Introduction
Septic arthritis of the temporomandibular joint
(SATMJ) is a rarely reported disease characterized by pain, fever,
swelling and even loss of TMJ function. In the past decades, only a
few dozen cases of SATMJ have been reported; however, an increasing
number of cases of SATMJ in children and adults have been reported
in recent years (1–4). The disease predominantly occurs in male
adults, the mortality rate of SATMJ is as high as 12%, and up to
75% of survivors develop significant functional disability in the
involved joints (5). The etiologies
of SATMJ include general and local predisposing factors. Certain
systemic and autoimmune diseases may cause septic arthritis, such
as rheumatoid arthritis, diabetes, systemic lupus erythematous and
hypogammaglobulinemia (6–8). On the other hand, direct extension of
head and neck infection, local trauma and burn wounds affecting the
TMJ obviously increase the morbidity of SATMJ (9–12).
Studies have reported that iatrogenic causes, such as third molar
abstraction and distraction osteogenesis, also lead to SATMJ
(13,14). On a microbiological level, the
pathogenesis of SATMJ involves hematogenous dissemination of
causative microorganisms or direct extension of a contiguous
infection (15), and the infection
was often caused by bacteria including Staphylococcus aureus,
Neisseria gonorrhea, Streptococcus and Aspergillus
flavus (6,16–19).
Although no criteria have been established on the diagnosis and no
consensus has been reached on the management of SATMJ, most studies
employed aspiration and analysis of TMJ fluid, blood chemistry,
imaging and clinical examination are for its diagnosis and
treatment. Failure to promptly treat SATMJ may result in
irreversible damage of the TMJ structure, leading to long-term
complications that result in impaired joint mobility and function,
including bone deformity, fibrosis and ankyloses (15,17,20,21).
The present study reported a case of acute and
severe SATMJ with pyogenic orofacial infections, which was not
improved after abscess incision drainage and antibiotic treatment.
Finally, the clinical signs were resolved by condylectomy and
debridement of the erosive septic TMJ, which completely restored
the function of the TMJ. A review of the literature associated with
this topic was also performed.
Case presentation
History
A non-immunocompromised 83-year-old woman presented
at the Department of Stomatology of the First Affiliated Hospital
of Dalian Medical University (Dalian, China) on October 14th 2014,
with a chief complaint of pain and swelling in the right buccal,
temporal, parapharyngeal and pre-auricular regions for twenty days,
including increasing difficulty opening her mouth. She had smoked
for 60 years and was still smoking on admission, but was otherwise
generally healthy. The patient reported that she initially had a
pustule with a slight swelling in the skin of the inside of the
right cheek; she then sought medical attention and was treated with
antibiotics at another hospital. However, ten days later, the
swelling subsequently aggravated and radiated to the right temporal
and pre-auricular region, alongside which she presented with
progressing dysphagia. Prior to the onset of the disease, the
patient did not feel any spontaneous pain in the tooth or residual
roots.
Clinical examination
The physical examination revealed a severe,
ill-defined swelling with tenderness in the right-side orofacial
region, mainly centering over the right buccal, pre-auricular and
temporal regions, which was only mildly warm and erythematous, and
which limited the patient's ability to open her mouth to 10–15 mm
(Fig. 1). A fistula with pyorrhea
was observed in the right cheek. Upon opening of the mouth, a
tumescent palatoglossal arch was also found. Pitting edema were
distributed over the patient's bilateral lower legs. The patient's
vital signs were as follows: Body temperature, 37.1°C; heart rate,
88 beats per minute; blood pressure, 140/80 mmHg; and oxygen
saturation, 98% in room air. A blood culture was obtained.
Hematological values were as follows: White blood cell count,
7,370/µl; neutrophils, 5,520/µl; hemoglobin, 98 g/dl; platelet
count, 399,000/µl; albumin, 31.5 g/l; globulin, 37.9 g/l; and a
reverse albumin/globulin ratio. All other laboratory values were
within normal limits. In order to confirm the diagnosis, further
investigation was pursued. Computed tomography (CT) scans of the
craniofacial region revealed a destructive soft tissue mass,
involving the articular eminence and condylar head. In addition,
abscesses were seen in the right buccal and temporal spaces as well
as parapharyngeal spaces (Fig. 2).
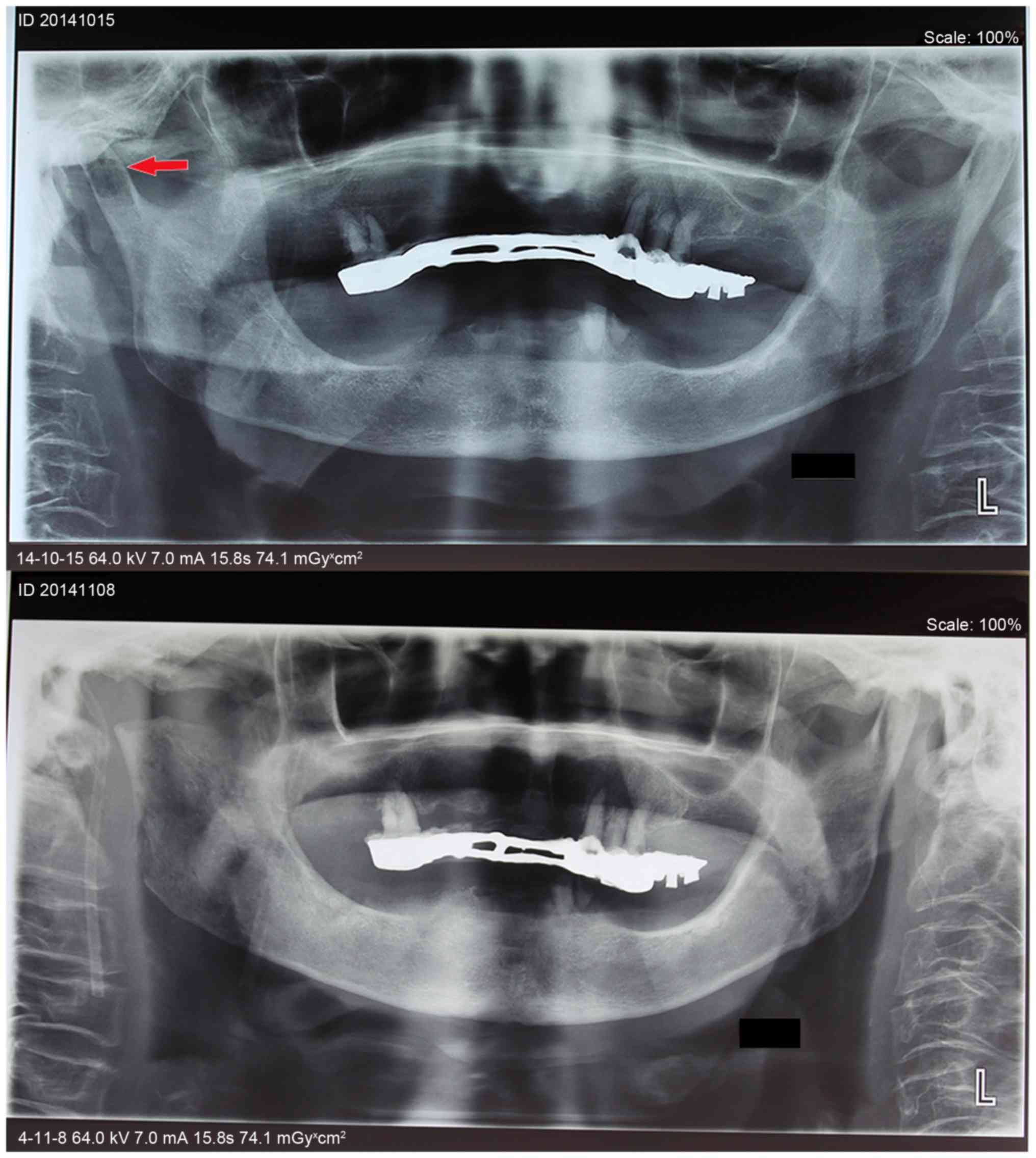
The CT scans and a panoramic radiograph showed that the condylar
head was diffusely radiolucent in the middle (Figs. 2–4).
Treatment
Based on the patient's history as well as the
clinical and radiographic examination, a presumptive diagnosis of
pyogenic orofacial infections was made. Thus, the patient was
admitted to the hospital and adequate drainage of the abscess was
performed under local anesthesia via two incisions at buccal (by
enlarging the fistula) and temporal regions. During the surgery,
~50 milliliters of purulent material was withdrawn from the abscess
and sent for culture and antibiotic sensitivity testing, and
another 5 milliliters of pus was aspirated from the
glossopharyngeal space with a long needle (Fig. 1B and C). The patient reported
immediate improvement of her symptoms. After evacuation of pus,
repeated irrigation with saline was performed each day through the
percutaneous catheters and empirical intravenous antibiotic therapy
was commenced (cefazolin sodium, 2 g/12 h; metronidazole, 0.5
g/day). Analgesics were also given to reduce the patient's pain. To
correct hypoproteinemia, the patient was treated with intravenous
human serum albumin and fresh frozen plasma. On the 7th day after
the abscess incision, purulent material culture and antibiotic
sensitivity testing showed positivity for Escherichia coli,
which was sensitive to imipenem and meropenem. According to the
test results, the patient was continually treated with intravenous
antibiotics, which was switched to imipenem (1 g/12 h). Although
the swelling of the right facial region decreased and pain was
relieved after a 25-day treatment, pyorrhea did not appear to
improve. As the symptoms had not resolved, another CT scan was
performed, which revealed that an abscess remained in the anterior
region of the right-side TMJ, suggesting that exploration of the
joint was necessary. After the patient gave her informed consent, a
right mandibular condylectomy and debridement via a pre-auricular
approach was performed to excise the lesion of the right-side
mandibular condyle under general anesthesia. During the surgery, a
large amount of pus was drained from the abscess and a right
mandibular condyle with bone erosion was seen (Fig. 5). After the condylectomy, a vertical
defect was observed but not repaired, as the patient was too old to
tolerate a bigger operative trauma and a longer general anesthesia.
The wound was lightly packed with an iodoform gauze used as a
drainage, which was gradually taken out over 1 week, and the wound
was later closed in layers. The tissue that filled the joint space
was sent for histopathological examination, which identified
inflammatory granulation tissue with patchy infiltrates of
lymphocytes (Fig. 5E and F).
Therefore, the final diagnosis was right-side SATMJ. At 2 months
after condylectomy, a CT scan revealed improvement of the abscess
around the right-side TMJ region (Fig.
2) and after another 2 months, her mouth-opening range reached
~35 mm without any right face pain (Fig.
1F). After 1 year of post-operative follow-up, neither
recurrence nor complications were observed. Unfortunately, the
superior buccal branches of the facial nerve (VII) were unwittingly
damaged during the course of treatment. While it is difficult to
state when this nerve damage occurred, it was likely to have
occurred during the process of abscess incision and draining,
although pseudo facioplegia or condylectomy may have also caused
damage. This damage was permanent, the symptoms were still present
1 year following treatment and although the damage did not affect
normal chewing and eating, the patient drooled when liquid was
gargled.
Discussion
Compared with hips and knees, TMJs are affected by
septic arthritis much less frequently (6,22,23).
Usually, trauma, head and neck infection or TMJ arthrosis are the
major causes of SATMJ, while it may also arise secondary to
mastoiditis and otitis media (13).
It is generally accepted that occurrence of SATMJ often occurs due
to either local spread or hematogenous dissemination from a distant
primary infection (5,6,8,15). In addition, the TMJ synovium is
highly vascular and has no limiting basement membrane.
Consequently, acute or blunt trauma with resulting capsular injury
is particularly likely to bring the infection into the joint space
via these two routes (13,24). As mentioned above, in the present
case, SATMJ appeared to originate from pyogenic orofacial
infections invading the TMJ. However, treatment by abscess incision
and drainage as well as intravenous antibiotic therapy was not
sufficient. It was therefore assumed that the occlusal trauma and
mechanical stress resulting from edentulous jaws of this patient
had damnified the aging mandibular condyle, which initiated
arthritic changes, including destruction and necrosis in the TMJ,
and created an environment supportive of bacterial infection.
Subsequently, abscess spreading from the joint space to adjacent
spaces caused the severely pyogenic orofacial infections (25). In other words, the present case
suggested that SATMJ itself may be an independent bacterial disease
and a source of infections to peripheral tissues, which is distinct
from aseptic inflammations similarly caused by occlusal trauma,
such as osteoarthritis of TMJ.
Misdiagnosis of SATMJ causes delays in its cure as
well as irreversible complications, which may even be life
threatening. Therefore, timely and correct diagnosis with early
treatment is critical to preventing acute complications and late
sequelae. Despite the lack of unified diagnostic criteria for
SATMJ, numerous studies have consistently described the
determination of clinical manifestations, imaging findings, joint
aspiration and joint fluid analysis and laboratory chemical and
microbiological tests as diagnostic approaches (5,26–29).
During the clinical examination, an erythematous and a warm
swelling in the pre-auricular region, which is frequently
associated with pain, malocclusion, limited maximum mouth opening
and mandibular deviation were common symptoms of SATMJ, which were
required to be identified, with differential diagnoses being dental
abscess or periapical dental abscess, bacterial or viral
pharyngitis, retropharyngeal abscess, peritonsillar abscess, acute
otitis media, mastoiditis, parotitis, submandibular sialadenitis
and lymphadenopathy (17,29,30). In
addition, when pre-auricular swelling occurs without obvious signs
of inflammation, SATMJ must be considered after excluding
cellulitis of the overlying soft tissues, synovial cysts of the
TMJ, parotid cysts and tumours, condylar hypertrophy and various
neoplastic processes arising in the mandibular condyle (31–33).
Furthermore, SATMJ must also be distinguished from certain
systematic diseases, which may present with similar clinical
features, such as rheumatoid arthritis, gout, pseudogout, rheumatic
fever and trauma (21,25,34–37).
Early diagnosis may not be easy as not all infected TMJs exhibit
signs of acute inflammation. Particularly in elderly and
immunosuppressed patients, the inflammatory response of SATMJ may
be muted (10). In the present case,
the signs of inflammation were moderated by fascial attachments
surrounding the TMJ until the fistula had formed in the right
cheek. As SATMJ was concealed by the abscess, which increased the
difficulty to establish the diagnosis, the patient's treatment was
delayed. In such circumstances, supplementary examinations were
required to confirm the diagnosis, as available findings were not
particularly significant.
Once SATMJ is suspected, joint aspiration is
recommended to confirm it. The aspirated fluid and turbidity are
required to be grossly examined for color as well as subjected to
Gram staining, culture and sensitivity analysis (29,38).
Owing to their relatively high accuracy and the high rate of the
occurrence of bacteria in the synovial fluid, cultures are
considered to be a credible method to definitively diagnose SATMJ,
but not gonococcal arthritis. Fasting synovial glucose estimation
is another form of evidence of infection, which is decreased to
less than half the serum value in most bacterial infections and
increased in gonococcal arthritis (10,29).
Radiological findings, such as panoramic
radiographs, CT and magnetic resonance imaging (MRI) were able to
distinguish between intra- and extra-articular causes of
pre-auricular swelling and to evaluate mandibular osteomyelitis and
deformity of the TMJ. A plain radiograph may reveal small areas of
joint damage obscured. A CT or MRI scan is highly sensitive to
early alterations and is useful to demonstrate widening of the
articular spaces, bone erosion, effusion and abscess formation in
the stage of TMJ infection. It is worth noting that at a time-point
at which bone abnormalities are radiographically detectable,
accumulation of inflammatory exudate and pus in the TMJ space may
have persisted for several weeks, and a CT and MRI scan is
therefore advantageous in the diagnosis of patients in the chronic
stage (25,30,39).
Laboratory investigations are also helpful for the
diagnosis of SATMJ. Increased serum leukocyte and neutrophil counts
prompt infections; however, they may be normal in patients treated
with antibiotics. C-reactive protein, as a non-specific indicator
of inflammation, may be elevated in the initial stage (5,26).
Besides, arthroscopy is advocated as an aid for diagnosing SATMJ
and a negative technetium 99 scan of the joint can rule out the
diagnosis of arthritis (10,27).
At present, no clear consensus on the management of
SATMJ has been reached, and thus, different methods have been
proposed for joint drainage and decompression in various SATMJ
cases, including needle aspiration, arthroscopy and arthrotomy
(40). To keep potential
therapy-induced risks of the TMJ to a minimum, needle aspiration
was often selected as an initial way to relieve the pain and clear
the inflammatory medium; particularly arthrocentesis with double
needles was advocated as more effective and irrigation may be
performed simultaneously. During needle aspiration, appropriate
pressure is suggested to avoid the spread of inflammation. For
patients with a mild-to-moderate complaint, arthroscopy can be
applied to remove fibrous adhesion and smoothen the articular
surface (5,13). However, as soon as the abscess is
established, exploration of the TMJ is necessary and surgical
drainage must be performed immediately, as the proximity of the TMJ
to the skull base facilitates the potential intracranial spread of
infection (6,10,17,41). If
severe erosion and destruction of the cartilage and bone is
identified, condylectomy and joint replacement will be required
(5,19,34).
Similarly, in the present case, purulent mandibular condyle and TMJ
disc were excised, and granulation tissues filled within the joint
space were debrided. Of note, a previous study demonstrated that
there was no significant difference between surgical drainage and
needle aspiration, and based on this viewpoint, it is suggested
that SATMJ of pediatric patients should be treated by aspiration
alone and washout in the acute setting (10,29,42).
An increasing body of evidence suggested that the
detection of pathogens by microbiological culture is important for
selecting a suitable and efficient antibiotic therapy (5,10,13,27,29,30,38,40).
Although empiric antibiotic therapy may not be effective against
certain pathogens, systemic antibiotics should be applied if a
provisional diagnosis of SATMJ has been made, and a negative result
of definitive bacterial culture should not delay early vigorous
treatment. Therefore, empiric antibiotics should be given until the
pathogenic examinations are performed. The antibiotic treatment
regime should be modified according to the culture and the
sensitivity results, if they are available (5,10,13,27,29,30,38,40).
It is generally advised that antibiotic therapy should be vigorous
and the length of treatment should be at least 30 days (10,34). It
is worth emphasizing that whatever type of antibiotic, the dosage
and length of course selected should be appropriate for the
individual patient. When systemic antimicrobials reach adequate
concentrations in normal joints, intra-articular irrigation with
antibiotics is controversial and may lead a concomitant risk of
introducing new microbes into the infected joint (30,34). In
the present case, the patient showed a poor response to
antimicrobial treatment alone prior to hospitalization, and it was
therefore suggested that antibiotics may only be used as an adjunct
therapeutic method, while the treatment of severe SATMJ requires
removal of the inflammatory lesions.
Joint immobilization is also helpful in the
treatment of SATMJ, and once the joint pain subsides and the acute
infectious process improves, physical therapy is necessary to
prevent the long-term limitation of mouth opening caused by
fibrosis and ankyloses of TMJ (5,10,15–17,26,43).
In conclusion, SATMJ may at times not have any
obvious early symptoms and its diagnosis is often delayed until
reaching an advanced stage. Hence, clinicians should pay more
attention to any diagnostic clues for SATMJ, including its clinical
manifestations, imaging findings and the results of joint
aspiration and joint fluid analysis and cultures. Once the
diagnosis of SATMJ is suspected, appropriate therapy must be
performed as soon as possible, such as needle aspiration,
arthroscopy, arthrotomy and antibiotic treatment, and even
condylectomy and joint replacement. Only in this way, an ideal
prognosis of SATMJ can be achieved.
Acknowledgements
The authors are grateful to Dr Ru Wang from the
Department of Stomatology, (The First Affiliated Hospital of Dalian
Medical University, Dalian, China) for her kind support and
assistance in the preparation of the present study.
References
|
1
|
Lohiya S and Dillon J: Septic arthritis of
the temporomandibular joint-unusual presentations. J Oral
Maxillofac Surg. 74:87–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gams K and Freeman P: Temporomandibular
joint septic arthritis and mandibular osteomyelitis arising from an
odontogenic infection: A case report and review of the literature.
J Oral Maxillofac Surg. 74:754–763. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martins J, Almeida S, Nunes P, Prata F,
Lobo ML and Marques JG: Grisel syndrome, acute otitis media, and
temporo-mandibular reactive arthritis: A rare association. Int J
Pediatr Otorhinolaryngol. 79:1370–1373. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al-Saadi NJ, Bakathir AA, Al-Hashmi AK and
Al-Ismaili MI: Temporomandibular joint ankylosis as a complication
of neonatal septic arthritis: Report of two cases. Sultan Qaboos
Univ Med J. 15:e554–e558. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cai XY, Yang C, Zhang ZY, Qiu WL, Chen MJ
and Zhang SY: Septic arthritis of the temporomandibular joint: A
retrospective review of 40 cases. J Oral Maxillofac Surg.
68:731–738. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Trimble LD, Schoenaers JA and Stoelinga
PJ: Acute suppurative arthritis of the temporomandibular joint in a
patient with rheumatoid arthritis. J Maxillofac Surg. 11:92–95.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
O'Meara PM and Bartal E: Septic arthritis:
Process, etiology, treatment outcome. A literature review.
Orthopedics. 11:623–628. 1988.PubMed/NCBI
|
|
8
|
Thomson HG: Septic arthritis of the
temporomandibular joint complicating otitis externa. J Laryngol
Otol. 103:319–321. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Borenstein DG and Simon GL: Hemophilus
influenzae septic arthritis in adults. A report of four cases and a
review of the literature. Medicine (Baltimore). 65:191–201. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bounds GA, Hopkins R and Sugar A: Septic
arthritis of the temporo-mandibular joint-a problematic diagnosis.
Br J Oral Maxillofac Surg. 25:61–67. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hilbert L, Peters WJ and Tepperman PS:
Temporomandibular joint destruction after a burn. Burns Incl Therm
Inj. 10:214–216. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McCain JP, Zabiegalski NA and Levine RL:
Joint infection as a complication of temporomandibular joint
arthroscopy: A case report. J Oral Maxillofac Surg. 51:1389–1392.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moses JJ, Lange CR and Arredondo A: Septic
arthritis of the temporomandibular joint after the removal of third
molars. J Oral Maxillofac Surg. 56:510–512. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scroggins SE, Baran AS, Angel MF and
Caloss R: Distraction osteogenesis as a treatment for retrognathia
and obstructive sleep apnea resulting from temporomandibular joint
septic arthritis: A case report. J Oral Maxillofac Surg.
70:e509–e515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sembronio S, Albiero AM, Robiony M, Costa
F, Toro C and Politi M: Septic arthritis of the temporomandibular
joint successfully treated with arthroscopic lysis and lavage: Case
report and review of the literature. Oral Surg Oral Med Oral Pathol
Oral Radiol Endod. 103:e1–e6. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cai XY, Yang C, Zhang ZY, Qiu WL, Ha Q and
Zhu M: A murine model for septic arthritis of the temporomandibular
joint. J Oral Maxillofac Surg. 66:864–869. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leighty SM, Spach DH, Myall RW and Burns
JL: Septic arthritis of the temporomandibular joint: Review of the
literature and report of two cases in children. Int J Oral
Maxillofac Surg. 22:292–297. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mayne JG and Hatch GS: Arthritis of the
temporomandibular joint. J Am Dent Assoc. 79:125–130. 1969.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Varghese L, Chacko R, Varghese GM and Job
A: Septic arthritis of the temporomandibular joint caused by
Aspergillus flavus infection as a complication of otitis externa.
Ear Nose Throat J. 94:E24–E26. 2015.PubMed/NCBI
|
|
20
|
Regev E, Koplewitz BZ, Nitzan DW and
Bar-Ziv J: Ankylosis of the temporomandibular joint as a sequela of
septic arthritis and neonatal sepsis. Pediatr Infect Dis J.
22:99–101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goldschmidt MJ, Butterfield KJ, Goracy ES
and Goldberg MH: Streptococcal infection of the temporomandibular
joint of hematogenous origin: A case report and contemporary
therapy. J Oral Maxillofac Surg. 60:1347–1353. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Studahl M, Bergman B, Kälebo P and
Lindberg J: Septic arthritis of the knee: A 10-year review and
long-term follow-up using a new scoring system. Scand J Infect Dis.
26:85–93. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deesomchok U and Tumrasvin T: Clinical
study of culture-proven cases of non-gonococcal arthritis. J Med
Assoc Thai. 73:615–623. 1990.PubMed/NCBI
|
|
24
|
Esterhai JL Jr and Gelb I: Adult septic
arthritis. Orthop Clin North Am. 22:503–514. 1991.PubMed/NCBI
|
|
25
|
Kito S, Hirashima S, Yoshioka I, Habu M,
Kodama M, Kokuryo S, Oda M, Tanaka T, Wakasugi-Sato N,
Matsumoto-Takeda S, et al: A case of chronic infectious arthritis
of the temporomandibular joint associated with osteomyelitis
without malocclusion. Open Dent J. 4:29–32. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Klüppel LE, Bernabé FB, Primo BT,
Stringhini DJ, da Costa DJ, Rebellato NL and Müller PR: Septic
arthritis of the temporomandibular joint. J Craniofac Surg.
23:1752–1754. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hincapie JW, Tobon D and Diaz-Reyes GA:
Septic arthritis of the temporomandibular joint. Otolaryngol Head
Neck Surg. 121:836–837. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cai XY, Yang C, Chen MJ, Zhang SY and Yun
B: Arthroscopic management of septic arthritis of temporomandibular
joint. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 109:24–30.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gayle EA, Young SM, McKenna SJ and
McNaughton CD: Septic arthritis of the temporomandibular joint:
Case reports and review of the literature. J Emerg Med. 45:674–678.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chuk R, Arvier J, Laing B and Coman D:
Septic arthritis of the temporomandibular joint in an infant. Clin
Pract. 5:7362015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Janecka IP and Conley JJ: Synovial cyst of
temporo-mandibular joint imitating a parotid tumour (A case
report). J Maxillofac Surg. 6:154–156. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ethell AT: A rare ‘parotid tumour’. J
Laryngol Otol. 93:741–754. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Papavasiliou A, Sawyer R, Lund V and
Michaels L: Benign conditions of the temporomandibular joint: A
diagnostic dilemma. Br J Oral Surg. 21:222–228. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hekkenberg RJ, Piedade L, Mock D, Baker G
and Freeman JL: Septic arthritis of the temporomandibular joint.
Otolaryngol Head Neck Surg. 120:780–782. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li-Yu J, Schumacher HR Jr and Gratwick G:
Invasive tophaceous pseudogout in the temporomandibular joint:
Misdiagnosis as tumor: Case report and review of the literature. J
Clin Rheumatol. 6:272–277. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nakagawa Y, Ishii H, Shimoda S and
Ishibashi K: Pseudogout of the temporomandibular joint. A case
report. Int J Oral Maxillofac Surg. 28:26–28. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Copeland M and Douglas B: Ganglions of the
temporomandibular joint: Case report and review of literature.
Plast Reconstr Surg. 81:775–776. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Al-Khalisy HM, Nikiforov I, Mansoora Q,
Goldman J and Cheriyath P: Septic arthritis in the
temporomandibular joint. N Am J Med Sci. 7:480–482. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Murakami K, Matsumoto K and Iizuka T:
Suppurative arthritis of the temporomandibular joint. Report of a
case with special reference to arthroscopic observations. J
Maxillofac Surg. 12:41–45. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bast F, Collier S, Chadha P and Collier J:
Septic arthritis of the temporomandibular joint as a complication
of acute otitis media in a child: A rare case and the importance of
real-time PCR for diagnosis. Int J Pediatr Otorhinolaryngol.
79:1942–1954. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rimoin DL and Wennberg JE: Acute septic
arthritis complicating chronic rheumatoid arthritis. JAMA.
196:617–621. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Goldenberg DL, Brandt KD, Cohen AS and
Cathcart ES: Treatment of septic arthritis: Comparison of needle
aspiration and surgery as initial modes of joint drainage.
Arthritis Rheum. 18:83–90. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nade S: Acute septic arthritis in infancy
and childhood. J Bone Joint Surg Br. 65:234–241. 1983.PubMed/NCBI
|