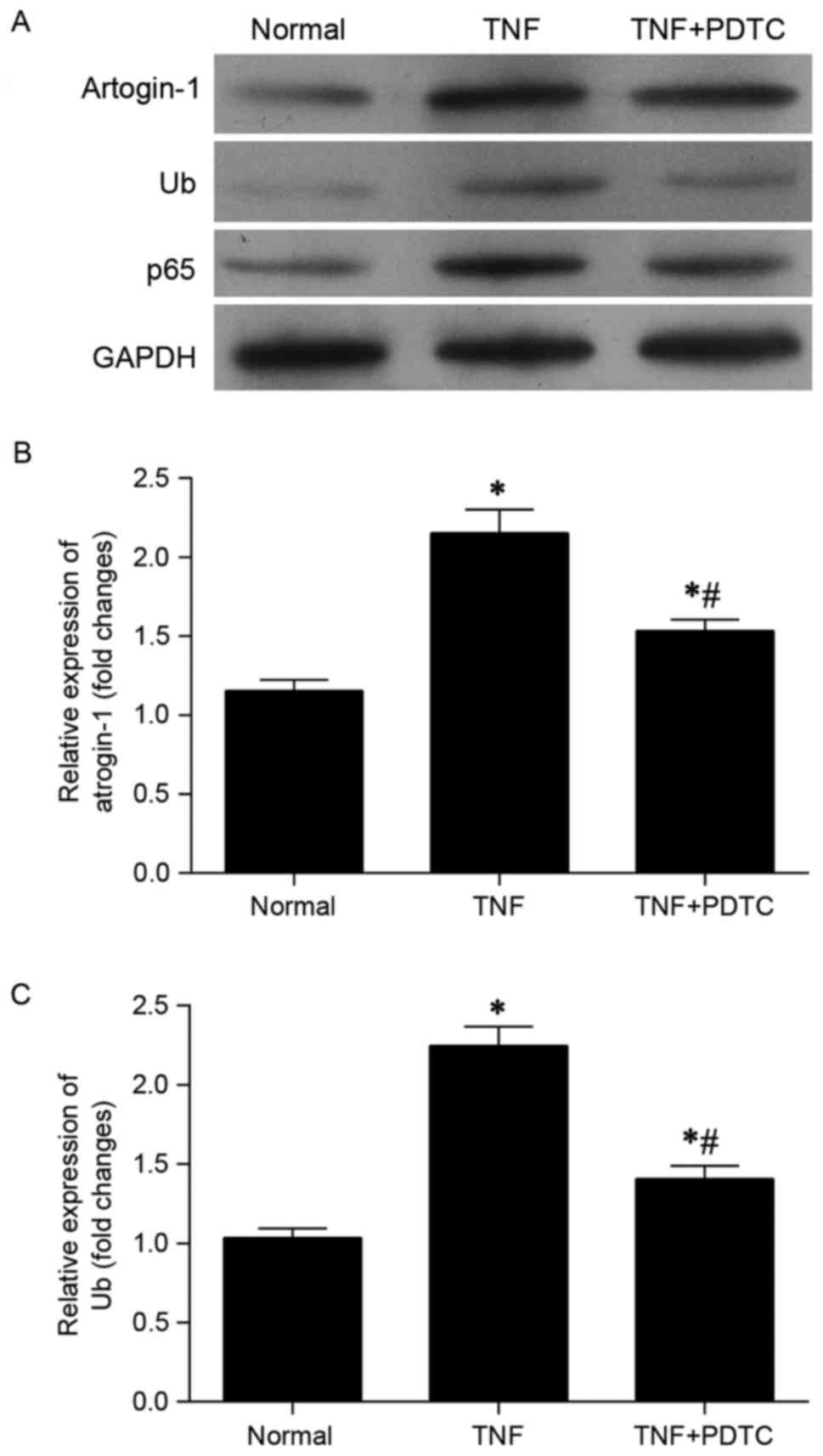
PDTC reduces atrogin-1 and ubiquitin
expression in vitro
At the protein level, it was observed that the
elevated levels of atrogin-1, ubiquitin and p65 induced by TNF-α
were markedly reduced by PDTC (Fig.
4A). Similarly, analysis of mRNA expression indicated that
upregulation of atrogin-1 (Fig. 4B)
and ubiquitin (Fig. 4C) mediated by
TNF-α was significantly inhibited 48 h following administration of
PDTC. In comparison with the normal control group, the TNF + PDTF
group exhibited significantly increased expression of atrogin-1 and
ubiquitin mRNA (both P<0.05).
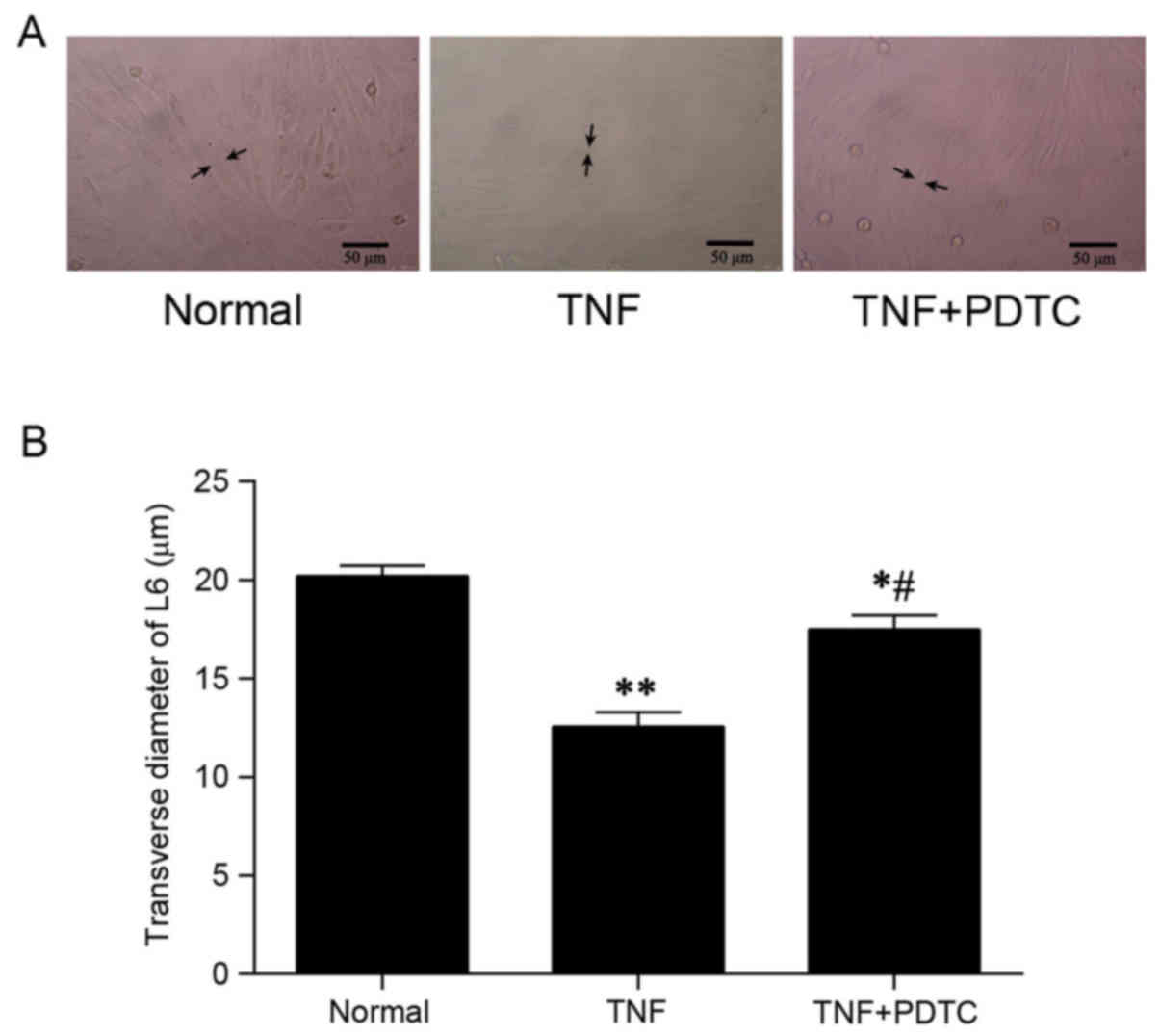
PDTC prevents cell atrophy in
vitro
Inverted fluorescent microscopy demonstrated that L6
rat myoblasts treated with TNF-α alone were atrophic compared with
normal control cells, while cell sizes in the TNF-α + PDTC
treatment group appeared unaffected (Fig. 5A). In addition, relative to the TNF-α
treatment group, the transverse diameters of the L6 myoblasts in
the PDTC + TNF-α group were significantly increased. In comparison
with the normal control group, TNF+PDTF group had a significantly
reduced transverse diameter (~90% relative to control group;
P<0.05; Fig. 5B).
Discussion
The results of the current study indicated that APS,
a component of traditional Chinese medicine, may protect muscle
cells in vivo and in vitro from atrophy associated
with CRF (cachexia). Other studies have reported that traditional
Chinese medicine have beneficial effect on treating CRF (19,20). Li
et al (21) reported that
icariin-treated human umbilical cord mesenchymal stem cells could
improve kidney function via reduced inflammatory responses and
oxidative damage in CRF rats. Zhang et al (22) indicated that Shenkang granules
ameliorate renal injury in a rat model of CRF through preventing
the accumulation of extracellular matrix, by decreasing the
expression of collagen I and III and inhibiting the expression of
matrix metalloproteinases-2 and −9 in the renal tissue. In the
present study, it was observed that APS reduced the expression of
atrogin-1 and ubiquitin in vivo and reversed muscle cell
atrophy following TNF-α pretreatment, while the NF-κB inhibitor
PDTC had similar effects in vitro. These results suggest
that APS may target atrogin-1 through inhibitory effects on the
NF-κB pathway, leading to reductions in ubiquitin expression and
reduced muscle cell atrophy. In addition, compared with other
investigations of traditional Chinese medicine in CRF, the
protective effect was via a different mechanism, suggesting a
combined therapeutic strategy for CRF may be effective.
Previous results support the use of the subtotal
nephrectomy CRF rat model, in which an impairment of glomerular
filtration rate and disturbances in calcium and phosphate
metabolism have been observed (23).
Clinical research has also indicated that cell atrophy is prevalent
in patients with CRF and is associated with the progression of
renal failure (1,2). One of the classic treatments for CRF is
the use of ketoacid analogs, and the current study compared the
administration of APS and the use of ketoacid analog KT. A similar
protective effect was observed in both groups, supporting the
proposal that APS may be used to treat CRF.
CRF is often associated with cachexia. It has been
demonstrated that serum obtained from patients with CRF activates
the UPP by a currently unknown mechanism (10,24). The
UPP and protein degradation is the primary mechanism by which NF-κB
signaling is regulated within skeletal muscle during CRF (10,25,26).
Furthermore, recent in vitro studies, animal models and
human studies have indicated that upregulation of NF-κB has a
pathogenic role in mediating chronic inflammation during chronic
renal disease (25,27). In the present study, a state of cell
malnutrition (cachexia) was established by pretreating rat L6
myoblasts with TNF-α. The results indicated that APS protected
cells from atrophy related to CRF (cachexia) via reducing the
expression of atrogin-1 and ubiquitin. This effect was similar to
direct inhibition of atrogin-1 using atrogin-1 siRNA.
Of particular relevance to the present study are the
recent findings that a combination of APS and another traditional
Chinese medicine, rhein, may alleviate the pathologies of CRF,
including functional damage to the glomeruli, interstitial
inflammation and the apoptosis of renal tubular cells (8).
Further clinical studies are warranted to confirm
the preliminary findings obtained from the in vitro and
in vivo models in the current study. In future studies, the
limitations of the present study need to be resolved, including
side-effect and dose-dependency evaluation, as well as time point
studies. It may also be useful to identify and develop components
of the UPP as serological markers in CRF patients, particularly
those being treated with traditional Chinese medicines such as APS,
since these will be important for developing therapeutic strategies
for patients with CRF.
In conclusion, APS may delay the progression of
muscle cell atrophy associated with malnutrition in CRF, possibly
by targeting the UPP and its downstream effector atrogin-1.
Acknowledgements
The authors wish to thank Shanghai GeneChem Co.,
Ltd., (Shanghai, China) for their assistance in gene-silencing
technology. The present study was supported by the National Natural
Science Foundation of China (grant no. 81173457).
References
|
1
|
Dane MJ, Khairoun M, Lee DH, van den Berg
BM, Eskens BJ, Boels MG, van Teeffelen JW, Rops AL, van der Vlag J,
van Zonneveld AJ, et al: Association of kidney function with
changes in the endothelial surface layer. Clin J Am Soc Nephrol.
9:698–704. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thomas R, Kanso A and Sedor JR: Chronic
kidney disease and its complications. Primary Care. 35:329–344vii.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seveso M, Grizzi F, Bozzini G, Mandressi
A, Guazzoni G and Taverna G: Open partial nephrectomy: Ancient art
or currently available technique? Int Urol Nephrol. 47:1923–1932.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Windecker S, Tijssen J, Giustino G,
Guimarães AH, Mehran R, Valgimigli M, Vranckx P, Welsh RC, Baber U,
van Es GA, et al: Trial design: Rivaroxaban for the prevention of
major cardiovascular events after transcatheter aortic valve
replacement: Rationale and design of the GALILEO study. Am Heart J.
184:81–87. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fan Q, Chen M, Zuo L, Shang X, Huang MZ,
Ciccarelli M, Raake P, Brinks H, Chuprun KJ, Dorn GW II, et al:
Myocardial ablation of g protein-coupled receptor kinase 2 (GRK2)
decreases ischemia/reperfusion injury through an anti-intrinsic
apoptotic pathway. PLoS One. 8:e662342013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Piccoli GB, Leone F, Attini R, Parisi S,
Fassio F, Deagostini MC, Ferraresi M, Clari R, Ghiotto S, Biolcati
M, et al: Association of low-protein supplemented diets with fetal
growth in pregnant women with CKD. Clin J Am Soc Nephrol.
9:864–873. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Piccoli GB, Ferraresi M, Deagostini MC,
Vigotti FN, Consiglio V, Scognamiglio S, Moro I, Clari R, Fassio F,
Biolcati M and Porpiglia F: Vegetarian low-protein diets
supplemented with keto analogues: A niche for the few or an option
for many? Nephrol Dial Transplant. 28:2295–2305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lian Y, Xie L, Chen M and Chen L: Effects
of an astragalus polysaccharide and rhein combination on apoptosis
in rats with chronic renal failure. Evid Based Complement Alternat
Med. 2014:2718622014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li YW, Zhang Y, Zhang L, Li X, Yu JB,
Zhang HT, Tan BB, Jiang LH, Wang YX, Liang Y, et al: Protective
effect of tea polyphenols on renal ischemia/reperfusion injury via
suppressing the activation of TLR4/NF-κB p65 signal pathway. Gene.
542:46–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mitch W: Mechanisms accelerating muscle
atrophy in catabolic diseases. Trans Am Clin Climatol Assoc.
111:269–270. 2000.
|
|
11
|
Ha YM, Chung SW, Kim JM, Kim DH, Kim JY,
Lee EK, Lee J, Kim YJ, Yoo MA, Jeong KS and Chung HY: Molecular
activation of NF-kappaB, pro-inflammatory mediators, and signal
pathways in gamma-irradiated mice. Biotechnol Lett. 32:373–378.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bodine S, Latres E, Baumhueter S, Lai VK,
Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K,
et al: Identification of ubiquitin ligases required for skeletal
muscle atrophy. Science. 294:1704–1708. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gomes MD, Lecker SH, Jagoe RT, Navon A and
Goldberg AL: Atrogin-1, a muscle-specific F-box protein highly
expressed during muscle atrophy. Proc Natl Acad Sci USA. 98:pp.
14440–14445. 2001; View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fleck C, Appenroth D, Jonas P, Koch M,
Kundt G, Nizze H and Stein G: Suitability of 5/6 nephrectomy
(5/6NX) for the induction of interstitial renal fibrosis in
rats-influence of sex, strain, and surgical procedure. Exp Toxicol
Pathol. 57:195–205. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dekelbab BH, Witchel SF and DeFranco DB:
TNF-alpha and glucocorticoid receptor interaction in L6 muscle
cells: A cooperative downregulation of myosin heavy chain.
Steroids. 72:705–712. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun H, Qiu J, Chen Y, Yu M, Ding F and Gu
X: Proteomic and bioinformatic analysis of differentially expressed
proteins in denervated skeletal muscle. Int J Mol Med.
33:1586–1596. 2014.PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Manickam P, Kaushik A, Karunakaran C and
Bhansali S: Recent advances in cytochrome c biosensing
technologies. Biosens Bioelectron. 87:654–668. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peng M, Cai P, Ma H, Meng H, Xu Y, Zhang X
and Si G: Chinese herbal medicine Shenqi Detoxification Granule
inhibits fibrosis in adenine induced chronic renal failure rats.
Afr J Tradit Complement Altern Med. 11:194–204. 2013.PubMed/NCBI
|
|
20
|
Lu JR, Han HY, Chen J, Xiong CX, Wang XH,
Hu J, Chen XF and Ma L: Protective effects of Bu-Shen-Huo-Xue
formula against 5/6 nephrectomy-induced chronic renal failure in
rats. Evid Based Complement Alternat Med. 2014:5898462014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li W, Wang L, Chu X, Cui H and Bian Y:
Icariin combined with human umbilical cord mesenchymal stem cells
significantly improve the impaired kidney function in chronic renal
failure. Mol Cell Biochem. 428:203–212. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang YU, Zhou N, Wang H, Wang S and He J:
Effect of Shenkang granules on the progression of chronic renal
failure in 5/6 nephrectomized rats. Exp Ther Med. 9:2034–2042.
2015.PubMed/NCBI
|
|
23
|
Oehring H, Widder J, Appenroth D,
Jirikowski GF and Fleck C: Ultrastructural and
ultraimmunohistochemical changes upon partial nephrectomy and
uranyl intoxication in the rat kidney. Exp Toxicol Pathol.
65:441–449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carbó C, Arderiu G, Escolar G, Fusté B,
Cases A, Carrascal M, Abián J and Díaz-Ricart M: Differential
expression of proteins from cultured endothelial cells exposed to
uremic versus normal serum. Am J Kidney Dis. 51:603–612. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rangan G, Wang Y and Harris D: NF-kappaB
signalling in chronic kidney disease. Front Biosci (Landmark Ed).
14:3496–3522. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang YQ, Feng B and Yuan FH: Effect of
chronic renal failure medium on the ubiquitin-proteasome pathway of
arterial muscle cells. Mol Med Rep. 7:1021–1025. 2013.PubMed/NCBI
|
|
27
|
Mohammed-Ali Z, Cruz GL and Dickhout JG:
Crosstalk between the unfolded protein response and NF-κB-mediated
inflammation in the progression of chronic kidney disease. J
Immunol Res. 2015:4285082015. View Article : Google Scholar : PubMed/NCBI
|