Introduction
Steroids are useful drugs that can be applied in
numerous serious diseases due to their ability to profoundly affect
the disease course, including improvement of symptoms and reduction
of disease duration (1). However,
high dosages and prolonged use of steroids induces high incidence
of complications and side effects, leading to severe consequences
(2). Steroid-induced avascular
necrosis of the femoral head (SANFH) is the most commonly reported
steroid-associated osteonecrosis complication (3). SANFH is mainly bilateral and involves
extensive necrosis, frequently occurring in young and middle-aged
individuals, and resulting in a high disability rate (4). Numerous theories have been proposed
concerning the pathogenesis of SANFH, including osteoporosis,
cumulative osteocyte dysfunction, enlarged fat cells and fat
embolism (5). However, the exact
mechanism remains incompletely understood.
Autophagy is a complex and evolutionarily conserved
process, in which abnormal cellular proteins and organelles are
engulfed in autophagosomes and fused with lysosomes, forming
autolysosomes (6). The fundamental
function of autophagy is to maintain the metabolic balance in the
cell, since autophagy is able to produce essential nutrients such
as amino acids under various stress conditions, including nutrient
starvation, oxidative stress and endoplasmic reticulum stress
(7). Autophagy is beneficial for
repair after injury; however, it is also known to also have
unfavorable results. For instance, the physiological levels of
autophagy are favorable to neuronal survival, but excessive or
inadequate levels can be harmful and cause injury. In addition, the
degree of autophagy is critical in ischemic stroke (8). In the field of cancer research,
autophagy has positive and negative effects in tumorigenesis and
serve an important role in the resistance of cancer cells to
chemotherapy (9). Recently, studies
have focused on the role of autophagy in the bone. For examples,
Liu et al observed that the natural flavonoid
isoliquiritigenin decreased microtubule-associated protein 1 light
chain 3 (LC3) and Beclin 1 accumulation, suppressed autophagy and
exerted anti-osteoclastogenic effects (10). Yang et al also identified that
autophagy protected osteoblasts from apoptosis induced by advanced
glycation end products through the intracellular reactive oxygen
species pathway (11). However, the
degree of autophagy activity in SANFH remains unclear.
Glucocorticoids, such as dexamethasone (DXM), may
regulate the autophagy activity. A previous study indicated that
autophagy may be induced by DXM in chondrocyte cells, associated
with a reduction in cell viability (12). Furthermore, DXM stimulates an early
activation of autophagy in L6 myotubes, regulating the muscle
atrophy program (13). To the best
of our knowledge, it is uncertain whether DXM induces autophagy in
osteoblast, or what the effect of autophagy is in SANFH.
The aim of the present study was to investigate the
autophagy activity in the femoral head of SANFH patients. In
vitro, a mouse osteoblast was used to investigate the effect of
DXM-induced autophagy on proliferation, integrity and
differentiation. It was demonstrated that the degree of autophagy
activity in the femoral head was overactivated, while inhibition of
autophagy attenuated DXM-induced cell injury in osteoblasts in
vitro.
Materials and methods
Hematoxylin and eosin (H&E)
staining and immunohistochemical (IHC) analysis of femoral head
samples
The protocol described herein were approved by the
Ethics Reviewing Council of Xiangya Hospital (Changsha, China). A
total of 6 femoral heads were collected from SANFH patients (4
males and 2 females; 64.3±6.4 years), and 8 femoral heads from
individuals undergoing total hip replacements (5 males and 3
females, 61.5±8.7 years) were collected as the control group
(between May 2013 and December 2013; Xiangya Hospital). All
patients signed an informed consent approved by the Institutional
Review Board of Xiangya Hospital. All harvested femoral head
samples were decalcified for 14 days in 10% EDTA (pH 7.4),
dehydrated and subsequently embedded in paraffin. The samples were
cut into 5-µm sections and stained with H&E at room temperature
to investigate the morphologic changes of femoral head from SANFH
patients. Subsequently, autophagy in the sections was evaluated by
IHC staining with a Beclin 1 primary antibody (sc-11427; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA; dilution, 1:100) applied
overnight at 4°C.
Cell culture
Mouse osteoblast MC3T3-E1 cell line was purchased
from the Institute of Cell Bank for Biological Sciences (Shanghai,
China). The cells were cultured in Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal-calf-serum (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 mg/l streptomycin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The cells were
incubated at 37°C in a humidified atmosphere with 5% CO2.
Cell proliferation assay
The MC3T3-E1 cells were seeded into 96-well culture
plates (5×103 cells/well) and treated with DXM (0.01,
0.1, 1 and 10 µmol/l; cat. no. D1756; Sigma-Aldrich; Merck KGaA)
with or without an autophagy inhibition (3-methyladenine; 3-MA; 2.5
mM; Sigma-Aldrichl Merck KGaA), for 24 or 48 h. Next, 10 µl cell
counting kit-8 reagent (CK04; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) was added to each well and incubated for 40 min.
The viability of the cells was measured at 450 nm using a
microplate reader (SpectraMax 250; GE Healthcare Life Sciences,
Chalfont, UK). Cell viability (%) was calculated based on the
optical density (OD) values, as follows: (OD of treated sample -
blank)/(OD of control sample-blank) × 100.
Collection of supernatant of cell
treatment
The MC3T3-E1 cells were seeded into 6-well culture
plates (1×106 cells/well) and treated with DXM (0.01,
0.1, 1 and 10 µmol/l) with or without 3-MA for 24 or 48 h at 37°C.
The cell culture medium was subsequently centrifuged at 2,500 × g
for 10 min at 4°C. The supernatant was collected for analysis.
Lactate dehydrogenase (LDH) activity
assay
Cell injury was evaluated using the LDH Cytotoxicity
Assay kit (C0016; Beyotime Institute of Biotechnology, Haimen,
China). Cells were seeded in 96-well culture plates
(1×104 cells/well) and the LDH activity was determined
following the protocol provided by the kit's manufacturer. The
absorbance of the samples at 490 nm was measured using a microplate
reader (SpectraMax 250; GE Healthcare Life Sciences).
Alkaline phosphatase (ALP)
activity
Cells were seeded into 12-well plates at a density
of 5×104 cells per well. Subsequent to culturing for 24
h or 48 h at 37°C, osteogenic differentiation of the cells was
measured on the basis of ALP activity using an ALP assay kit
(Sigma-Aldrich; Merck KGaA).
Protein extraction and western blot
analysis
Total proteins were isolated from cells using
radioimmunoprecipitation assay lysis buffer (P0013; Beyotime
Institute of Biotechnology). A BCA protein assay (Thermo Fisher
Scientific, Inc.) was adapted to measure the total protein
concentration in the samples. Equal amounts of proteins (40 µg)
were separated by 10% SDS-PAGE (Amresco, LLC, Solon, OH, USA) and
transferred onto polyvinylidene difluoride membranes (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China). The
membranes were then blocked with 5% w/v non-fat dried milk
dissolved in Tris-buffered saline and 0.1% Tween-20 (pH 8.3;
Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) for 1 h.
Subsequently, the membranes were incubated with primary antibodies
at 4°C overnight, following standard procedures. The primary
antibodies used were: Bone morphogenetic protein-2 (BMP-2; sc-9003;
1:1,000), Beclin 1 (sc-11427; 1:1,000) and LC3 (sc-292354; 1:1,000;
all from Santa Cruz Biotechnology, Inc.). β-actin was used as an
internal control (SAB5500001, Sigma-Aldrich; Merck KGaA; 1:7,500).
Samples were then incubated with horseradish peroxidase-conjugated
anti-rabbit IgG secondary antibody (A0545, Sigma-Aldrich; Merck
KGaA; 1:1,000) at room temperature. Protein band intensities were
quantified using Quantity One® software (version 4.62;
Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation
and were analyzed using one-way analysis of variance. Statistical
analyses were performed using SPSS version 19.0 for Windows (IBM
SPSS, Armonk, NY, USA). For all tests, differences with P<0.05
were considered as statistically significant. Each experiment was
repeated at least three times.
Results
Autophagy is overactivated in
SANFH
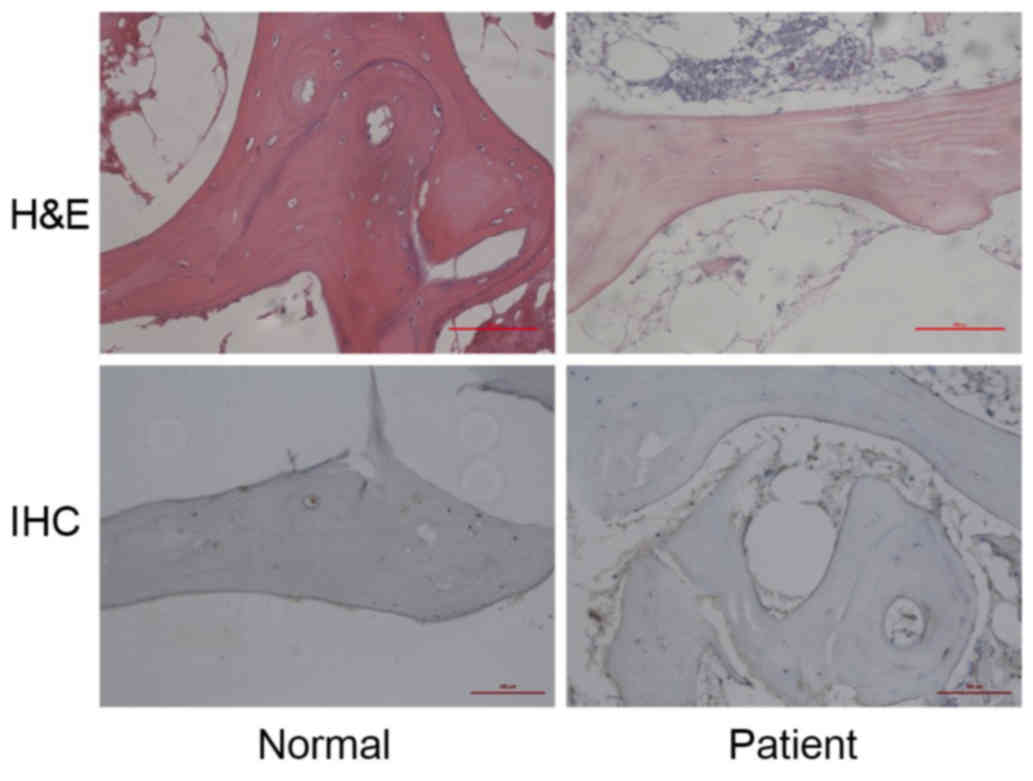
In the control group, there were rich hematopoietic
cells and relatively fewer lipocytes. The bone trabeculas were
regularly arrayed, with complete structure and clearly visible
osteocytes. However, in the patient group, H&E staining
demonstrated bone marrow structure disturbance, marrow cell
necrosis and debris assembly (Fig.
1, upper images). Subsequently, Beclin 1 expression in the
femoral head sections was detected by IHC in order to evaluate the
autophagy activity. It was observed that Beclin 1 expression was
upregulated in the patient group when compared with the normal
group (Fig. 1, lower images). These
results indicate that overactivated autophagy may be associated
with the pathology of SANFH.
DXM impairs the proliferation,
integrity and differentiation of mouse osteoblasts
DXM is one of the most commonly used steroids in
clinical practice and experimental research. Various concentrations
of DXM (0.01, 0.1, 1 and 10 µmol/l) were added to MC3T3-E1
osteoblast cultures for 24 or 48 h. The results indicated that DXM
inhibited the proliferation of osteoblasts in a dose-depended
manner (Fig. 2A and B). In addition,
the inhibition ratio of DXM was significantly higher at 48 h in
comparison with that after 24-h incubation (P<0.05; Fig. 2C).
LDH is released from the cells when the cellular
integrity is damaged, thus the LDH release from osteoblasts was
also investigated in the present study. LDH activation in the
supernatant of osteoblasts was significantly increased after DXM
treatment (0.1, 1 and 10 µmol/l) for 24 h, and this increase was
more apparent following DXM treatment for 48 h (Fig. 2D and E).
ALP activity and BMP-2 expression are typically used
as the markers of osteoblast differentiation. In the current study,
DXM was not found to affect the ALP activity after treatment for 24
h; however, ALP activity was significantly reduced after treatment
with DXM (0.01, 0.1, 1 and 10 µmol/l) for 48 h (Fig. 2F and G). Furthermore, the BMP-2
expression was also significantly decreased after treatment with
DXM (1 and 10 µmol/l) for 48 h (Fig.
3). These results indicate that DXM treatment impairs the
proliferation, integrity and differentiation of mouse
osteoblasts.
DXM triggers autophagy in
osteoblasts
Subsequently, the present study investigated the
potential mechanism underlying the osteoblast injury induced by
DXM. As the autophagy is overactivated in SANFH, it was presumed
that DXM triggers autophagy contributing to osteoblast injury.
Beclin 1 and LC3 were selected as the markers for autophagy. The
results revealed that DXM (1 and 10 µmol/l) significantly increased
Beclin 1 and LC3 expression levels after treatment for 24 h
(P<0.05), whereas lower concentrations of DXM (0.01 and 0.1
µmol/l) had no marked effect (P>0.05). However, after treatment
for 48 h, 0.1 µmol/l DXM treatment also significantly increased
Beclin 1 and LC3 expression levels (P<0.05; Fig. 4). These results indicate that DXM
triggers autophagy in osteoblasts in dose- and time-depended
manners.
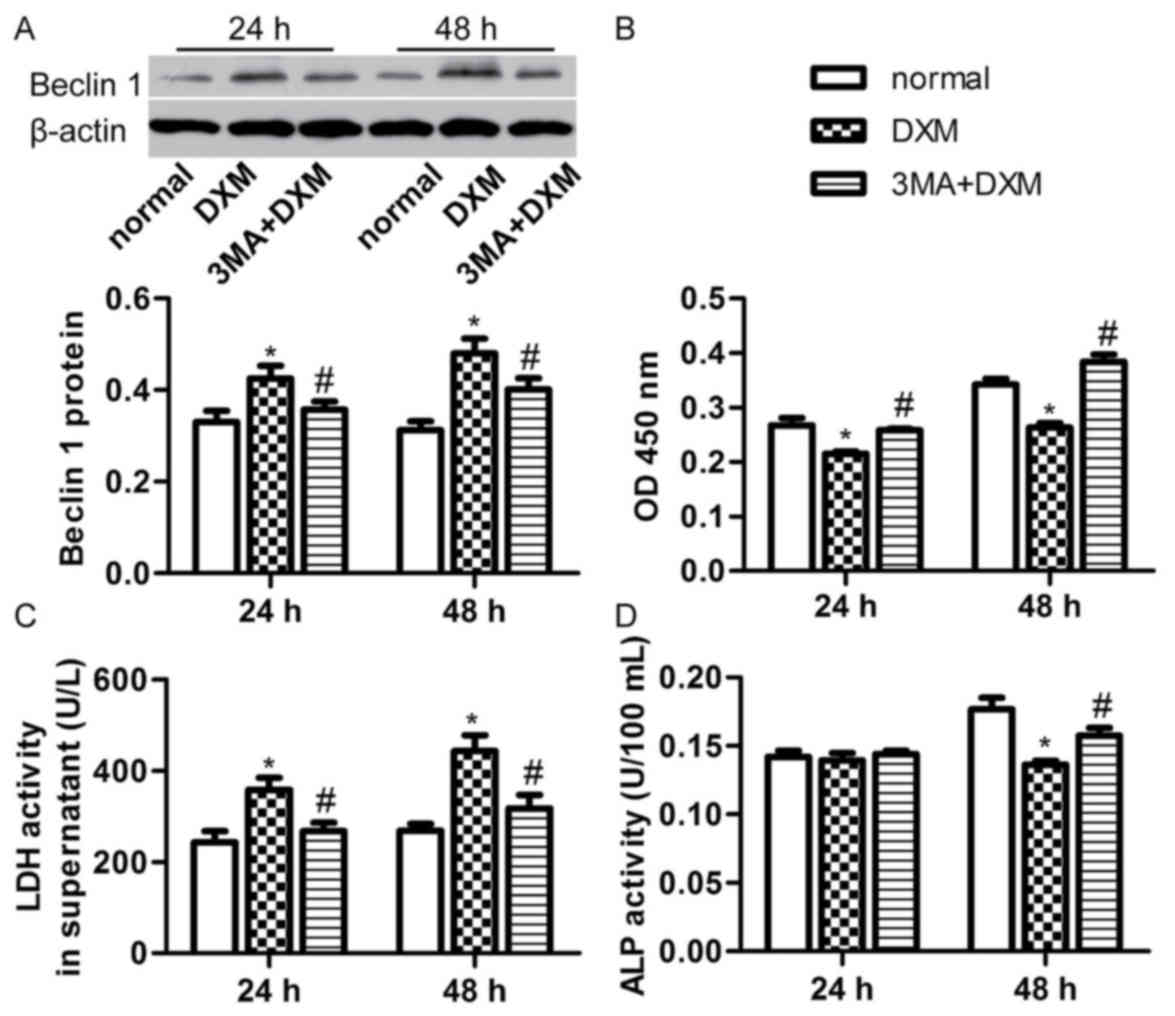
Inhibition of autophagy rescues
osteoblast cell injury induced by DXM
Since DXM was found to induce osteoblast cell injury
and trigger autophagy, the current study further investigated
whether inhibiting autophagy rescues from cell injury induced by
DXM. Thus, 2.5 mM 3-methyladenine (3-MA) was added to inhibit
autophagy. The 3-MA treatment significantly decreased the DXM (1
µmol/l)-induced Beclin 1 expression after 24 and 48 h incubation
(P<0.05; Fig. 5A). In addition,
the number of osteoblasts was evidently increased in the 3-MA + DXM
group compared with the DXM alone group at the two time points
(P<0.05; Fig. 5B). 3-MA also
reduced the LDH activity in the supernatant of osteoblasts treated
with DXM (1 µmol/l) for 24 and 48 h (P<0.05; Fig. 5C), while it significantly increased
the ALP activity of osteoblasts treated with DXM (1 µmol/l) for 48
h (P<0.05; Fig. 5D). These
results indicate that inhibition of autophagy with 3-MA is able to
rescue cell proliferation, integrity and differentiation of
osteoblasts induced by DXM.
Discussion
The present study revealed that autophagy was
overactivated in SANFH samples. In addition, DXM was demonstrated
to trigger autophagy, as well as to decrease cell proliferation,
cell integrity and differentiation of osteoblasts in dose- and
time-depended manners. Inhibition of autophagy with 3-MA was shown
to rescue from osteoblast cell injury induced by DXM. To the best
of our knowledge, this is the first study reporting that
overactivated autophagy is a mechanism underlying osteoblast loss
in SANFH.
SANFH is a progressive disease with bone marrow cell
and osteocyte death, resulting in collapse of the femoral head.
Osteonecrosis can result in debilitation and adversely affect the
quality of life, frequently requiring surgical intervention. Thus
far, there are no effective preventive measures for SANFH (14). Although multiple theories underlying
this complication have been proposed, no pathophysiologic mechanism
has been identified as the etiology for the development of
osteonecrosis of the femoral head (15). In the present study, the IHC results
revealed that Beclin 1 protein expression was increased in the
femoral head of SANFH patients, indicating that autophagy was
overactivated. Autophagy is an evolutionarily conserved mechanism
that links to several cellular pathways (16). An increasing number of studies
support that autophagy can have both positive and negative effects
in various diseases, such as in anti-angiogenesis therapy (17), the development of cancer (18) and cerebral ischemia (8). However, it remains unclear whether or
not overactivated autophagy serves a beneficial role in SANFH.
Glucocorticoid administration is often overlooked as
the most common cause of nontraumatic osteonecrosis.
Glucocorticoids are a class of corticosteroids that are prescribed
for numerous diseases, including rheumatoid arthritis (19), septic shock (20) and IgA nephropathy (21). However, high-dose or abnormal use of
glucocorticoids results in bone disease. DXM is one of the most
commonly administered glucocorticoids in clinical practice. The
current study observed that DXM inhibited the cell proliferation of
osteoblasts, increased the LDH activity in the supernatant, and
decreased the ALP activity and BMP-2 expression in osteoblasts.
These results indicate that DXM is an injury factor in osteoblasts.
Similarly, Ding et al demonstrated that DXM induced
apoptosis of osteocytic and osteoblastic cells via activating TAK1
(22), which supports the findings
of the present study. The current results also revealed that DXM
triggered autophagy in osteoblasts; therefore, in consideration of
the overactivated autophagy in the femoral head, it is presumed
that autophagy serves an important role in DXM-induced osteoblast
cell injury. Finally, incubation of osteoblasts with 3-MA, an
inhibitor of autophagy (23),
resulted in decreased expression of Beclin 1, while it also rescued
the cell proliferation, integrity and differentiation of
osteoblasts induced by DXM.
However, the effect of autophagy in DXM-induced
osteonecrosis remain controversial. Shen et al observed that
DXM induced apoptosis in chondrocytes, and autophagy protected
chondrocytes from glucocorticoids-induced apoptosis via
ROS/Akt/FOXO3 signaling (24). Zhao
et al also demonstrated that high doses of DXM reduce the
ATDC5 chondrocyte cell viability by inducing autophagy (25). Therefore, in the current study, it is
hypothesized that the degree of autophagy determines the protective
or adverse role in DXM-induced cell injury.
In conclusion, the present study revealed that
autophagy was overactivated in the femoral head of SANFH patients,
while DXM treatment impaired the cell proliferation, integrity and
differentiation of osteoblasts in vitro. Finally, inhibiting
autophagy with 3-MA attenuated the cell injury induced by DXM
treatment. Thus, autophagy may be a potential target for the
prevention of SANFH.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81201420, 81272034,
81472130, 81402224 and 81501923), the Provincial Science Foundation
of Hunan (nos. 14JJ3032 and 2015JJ3139), the Scientific Research
Project of the Development and Reform Commission of Hunan Province
[no. (2013) 1199], the Scientific Research Project of Science and
Technology Office of Hunan Province (no. 2013SK2018), the Health
and Family Planning Commission of Hunan Province (B2014-12), the
Administration of Traditional Chinese Medicine of Hunan Province
(no. 2015116), the China Scholarship Council (student ID:
201606375101), and the Doctoral Scientific Fund Project of the
Ministry of Education of China (no. 20120162110036).
References
|
1
|
Kozlov N, Benzon HT and Malik K: Epidural
steroid injections: Update on efficacy, safety, and newer
medications for injection. Minerva Anestesiol. 81:901–909.
2015.PubMed/NCBI
|
|
2
|
Póvoa P and Salluh JI: What is the role of
steroids in pneumonia therapy? Curr Opin Infect Dis. 25:199–204.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang SL, Jiao J and Yan HW: Hydrogen-rich
saline attenuates steroid-associated femoral head necrosis through
inhibition of oxidative stress in a rabbit model. Exp Ther Med.
11:177–182. 2016.PubMed/NCBI
|
|
4
|
Fukushima W, Fujioka M, Kubo T, Tamakoshi
A, Nagai M and Hirota Y: Nationwide epidemiologic survey of
idiopathic osteonecrosis of the femoral head. Clin Orthop Relat
Res. 468:2715–2724. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kerachian MA, Cournoyer D, Harvey EJ, Chow
TY, Bégin LR, Nahal A and Séguin C: New insights into the
pathogenesis of glucocorticoid-induced avascular necrosis:
Microarray analysis of gene expression in a rat model. Arthritis
Res Ther. 12:R1242010. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang C, Hu Q and Shen HM: Pharmacological
inhibitors of autophagy as novel cancer therapeutic agents.
Pharmacol Res. 105:164–175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li M, Gao P and Zhang J: Crosstalk between
Autophagy and Apoptosis: Potential and emerging therapeutic targets
for cardiac diseases. Int J Mol Sci. 17:3322016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen W, Sun Y, Liu K and Sun X: Autophagy:
A double-edged sword for neuronal survival after cerebral ischemia.
Neural Regen Res. 9:1210–1216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang M, Zeng P, Kang R, Yu Y, Yang L, Tang
D and Cao L: S100A8 contributes to drug resistance by promoting
autophagy in leukemia cells. PLoS One. 9:e972422014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu S, Zhu L, Zhang J, Yu J, Cheng X and
Peng B: Anti-osteoclastogenic activity of isoliquiritigenin via
inhibition of NF-κB-dependent autophagic pathway. Biochem
Pharmacol. 106:82–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang L, Meng H and Yang M: Autophagy
protects osteoblasts from advanced glycation end products-induced
apoptosis through intracellular reactive oxygen species. J Mol
Endocrinol. 56:291–300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao Y, Zuo Y, Huo HJ, Xiao YL, Yang XJ
and Xin DQ: Glucocorticoid induced autophagy in N1511 chondrocyte
cells. Eur Rev Med Pharmacol Sci. 18:3573–3579. 2014.PubMed/NCBI
|
|
13
|
Troncoso R, Paredes F, Parra V, Gatica D,
Vásquez-Trincado C, Quiroga C, Bravo-Sagua R, López-Crisosto C,
Rodriguez AE, Oyarzún AP, et al: Dexamethasone-induced autophagy
mediates muscle atrophy through mitochondrial clearance. Cell
Cycle. 13:2281–2295. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hao C, Yang S, Xu W, Shen JK, Ye S, Liu X,
Dong Z, Xiao B and Feng Y: MiR-708 promotes steroid-induced
osteonecrosis of femoral head, suppresses osteogenic
differentiation by targeting SMAD3. Sci Rep. 6:225992016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mont MA, Cherian JJ, Sierra RJ, Jones LC
and Lieberman JR: Nontraumatic Osteonecrosis of the Femoral Head:
Where Do We Stand Today? A Ten-Year Update. J Bone Joint Surg Am.
97:1604–1627. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tai S, Hu XQ, Peng DQ, Zhou SH and Zheng
XL: The roles of autophagy in vascular smooth muscle cells. Int J
Cardiol. 211:1–6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu J, Fan L, Wang H and Sun G: Autophagy,
a double-edged sword in anti-angiogenesis therapy. Med Oncol.
33:102016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zarzynska JM: The importance of autophagy
regulation in breast cancer development and treatment. Biomed Res
Int. 2014:7103452014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mackie SL, Pease CT, Fukuba E, Harris E,
Emery P, Hodgson R, Freeston J and McGonagle D: Whole-body MRI of
patients with polymyalgia rheumatica identifies a distinct subset
with complete patient-reported response to glucocorticoids. Ann
Rheum Dis. 74:2188–2192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cohen J, Pretorius CJ, Ungerer JP,
Cardinal J, Blumenthal A, Presneill J, Gatica-Andrades M, Jarrett
P, Lassig-Smith M, Stuart J, et al: Glucocorticoid Sensitivity Is
Highly Variable in Critically Ill Patients With Septic Shock and Is
Associated With Disease Severity. Crit Care Med. 44:1034–1041.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ayoub I, Hebert L and Rovin BH: Intensive
Supportive Care plus Immunosuppression in IgA Nephropathy. N Engl J
Med. 374:991–993. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ding H, Wang T, Xu D, Cha B, Liu J and Li
Y: Dexamethasone-induced apoptosis of osteocytic and osteoblastic
cells is mediated by TAK1 activation. Biochem Biophys Res Commun.
460:157–163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li B, Duan P, Li C, Jing Y, Han X, Yan W
and Xing Y: Role of autophagy on bone marrow mesenchymal stem-cell
proliferation and differentiation into neurons. Mol Med Rep.
13:1413–1419. 2016.PubMed/NCBI
|
|
24
|
Shen C, Cai GQ, Peng JP and Chen XD:
Autophagy protects chondrocytes from glucocorticoids-induced
apoptosis via ROS/Akt/FOXO3 signaling. Osteoarthritis Cartilage.
23:2279–2287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao Y, Zuo Y, Huo H, Xiao Y, Yang X and
Xin D: Dexa-methasone reduces ATDC5 chondrocyte cell viability by
inducing autophagy. Mol Med Rep. 9:923–927. 2014.PubMed/NCBI
|