Introduction
Bronchial asthma is a chronic inflammatory disease
involving multiple inflammatory cells such as mastocytes,
eosinophilic granulocytes and T lymphocytes. The characteristic
airway inflammation, tissue damage and airway dysfunction are a
result of local aggregation of inflammatory cells in the airways
and release of inflammatory mediators and cytokines (1). The T lymphocytes play a core role in
asthma airway inflammation (2).
Bronchial asthma is a chronic inflammatory disease mediated by
excessively activated Th2 cells. In contrast, a Th1 reaction is
considered a protective factor of allergic diseases including
asthma. In addition, the immunotherapy for allergic diseases aims
at transforming the Th2 cytokine phenotype into a Th1 phenotype
(3). Thus, the idea of a Th1/Th2
imbalance in asthma is widely accepted. Nevertheless, there still
are experimental and clinical phenomena related to asthma that
remain unexplained by the altered Th1/Th2 model. Research has found
that the Th1 cells can exacerbate allergies and asthma. The Th1
cytokine IFN-γ is associated with airway hyper-responsiveness
induced by antigens and infiltration of eosinophilic granulocytes
(4). The regulatory T (Treg) cells
cannot only inhibit the Th1 cells but can inhibit the Th2 cells as
well (5). Therefore, research on
Treg cells has become a priority to better understand the
pathogenesis of asthma.
The Th17 cells mediate the incidence and progression
of inflammatory reactions, autoimmune diseases, tumors and
transplant rejection (6). The major
biological function of the secreted IL-17 is to promote
inflammatory reactions. IL-17 is a pre-inflammatory cytokine and
also an early activating factor of the inflammatory reactions
induced by the T cells (7). It can
amplify inflammatory reactions by promoting the release of other
pre-inflammatory cytokines. It can also recruit neutrophils,
promote release of inflammatory factors by multiple cells, promote
secretion of mucus by the mucous glands, and strengthen the airway
hyper-responsiveness. IL-17 is closely associated with the
incidence and progression of the chronic inflammatory diseases
related to the airways (8). In the
pulmonary tissues of asthma model rats, the IL-17 secreted by Th17
cells is significantly and positively correlated with an increase
in the eosinophilic granulocytes in the bronchoalveolar lavage
fluid (BALF). Thus, the Th17 cells and IL-17 may induce the
expression of eosinophilic granulocytes in the eosinophilic
asthma.
Moreover, research surveys in recent years have
shown that allergic asthma accounts only for ~41% of all asthma
attacks (9) and the remaining 59% of
episodes are associated with neutrophils, indicating that
approximately half of the attacks are not attributable to allergens
or atopy (10). Given this complex
pathogenic scenario, the roles of neutrophils and their powerful
recruiting factor, IL-17, remain to be clarified. For example,
activation in ovalbumin (OVA)-sensitized mice leads to increased
expression of IL-17 mRNA in the pulmonary tissue and significant
neutrophil infiltration (11). The
high expression of IL-17 in BALF of asthma patients (12) indicates that the Th17 cells may
participate in the onset of asthma.
Transcription factors are a class of nucleoproteins
that identify and are bound to specific DNA regulatory sequences
thereby stimulating or inhibiting transcription. Any changes in
their quantity or activity may lead to abnormal expression of genes
critical to cellular growth and differentiation.
Among the transcription factors promoting the
differentiation of Treg and Th17, Foxp3 and retinoic acid
receptor-related orphan nuclear receptor (ROR)γt are the most
important ones (13). RORγt is a
critical transcription factor that controls differentiation in Th17
cells. Research indicates that the level of RORγt mRNA increases
gradually when the initial T cells are differentiating into Th17
(14). T cells with a deletion of
the RORγt are not able to differentiate into Th17 in in
vitro experiments. There is a complex mutual relationship
between the Treg and the Th17 cells. They are correlated during
differentiation but display antagonistic functions (13). Other cytokines such as IL-6, and
TGF-β can determine whether the immune response of the Treg or that
of the Th17 is dominant. The TGF-β produced by the immune system
inhibits proliferation of the effector T cells, and induces
differentiation of the naive T cells into the Treg cells (15). Treg cells secrete TGF-β and express
Foxp3, inhibit inflammatory responses, maintain the immune
tolerance of the organism, and prevent the incidence of autoimmune
diseases (16) when the immune
system is in a steady state or subject to no inflammatory injury.
However, the acute stage protein IL-6 will be substantially
produced when infection or inflammation occur. IL-6 inhibits
proliferation of the Treg cells. IL-6 and TGF-β jointly induce
differentiation of Th17 cells and secrete IL-17 and IL-6.
Experiments have demonstrated that once the Th17 cells are produced
and IL-17 is secreted, a positive feedback develops. It induces
IL-6, further producing Th17, mediates pre-inflammatory responses,
and participates in autoimmune diseases (17).
It has been established that the Th1 and Th2 cell
subsets play important roles in the pathogenesis of bronchial
asthma. However, the function of other cell subsets like the Treg
and Th17, and the significance of the changes to their ratio are
the subject of research in our study. We believe a probe into the
balance of Treg/Th17 will supplement and improve the current
understanding of the immunological pathogenesis of bronchial asthma
and aid in the search for more effective treatment strategies. To
this end, we focused on the specific transcription factors of Foxp3
and RORγt that determine the differentiation of Treg/Th17.
Materials and methods
Experimental animals
SPF inbred strain female BALB/c mice were purchased
from the Laboratory Animal Center of Cheeloo College of Medicine,
Shandong University and raised in a laboratory animal center. Mice
aged 6–8 weeks were selected for the experiments, and were divided
into three groups according to a random number table method: normal
control, asthma, and dexamethasone treatment group (10 mice in each
group). The mice were raised in separate cages and fed with special
food containing no allergens. This study was approved by the Animal
Ethics Committee of Binzhou People's Hospital.
Sensitization and activation in
mice
Each mouse was sensitized 3 times, at 0, 7, and 14
days of the experiments, as follows. Each mouse in the asthma group
was subjected to intraperitoneal and bilateral femoral subcutaneous
injections of a total of 200 µl sensitization solution containing
20 mg OVA and 20 mg Al(OH)3 (ICN Biomedicals, Inc.,
Costa Mesa, CA, USA). The mice in the dexamethasone treatment group
(Sanyao Science & Technology Development Co., Beijing, China)
were sensitized in the same way. Finally, the mice in the normal
control group were injected with normal saline for sensitization at
the same sites and dosage as those of the other groups.
Mice in the asthma group were placed in an in house
aerosol inhalation box and subjected to 5% (m/v) OVA aerosol
inhalation activation for 7 consecutive days for 45 min daily from
the 21st day after the experiment. A Paxiboy high-frequency
atomizer (PE Applied Biosystems, Foster City, CA, USA) was used.
The asthma attacks in mice were observed and recorded. The mice in
the dexamethasone treatment group were injected intraperitoneally
with dexamethasone (1 mg/kg) (Sanyao Science & Technology
Development Co.) 30 min before being subjected to aerosol
inhalation of 5% OVA for activation from days 21 to 27. The mice in
the normal control group had normal saline instead of OVA
atomization for activation.
Determination of airway
responsiveness
The Buxco mouse plethysmograph (Thermo Fisher
Scientific, Inc., Waltham, MA, USA), flow sensor, and atomization
device were properly connected. The connection between the sensor
and the amplifier and that between the amplifier and the computer
were checked. All interfaces were well sealed. The software
BioSystem XA analysis module (version 10.2; Tree Star, Inc.,
Ashland, OR, USA) was established. Each experimental mouse was
placed in a non-invasive plethysmograph box. The %∆Penh value for
each mouse was recorded continuously after activation with
atomization of normal saline and acetylcholine at different
concentrations. The experimental results were collected and saved.
The mice were returned to the cage.
Counting and classification of
leukocytes
A total of 100 ml of cold PBS were used to dilute
the BALF cell sediment. Thirty milliliters were taken and the cells
were counted with a cell counting chamber (Miltenyi Biotec GmbH,
Bergisch Gladbach, Germany). A 50 ml sample of the cell sediment
smear was dried and fixed. Two hundred cells were counted under the
microscope for differential counting. The cells were divided into
eosinophilic granulocytes, neutrophils, and lymphocytes based on
morphological characteristics.
Detection of IL-10 and IL-17 in BALF
and serum
The BALF blood sample from the mice was not diluted.
The standard was diluted as required. The sample or standard was
added to the ELISA plate pre-embedded with 100 ml/well anti-IL-10
and IL-17 antibodies (EMD Millipore, Billerica, MA, USA). A total
of 100 ml/well of biotinylated antibodies IL-10 and IL-17
(Polysciences, Inc., Warrington, PA, USA) were added. The plates
were incubated for 2 h at room temperature. Next, each plate was
washed 3 times. Then, 100 ml streptavidin horseradish peroxidase
solution (Wuhan Boster Biological Engineering Co., Ltd., Wuhan,
China) was added to each well. The plate was incubated for 1 h at
room temperature. Then, the plate was washed 3 times. The TMB
substrate solution was added as per 100 ml/well (Wuhan Boster
Biological Engineering Co., Ltd.). It was left to develop for 15
min in the dark at room temperature. A total of 100 ml of the stop
buffer were finally added. The OD value was measured at an
absorption wavelength of 450 nm (Thermo Fisher Scientific, Inc.). A
standard curve was plotted to obtain the content of IL-10 and IL-17
in the sample.
Pathological sections of pulmonary
tissue
Paraffin sections of mouse pulmonary tissue were
subjected to xylene dewaxing, and immersed into gradient grade
ethanol 3 times for 5 min each. The sections were stained with
hematoxylin (Beifang Biotechnology Research Institute, Beijing,
China) for 10 min following standard procedures and then with eosin
stain. The sections were routinely dehydrated, cleared with xylene,
mounted with neutral gum, and observed under an optical microscope
(Olympus Corp., Tokyo, Japan).
Flow cytometry
The spleen was removed from sacrificed mice under
sterile conditions, placed in a sterile plate containing RPMI-1640
culture medium (Invitrogen Life Technologies Inc., Carlsbad, CA,
USA), cut into 1–2 mm pieces with curved scissors, and ground
gently with the inner core of a 2 ml injection syringe. The ground
spleen cells were washed with 5–6 ml of the RPMI-1640 culture
medium containing 2% FBS (Invitrogen Life Technologies, Inc.), and
centrifuged for 10 min at 4°C at 1,350 × g to discard the
supernatant. A total of 7–8 ml of the erythrocytes lysis buffer
(Sigma-Aldrich, St. Louis, MO, USA) were added and the sample was
allowed to stand still for 4–5 min. Twenty-five milliliters of the
RPMI-1640 culture medium containing 2% FBS (Hyclone, Logan, UT,
USA) were added. The sample was centrifuged at 850 × g for 10 min
at 4°C and the supernatant was discarded. Next, 15 ml of the
culture medium was added. Twelve milliliters of the upper cells
were re-suspended and centrifuged at 850 × g for 10 min at 4°C.
Five milliliters of the RPMI-1640 complete medium was added and the
cells were re-suspended. A total of 20 ng/ml PMA (Sigma-Aldrich), 1
mg/ml inonmycin (Sigma-Aldrich), and 10 mg/ml BFA (Sigma-Aldrich)
were added. After the appropriate fluorescence-labeled monoclonal
antibodies, anti-CD4-FITC and anti-CD25-PE (BD Biosciences,
Heidelberg, Germany) were added to the 100 ml samples, each sample
was well mixed gently, incubated for 20 min at room temperature,
and then further incubated for 10 min in the dark at room
temperature. The samples were centrifuged at 850 × g for 5 min and
the supernatants were discarded. Two milliliters of cell staining
buffer solution was added and well mixed before centrifuging again
at 850 × g for 5 min. The supernatant was discarded. A total of 0.5
ml of cell fixation/membrane rupture buffer solution
(Sigma-Aldrich) was added to each sample. The samples were then
incubated for 20 min in the dark at room temperature. The
appropriate fluorescence labeled intracellular cytokine antibodies
anti-IL-17-PE and anti-Foxp3-APC (BD Biosciences, Mountain View,
CA, USA) were added. The solutions were well mixed and incubated
for 20 min in the dark at room temperature. A total of 0.5 ml of
the fixation buffer solution was added to re-suspend the cells. A
flow cytometer (BD Biosciences, San Jose, CA, USA) was used for
detection and analysis.
Immunohistochemical method
Sections were routinely dewaxed and dehydrated. Then
the endogenous oxidases were removed. Sections were subjected to
microwave antigen retrieval and incubated for 10 min at room
temperature. The sections were washed 3 times for 3 min each with
PBS. Next, 50 ml of non-immune animal serum (Bioss Biological
Technology Co., Beijing, China) were added, the samples were
incubated for 10 min at room temperature, and washed once with the
buffer solution. Then after addition of 50 ml of dilute antibody
Foxp3, RORγt (Abcam, Cambridge, UK), each section was incubated
overnight at 4°C. The next day, each section was washed 3 times for
3 min each with PBS. Fifty milliliters of the biotin-marked
secondary antibody (Wuhan Boster Biological Engineering Co., Ltd.)
was added to the sections and they were then incubated for 10 min
at room temperature. The sections were washed 3 times with PBS for
3 min each. After addition of 50 µl streptavidin-peroxidase
solution, each section was incubated for 10 min at room temperature
(Wuhan Boster Biological Engineering Co., Ltd.). The sections were
washed 3 times for 3 min each with PBS. Finally, each section was
observed for 3–10 min under the microscope after addition of 100 ml
of freshly prepared DAB solution. Then, each section was washed
with water, lightly stained with hematoxylin (Beifang Biotechnology
Research Institute), washed with water, dehydrated with gradient
ethanol, dried, and mounted with neutral gum. The Image-Pro Plus
microscope image analysis software (version 12.0; Tree Star, Inc.)
was used for analysis of the expression intensity of the proteins
studied. Five fields of view were determined for each section. The
count of the positive cells in each field of view was computed and
expressed by an average.
Western blot analysis
One milligram of whole pulmonary tissue protein
sample was subjected to SDS-PAGE. After completion of SDS-PAGE, two
pieces of gel were removed carefully. The spongy cushion, filter
paper, gel, film, filter paper, and spongy cushion were set up in
sequence for preparation of the gel transfer interlayer. After
expelling the bubbles with a glass rod, the sandwich was placed in
an electroblotting tank and transferred for 60–90 min at 100 V (350
mA). After completion of membrane transfer, the membrane was
transferred with tweezers to a plate containing 25 ml blocking
buffer and shaken gently for 2 h decoloration on a shaking table at
room temperature. After addition of the primary antibodies of Foxp3
and RORγt (Abcam), the membranes were incubated overnight at 4°C.
The next day, each membrane was washed 3 times for 5 min each with
TBST, on a shaking table, at room temperature. After addition of
the secondary antibody (Wuhan Boster Biological Engineering Co.,
Ltd.), each membrane was incubated on a shaking table for 1 h at
37°C. The membranes were washed with TBST, 3 times for 5 min each.
A developer was added (Thermo Fisher Scientific, Inc.) for
observation.
Statistical analysis
SPSS 19.0 software (IBM SPSS, Armonk, NY, USA) was
used for statistical processing. The data are expressed with mean ±
SD. The one-way analysis of variance and multiple comparisons were
used for comparative analysis in various groups. The SNK method was
used for multiple comparisons in the case of homogeneity of
variance. The Welch's method was used for analysis and the
Dunnett's T3 method was used for multiple comparisons in the case
of heterogeneity of variance. A difference was considered
significant at P<0.05.
Results
Airway responsiveness of the mice in
various groups
The results indicated that the level of %∆Penh in
various groups increased with the amount of the acetylcholine
concentration and there were significant differences among the mice
with different concentrations (F=893.456, P<0.01). The level of
%∆Penh of the mice in the asthma group was significantly higher
than that in the normal control group (F=265.558, P<0.01). There
were no significant differences between the normal saline asthma
group and normal control group (F=1.817, P=0.182). %∆Penh in the
asthma group was high in the case of other concentrations of
acetylcholine (P<0.01). An interaction effect existed between
different acetylcholine concentrations and different groups
(F=54.160, P<0.001) (Table
I).
 | Table I.%∆Penh values of the BALB/c mice in
various groups under different concentrations of acetylcholine
(mean ± SD). |
Table I.
%∆Penh values of the BALB/c mice in
various groups under different concentrations of acetylcholine
(mean ± SD).
|
| %∆Penh |
|
|
|
|---|
|
|
|
|
|
|
|---|
|
|
| Acetylcholine
concentration (mg/ml) |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Group | Normal saline | 3.125 | 6.25 | 12.5 | 25 | 50 | Sum | F | P-value |
|---|
| Asthma group | 13.25±2.56 | 106.39±10.25 | 198.14±33.45 | 156.43±52.39 | 585.87±50.27 | 887.09±85.70 | 348.34±35.97 | 469.478 | <0.001 |
| Dexamethasone
group | 12.59±3,87 | 27.43±10.53 | 84.34±35.53 | 328.57±50.44 | 336.78±54.98 | 568.16±55.76 | 187.93±20.91 | 265.347 | <0.001 |
| Normal control
group | 12.58±3.77 | 21.38±5.32 | 59.58±15.19 | 125.68±30.46 | 196.67±51.35 | 384.46±3.35 | 135.38±34.54 | 112.967 | <0.001 |
| Sum | 12.45±3.36 | 51.47±36.21 | 113.49±64.47 | 214.35±105.38 | 205.27±102.98 | 370.56±168.53 | 613.46±226.57 |
236.438a |
<0.001a |
| F | 1.776 | 244.57 | 55.34 | 61.47 | 135.46 | 113.57 |
266.639a |
F=55.469b |
| P-value | 0.156 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
<0.001a |
P<0.001b |
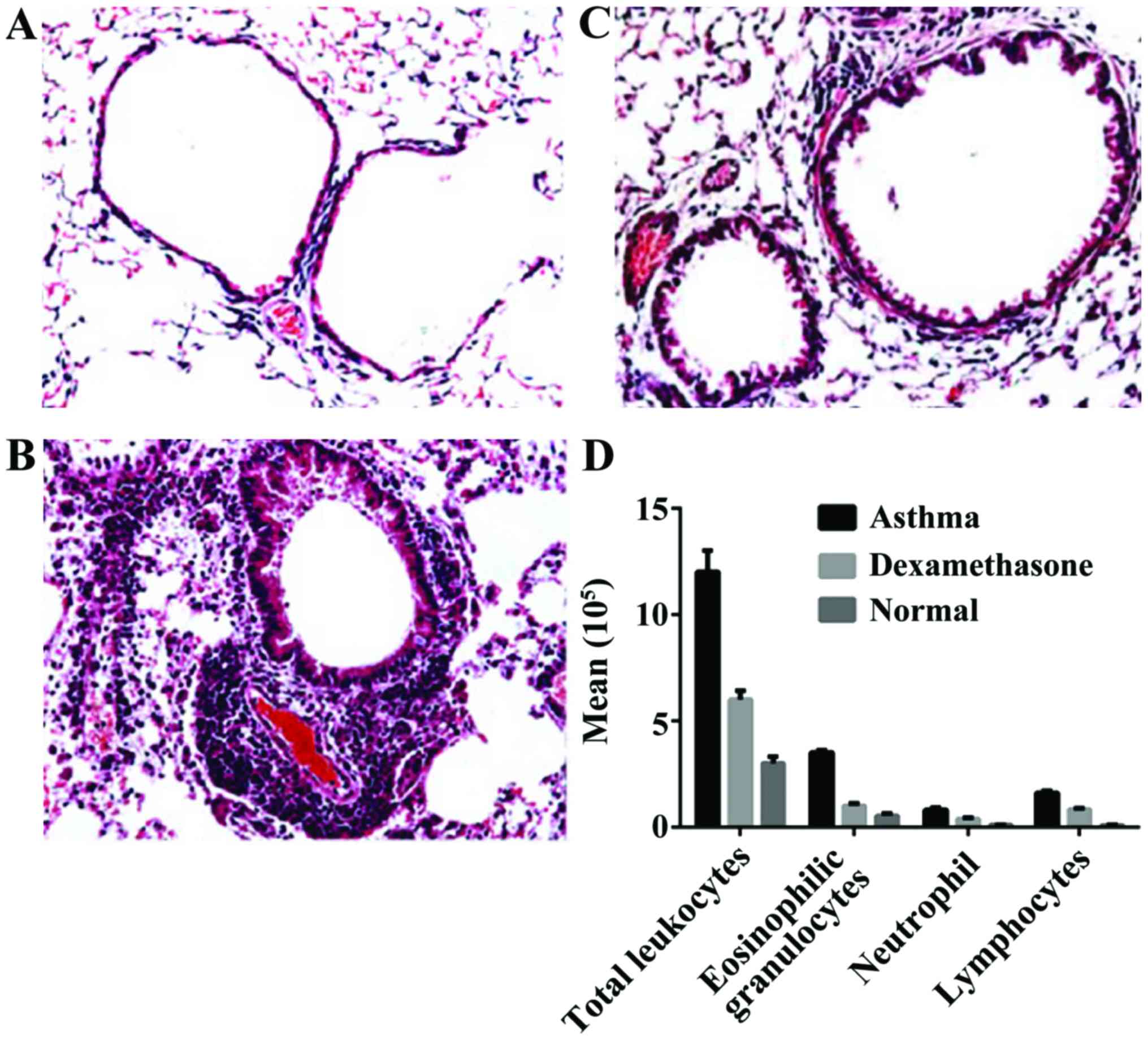
Morphological changes in pulmonary
tissue and differential count of BALF leukocytes
Based on the pathological sections of pulmonary
tissues, we observed infiltration of a large number of inflammatory
cells around the bronchiole and concomitant vessels in the mice of
the asthma group. The infiltration by a large number of
inflammatory cells was dominated by eosinophilic granulocytes and
lymphocytes in the airway wall and pulmonary tissue, additionally
there was a thickened bronchial wall, luminal stenosis, mucous
plugs in the bronchial lumens, reduced mucosa plicae, goblet cell
hyperplasia, widened alveolar septum, partial rupture, and
development of emphysema.
In the dexamethasone treatment group, we observed
orderly arranged bronchiole cilia, round lumens, occasional
infiltration of eosinophilic granulocytes in the vessel lumens,
alveolar cavities, and pulmonary interstitium, infiltration of a
small number of lymphocytes, and no thickened basilar membrane. The
total count of cells in BALF and the count of eosinophilic
granulocytes of mice in the asthma group were significantly larger
than that in the normal control group, indicating that significant
inflammation dominated by eosinophilic granulocytes occurred
locally in the airway of the mice in the asthma group. The total
counts of cells and the count of neutrophils, eosinophilic
granulocytes, and lymphocytes increased significantly when compared
with those in the normal control group (P<0.01). The counts of
neutrophil, eosinophilic granulocytes, and lymphocytes in the
dexamethasone treatment group decreased significantly when compared
with those in the asthma group (P<0.01). The counts of
neutrophils, eosinophilic granulocytes, and lymphocytes in the
dexamethasone treatment group were still large when compared with
those in the normal control group and the differences were
significant (P<0.01) (Fig.
1).
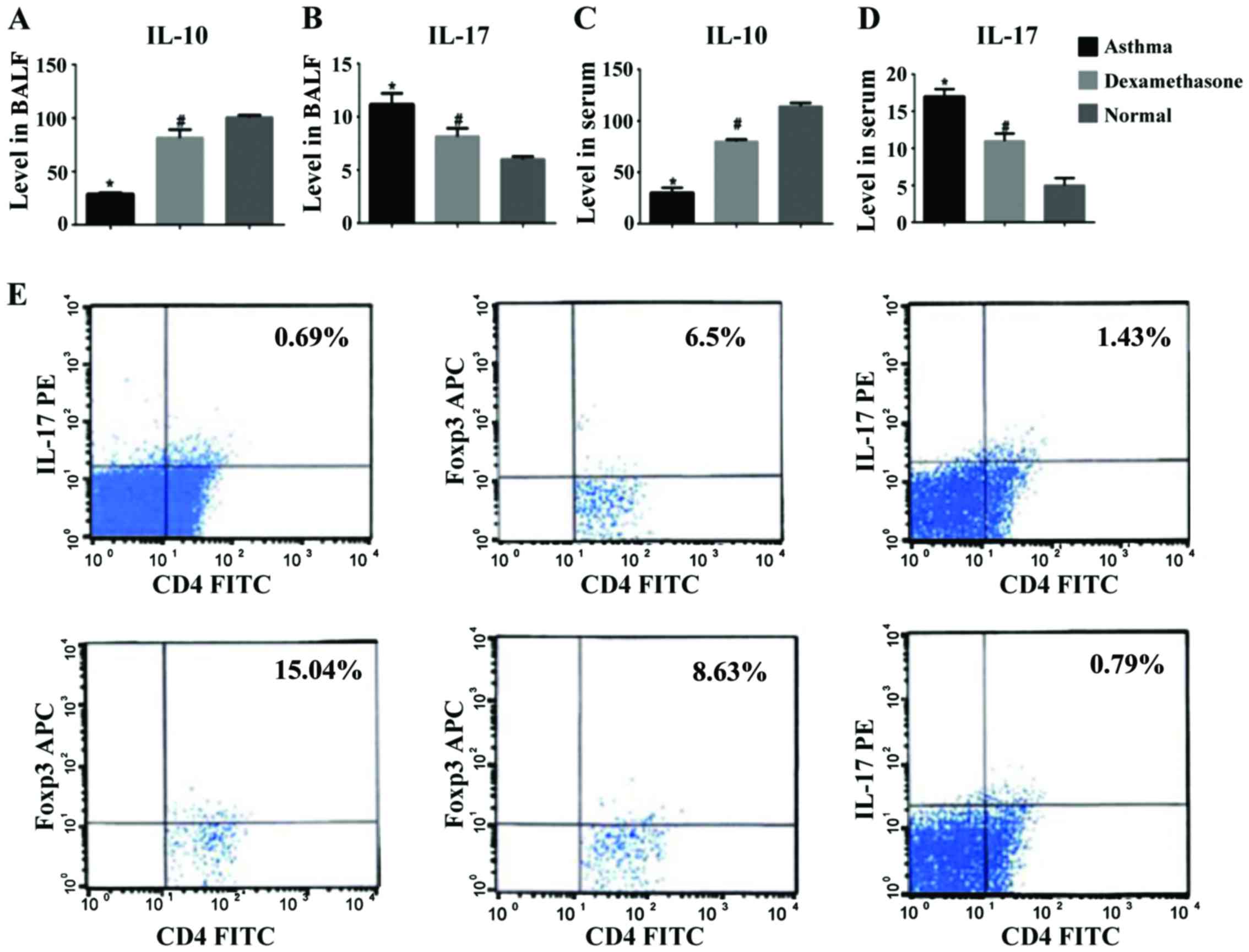
Changes in the content of IL-10 and
IL-17 in the BALF and serum, and the proportion of the
CD4+CD25+Foxp3+ and
CD4+IL-17+ cells of the lymphocyte population
in the CD4+ T cells
Based on the determination of content of the IL-10
and IL-17 cytokines in BALF and serum, it was found that the level
of IL-17 in BALF and serum of the mice in the asthma group were
increased significantly. There were significant differences between
the asthma group, and the dexamethasone treatment group and the
normal control group (P<0.01). The level of IL-17 in BALF and
serum in the dexamethasone treatment group decreased slightly when
compared with that in the asthma group but it was still higher when
compared with that in the normal control group. The level of IL-10
in BALF and serum of the mice in the asthma group was the lowest.
There were significant differences between the asthma group, and
the dexamethasone treatment group and the normal control group
(P<0.01). The level of IL-10 in BALF and serum in the
dexamethasone treatment group was increased slightly when compared
with that in the asthma group (Fig.
2A-D). The lymphocyte subset
CD4+CD25+Foxp3+
cells/CD4+ T cells in the asthma group were
significantly fewer than those in the normal control group. The
CD4+CD25+Foxp3+
cells/CD4+ cells in the dexamethasone treatment group
were increased slightly when compared with those in the asthma
group, but were still fewer than those in the normal control group
(P<0.01). The lymphocyte subset CD4+IL-17+
cells/CD4+ T cells in the asthma group were higher than
those in the normal control group. The ratio of
CD4+IL-17+ cells/CD4+ cells in the
dexamethasone treatment group was decreased slightly when compared
with that in the asthma group, but was still higher when compared
with that in the normal control group. There were statistically
significant differences between the two groups (P<0.01). The
ratio of CD4+CD25+Foxp3+
cells/CD4+IL-17+ cells in the asthma group
(50.4±0.45) was decreased when compared with that in the normal
control group (50.4±0.45). The ratio of
CD4+CD25+Foxp3+
cells/CD4+IL-17+ cells in the dexamethasone
treatment group was increased slightly when compared with that in
the asthma group but was still lower than that in the normal
control group. There were statistically significant differences
between the two groups.
Expression of the mRNAs of the
transcription factors Foxp3 and RORγt in pulmonary tissue of the
mice
The expression of the mRNA of RORγt in pulmonary
tissue of the mice in the asthma group was increased significantly
when compared with that in the normal control group. The expression
of the RORγt mRNA in the pulmonary tissue of the mice in the
dexamethasone treatment group was decreased and was significantly
lower than that in the asthma group. However, it was still higher
than that in the normal control group. There were statistically
significant differences between the two groups (P<0.01). The
expression of the mRNA of Foxp3 in the pulmonary tissue of mice in
the asthma group was decreased significantly when compared with
that in the normal control group. The expression of Foxp3 mRNA in
the pulmonary tissue of the mice in the dexamethasone treatment
group was increased slightly when compared with that in the asthma
group but was still lower than that in the normal control group.
There were statistically significant differences between the two
groups (P<0.01) (Table II).
 | Table II.Expression of IL-17, RORγt, IL-10,
and Foxp3 mRNA (mean ± SD) in the pulmonary tissue of the mice. |
Table II.
Expression of IL-17, RORγt, IL-10,
and Foxp3 mRNA (mean ± SD) in the pulmonary tissue of the mice.
| Group | n | IL-17 | IL-10 | RORγt | Foxp3 | Foxp3/RORγt |
|---|
| Asthma group | 10 |
0.30±0.05a,b |
0.22±0.07a,b |
0.64±0.08a,b |
0.11±0.05a,b |
0.18±0.05a,b |
| Dexamethasone
group | 10 |
0.16±0.04a |
0.58±0.08a |
0.52±0.07a |
0.31±0.08a |
0.59±0.14a |
| Normal control
group | 10 | 0.09±0.04 | 0.74±0.08 | 0.28±0.10 | 0.53±0.15 | 1.97±0.58 |
| F |
| 65.306 | 129.192 | 42.954 | 42.311 | 72.437 |
| P-value |
| 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Expression of the pulmonary
transcription factors of Foxp3 and RORγt
The transcription factors of Foxp3 and RORγt
expressed in the pulmonary tissue of the mice in the normal control
group were located in the cytoplasm and nuclei of the cells such as
lymphocytes, around the airways and in the pulmonary tissue. The
expression intensity of the transcription factor Foxp3 of the mice
in the asthma group was significantly lower and the expression
range decreased. The expression intensity and range of the
transcription factor of the mice in the dexamethasone treatment
group was slightly higher than that in the asthma group (P=0.015).
The intensity and range were still decreased when compared with
those in the normal control group. There were statistically
significant differences between the two groups (P<0.01). The
expression intensity and expression range of the positive cells of
RORγt of the mice in the dexamethasone treatment group was
decreased slightly when compared with that in the asthma group and
the differences were statistically significant (P<0.01).
The western blot analysis indicated that the
transcription factor Foxp3 protein was clearly expressed in the
pulmonary tissue of the mice in the normal control group but only
mildly expressed (or even not expressed) in the asthma group and
the differences were statistically significant (P<0.01).
Following the dexamethasone intervention, the expression of the
Foxp3 protein in the pulmonary tissue of the mice was increased and
was higher than that in the asthma group (P<0.01) (Fig. 3).
In contrast, the transcription factor protein RORγt
was lowly expressed in the pulmonary tissue of the mice in the
normal control group and highly expressed in the asthma group
(P<0.01). After dexamethasone intervention treatment, the
expression of the RORγt protein decreased and was lower than that
in the asthma group. However, it was still higher than that in the
normal control group. The differences between the two groups were
statistically significant (P<0.01).
Analysis of the correlation between
the Foxp3/RORγt protein expression ratio and airway
inflammations
An analysis was conducted for the correlation
between Foxp3/RORγt protein expression ratio and airway
inflammation indexes. The result indicated that the Foxp3/RORγt
protein expression ratio and airway responsiveness (%∆Penh) were
negatively correlated (Table
III).
 | Table III.Analysis of the correlation between
the Foxp3/RORγt protein expression ratio and the airway
inflammation. |
Table III.
Analysis of the correlation between
the Foxp3/RORγt protein expression ratio and the airway
inflammation.
| Item | r | P-value |
|---|
| %∆Penh |
|
|
|
3.125 | −0.739 | <0.001 |
|
6.25 | −0.752 | <0.001 |
|
12.5 | −0.733 | <0.001 |
| 25 | −0.854 | <0.001 |
| 50 | −0.867 | <0.001 |
| Count of
eosinophilic granulocytes | −0.815 | <0.001 |
| Count of
lymphocytes | −0.865 | <0.001 |
| Count of
neutrophil | −0.867 | <0.001 |
| Serum IL-17 | −0.898 | <0.001 |
| BALF IL-17 | −0.854 | <0.001 |
| Serum IL-10 | −0.717 | <0.001 |
| BALF IL-10 | −0.819 | <0.001 |
When different concentrations (3.125, 6.25, 12.5,
25, and 50 mg/ml) of acetylcholine were used for activation, the
correlation coefficients of the expression of the appropriate
airway responsiveness (%ΔPenh) and Foxp3/RORγt protein expression
ratio were respectively: −0.739, 0.752, 0.733, 0.854, and 0.867
(P<0.01). The Foxp3/RORγt protein expression ratio and the
counts of eosinophilic granulocytes and lymphocytes and neutrophils
were negatively correlated (r=−0.815, −0.865, −0.867, P<0.01).
The Foxp3/RORγt protein expression ratio and serum and the content
of IL-17 in BALF were negatively correlated (r=−0.898, −0.854,
P<0.01). They were positively correlated with the content of
IL-10 in the serum and BALF (r=−0.717, −0.819, P<0.01).
Discussion
Bronchial asthma is a chronic allergic reaction
disease displaying increased responsiveness of the airways to
multiple stimulating factors involving inflammatory cells such as
eosinophilic granulocytes, lymphocytes and mastocytes (18).
After OVA stimulation, the %∆Penh value of asthma
model mice increased significantly. Based on pathological sections
of pulmonary tissue, we observed infiltration of a larger number of
inflammatory cells around the bronchioles and concomitant vessels,
eosinophilic granulocytes within the pulmonary mesenchymal and
alveolar cavity, mucous plugs within the bronchial lumen, decreased
mucosa plicae, hyperplasia of goblet cells, thickened basilar
membrane, widened alveolar septum, partial rupture, and formation
of emphysema. The total count of leukocytes in BALF and the count
of eosinophilic granulocytes increased significantly. The IL-5 of
the Th2 cytokines in BALF increased significantly as the Th1
cytokines decreased. The immune abnormalities discovered are
consistent with a state of Th2 hyperfunction and Th1
hypofunction.
In recent years, increasing research shows that Treg
cells affect the incidence and progression of asthma and allergic
diseases (19–22). The CD4+CD25+
Treg cells have become one of the research highlights as they can
inhibit Th1, Th2, and CD8+ T cells. Substantial research
has found that the quantity and functions of Treg cells are
decreased in autoimmune diseases (23). In our study, we found that the
proportion of CD4+CD25+Foxp3+
cells from the CD4+ cells is decreased significantly
when compared with the same proportion in the mice of the normal
control group. In addition, the levels of IL-10 in BALF and serum
in the asthma group are decreased significantly when compared with
those in the normal control group. The level of IL-10 and the
proportion of CD4+CD25+Foxp3+
cells in CD4+ cells are positively correlated. This
indicates that the amount and functions of Treg cells in asthma are
decreased significantly when compared with those in the normal
control group, finding consistent with other researchers results.
The conclusion is that Treg cells seem to protect the organism in
asthma. The decline in the numbers and functions of the Treg cells
is closely associated with the incidence and progression of
asthma.
The Th17 cells are another new subtype of
CD4+ T cells different from Th1 and Th2. The Th17 cells
play an important role in asthma. Studies indicate that the levels
of mRNA and protein IL-17 in pulmonary tissue, BALF, and serum in
asthma patients increase significantly and are positively
correlated with airway hyper-responsiveness (24,25).
Studies have shown the infiltration of pulmonary eosinophilic
granulocytes, the level of Th2-like cytokines, and the airway
hyper-responsiveness of mice with deletion of the IL-17 gene
decrease significantly (26).
Another study found that the eosinophilic granulocytes increase
when IL-17 is secreted during the onset of asthma. In our
experiments, we found that the proportion of the
CD4+IL-17+ T cells out of the CD4+
cells in the asthma group was increased significantly when compared
with that in the normal control group. The level of the
Th17-related cytokine IL-17 in BALF and serum was increased
significantly when compared with that in the normal control group,
suggesting that Th17 participates in the onset of asthma.
The number of
CD4+CD25+Foxp3+ cells in the
asthmatic mice was decreased significantly. There were
statistically significant differences between the mice in the
asthma group and those in the normal control group. The
CD4+IL-17+ cells were fewer in the normal
mice but highly expressed in the asthma mice. Our research also
found that the count of
CD4+CD25+Foxp3+ cells and that of
CD4+IL-17+ cells varied, while the levels of
their respective cytokines, IL-10 and IL-17, were changing.
Once asthma occurs in the mice, the
CD4+IL-17+ cells are increased and the
CD4+CD25+Foxp3+ cells decrease.
Disruption of the normal immune balance occurs in the
CD4+CD25+Foxp3+ (Treg) and the
CD4+IL-17+ cells, (Th17). The ratio of the
CD4+CD25+Foxp3+/CD4+IL-17+
cells is closely associated with various airway inflammation
indexes. A smaller ratio of
CD4+CD25+Foxp3+/CD4+IL-17+
cells leads to a larger value of %ΔPenh activated by acetylcholine.
A smaller ratio of
CD4+CD25+Foxp3+/CD4+IL-17+
cells leads to larger count of eosinophilic granulocytes,
neutrophils, and lymphocytes in BALF. Also, a smaller ratio leads
to a higher content of IL-17 in the serum and BALF and higher
expression of the IL-17 gene and protein in the pulmonary tissues.
The same ratio is negatively correlated with IL-17. This means that
an altered Treg/Th17 ratio may be one of the important causes of
airway inflammation in bronchial asthma.
The transcription factors are a class of
nucleoproteins that identify and are bound to specific DNA
regulatory sequences and stimulate or inhibit transcription. Any
abnormalities in their quantity or activity lead to abnormal
expression of the genes critical to cellular growth and
differentiation. Foxp3 and RORγt are key transcription factors
regulating differentiation of Treg and Th17 cells, respectively.
Based on our results, RORγt is highly expressed in the pulmonary
tissues of asthma mice, indicating that epithelial cells,
mononuclear macrophages and other cell types in the pulmonary
tissues secrete substantial amounts of TGF-β and IL-6 in response
to allergic stimulation. Locally released TGF-β and IL-6 result in
a high expression of the RORγt gene and protein in the
CD4+ T cells, thus inducing differentiation of the naive
T cells towards Th17. In addition, RORγt can also upregulate the
expression of the IL-23 receptor on the surface of CD4+
T cells, promote binding between IL-23 and its receptor, maintain
the survival of Th17, promote proliferation of Th17, and secrete
IL-17. IL-17, in turn, is bound to the IL-17 receptor on the
surface of such inflammatory cells as epithelial cells,
neutrophils, and eosinophilic granulocytes, thereby allowing for
release of various chemotactic factors and inflammatory mediators,
inducing aggregation of substantial inflammatory cells towards the
inflammatory site, and further promoting the incidence of the
pulmonary tissue inflammation. Our results indicate that the
transcription factors Foxp3 and RORγt in the normal mice are
uniformly expressed in an immunologic balanced state. Once asthma
in mice occurs, both high expression of RORγt and low expression of
the Foxp3, result in an imbalanced expression of the Foxp3/RORγt
ratio. Our research has also found that during asthma, while the
expression of the transcription factors Foxp3 and RORγt changes,
the levels of the cytokines IL-10 and IL-17 are also changing in a
manner consistent with a dominant differentiation of Th0 towards
Th17 creating a special immunological picture for asthma.
In conclusion, the imbalanced expression of the
transcription factors Foxp3/RORγt is closely associated with the
airway inflammations of asthma. This indicates that, in addition to
the known change in the Th1/Th2 ratio that occurs in the
pathogenesis of asthma, there is also a deregulation of the
Treg/Th17 ratio. At the same time, the levels of the corresponding
transcription factors T-bet/GATA3 and Foxp3/RORγt also reflect the
immunologic balance of Th1/Th2 and Treg/Th17, respectively. Changes
in the state of the Th1/Th2 and Treg/Th17 ratios play important
roles in the immunological mechanisms of asthma and probably
provide good novel targets for intervention strategies against
asthma.
Acknowledgements
The present study was funded by the Science and
Technology Development Plan Project of Shandong (grant no.
2012YD18113).
References
|
1
|
Barnes PJ: Therapeutic approaches to
asthma-chronic obstructive pulmonary disease overlap syndromes. J
Allergy Clin Immunol. 136:531–545. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Postma DS, Weiss ST, van den Berge M,
Kerstjens HA and Koppelman GH: Revisiting the Dutch hypothesis. J
Allergy Clin Immunol. 136:521–529. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Busse W, Banks-Schlegel S, Noel P, Ortega
H, Taggart V and Elias J; NHLBI Working Group, : Future research
directions in asthma: An NHLBI Working Group report. Am J Respir
Crit Care Med. 170:683–690. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gauthier M, Ray A and Wenzel SE: Evolving
concepts of asthma. Am J Respir Crit Care Med. 192:660–668. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Raedler D, Ballenberger N, Klucker E, Böck
A, Otto R, da Costa O Prazeres, Holst O, Illig T, Buch T, von
Mutius E, et al: Identification of novel immune phenotypes for
allergic and nonallergic childhood asthma. J Allergy Clin Immunol.
135:81–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ramstein J, Broos CE, Simpson LJ, Ansel
KM, Sun SA, Ho ME, Woodruff PG, Bhakta NR, Christian L, Nguyen CP,
et al: IFN-γ-producing T-helper 17.1 cells are increased in
sarcoidosis and are more prevalent than T-helper type 1 cells. Am J
Respir Crit Care Med. 193:1281–1291. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park SY, Jing X, Gupta D and Dziarski R:
Peptidoglycan recognition protein 1 enhances experimental asthma by
promoting Th2 and Th17 and limiting regulatory T cell and
plasmacytoid dendritic cell responses. J Immunol. 190:3480–3492.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shum AK: T cell types that take your
breath away. Sci Transl Med. 7:301fs332015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hinks T, Zhou X, Staples K, Dimitrov B,
Manta A, Petrossian T, Lum P, Smith C, Ward J, Howarth P, et al:
Multidimensional endotypes of asthma: Topological data analysis of
cross-sectional clinical, pathological, and immunological data.
Lancet. 385 Suppl 1:S422015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Keely S and Foster PS: Stop press:
Eosinophils drafted to join the th17 team. Immunity. 43:7–9. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hinks TS, Zhou X, Staples KJ, Dimitrov BD,
Manta A, Petrossian T, Lum PY, Smith CG, Ward JA, Howarth PH, et
al: Innate and adaptive T cells in asthmatic patients: Relationship
to severity and disease mechanisms. J Allergy Clin Immunol.
136:323–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chesné J, Braza F, Chadeuf G, Mahay G,
Cheminant MA, Loy J, Brouard S, Sauzeau V, Loirand G and Magnan A:
Prime role of IL-17A in neutrophilia and airway smooth muscle
contraction in a house dust mite-induced allergic asthma model. J
Allergy Clin Immunol. 135:1643–1643.e3. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tao B, Ruan G, Wang D, Li Y, Wang Z and
Yin G: Imbalance of peripheral th17 and regulatory t cells in
children with allergic rhinitis and bronchial asthma. Iran J
Allergy Asthma Immunol. 14:273–279. 2015.PubMed/NCBI
|
|
14
|
El-Zein M, Conus F, Benedetti A, Parent ME
and Rousseau MC: Evaluating the validity of a two-stage sample in a
birth cohort established from administrative databases.
Epidemiology. 27:105–115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Son HL, Park HR, Park YJ and Kim SW:
Effect of retinoic acid in a mouse model of allergic rhinitis.
Allergy Asthma Immunol Res. 7:590–598. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jacquet A: Innate immune responses in
house dust mite allergy. ISRN Allergy. 2013:7350312013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Everaere L, Ait-Yahia S, Molendi-Coste O,
Vorng H, Quemener S, LeVu P, Fleury S, Bouchaert E, Fan Y, Duez C,
et al: Innate lymphoid cells contribute to allergic airway disease
exacerbation by obesity. J Allergy Clin Immunol. 138:1309–1318.e11.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Houghton LA, Lee AS, Badri H, DeVault KR
and Smith JA: Respiratory disease and the oesophagus: Reflux,
reflexes and microaspiration. Nat Rev Gastroenterol Hepatol.
13:445–460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kinoshita T, Baatjes A, Smith SG, Dua B,
Watson R, Kawayama T, Larche M, Gauvreau GM and O'Byrne PM: Natural
regulatory T cells in isolated early responders compared with dual
responders with allergic asthma. J Allergy Clin Immunol.
133:696–703. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lluis A, Depner M, Gaugler B, Saas P,
Casaca VI, Raedler D, Michel S, Tost J, Liu J, Genuneit J, et al
Protection Against Allergy, : Study in Rural Environments Study
Group: Increased regulatory T-cell numbers are associated with farm
milk exposure and lower atopic sensitization and asthma in
childhood. J Allergy Clin Immunol. 133:551–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Frischmeyer-Guerrerio PA, Guerrerio AL,
Oswald G, Chichester K, Myers L, Halushka MK, Oliva-Hemker M, Wood
RA and Dietz HC: TGFβ receptor mutations impose a strong
predisposition for human allergic disease. Sci Transl Med.
5:195ra942013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brandt EB, Kovacic MB, Lee GB, Gibson AM,
Acciani TH, Le Cras TD, Ryan PH, Budelsky AL and Hershey GK
Khurana: Diesel exhaust particle induction of IL-17A contributes to
severe asthma. J Allergy Clin Immunol. 132:1194–1204.e2. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Roychoudhuri R, Hirahara K, Mousavi K,
Clever D, Klebanoff CA, Bonelli M, Sciumè G, Zare H, Vahedi G, Dema
B, et al: BACH2 represses effector programs to stabilize
T(reg)-mediated immune homeostasis. Nature. 498:506–510. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Massoud AH, Charbonnier LM, Lopez D,
Pellegrini M, Phipatanakul W and Chatila TA: An asthma-associated
IL4R variant exacerbates airway inflammation by promoting
conversion of regulatory T cells to TH17-like cells. Nat Med.
22:1013–1022. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han RF, Li HY, Wang JW and Cong XJ: Study
on clinical effect and immunologic mechanism of infants capillary
bronchitis secondary bronchial asthma treated with bacterial
lysates Broncho-Vaxom. Eur Rev Med Pharmacol Sci. 20:2151–2155.
2016.PubMed/NCBI
|
|
26
|
Finotto S: T-cell regulation in asthmatic
diseases. Chem Immunol Allergy. 94:83–92. 2008. View Article : Google Scholar : PubMed/NCBI
|