Introduction
Numerous ocular diseases, including age-associated
macular degeneration, glaucoma and retinitis pigmentosa, cause a
severe and irreversible loss of visual function. Patients with
these conditions suffer progressive visual decline resulting from
irreversible loss of retinal neurons; however, at present, no
therapies are available to repair or replace the damaged retinal
cells (1). Advances in molecular
biology have identified innovative approaches, including stem cell
therapy, which may potentially repair or regenerate diseased retina
and subsequently restore visual function in eyes with degenerative
retinal disorders (2,3). Various cell sources for replacement of
retinal neurons have been identified, including retinal progenitor
cells (RPCs), brain neural stem cells (BNSCs), embryonic stem
cells, induced pluripotent stem cells and mesenchymal stem cells
(4,5). Among these, RPCs and BNSCs, which are
derived from the committed central nervous tissue, are two
promising types of stem cell for retinal replacement therapy. These
cells can be expanded to generate large numbers of cells, which can
then be differentiated into major neural retinal cell types,
including photoreceptor cells (6,7). When
transplanted, RPCs and BNSCs are incorporated into the neural
retina have been shown to rescue vision in animal models.
Furthermore, it was revealed that RPCs from younger donors show
better integration than those derived from older donors, and more
extensive integration occurs when the host retina is diseased or
injured (8,9). As with RPCs, integration of BNSCs is
increased when transplanted into young or injured host retina;
however, BNSCs transplanted into healthy adult monkeys showed
little migration or integration, forming a monolayer of stable
BNSCs (10,11). This phenomenon suggested that more
attention should be paid to changes in the local micro-environment
that occur in response to degeneration, trauma or ischemia, hypoxia
and ischemia/reperfusion, which lead to pathological changes that
produce free radicals, intracellular calcium overload and finally
induce cell apoptosis.
Compound anisodine (CA) is a Traditional Chinese
Medicine, which is a compound preparation made from hydrobromide
anisodine and procaine hydrochloride. CA have previously been shown
to regulate the vegetative nervous system, improve
microcirculation, scavenge reactive oxygen species and has been
commonly utilized as a neuroprotective agent to treat primary and
secondary chemic optic neuropathy and choroidoretinopathy,
including central retinal vein occlusion, occlusion, blepharospasm,
glaucoma, optic atrophy, senile macular degeneration, childhood
amblyopia, improving the anterior ischemic optic neuropathy of the
vessels, reducing vascular resistance and restoring patients'
vision (12–15). However, whether CA has an effect on
stem cells has remained elusive. The present study aimed to
investigate the neuroprotective effects of CA by assessing its
restorative effects on the proliferation and calcium overload of
hypoxia-induced rat RPCs and BNSCs.
Materials and methods
Isolation and culture of neonatal rat
RPCs and BNSCs
A total of eighty neonatal Sprague Dawley (SD) rats
on postnatal day 0 (P0) were obtained from the Laboratory Animal
Center of Xi'an Jiaotong University Health Science Center (Xi'an,
China) and housed in a room with a constant temperature of 24°C and
a relative humidity of 50±15% under a 12-h light/dark cycle, and
handling protocols were approved by the Institutional Animal Care
and Use Committee of Xi'an Jiaotong University Health Science
Center. Eyes and brains from 80 neonatal SD rats, which were
sacrificed by decapitation were enucleated and washed several times
with PBS. The neuroretinas and cerebral cortex were respectively
dissected from each eye and the brain, minced into small pieces,
centrifuged at 398.3 × g at 4°C for 5 min and resuspended.
Suspensions of RPCs and BNSCs were collected after passing them
through a 100-mm mesh strainer, followed by culture in Dulbecco's
Modified Eagle's Medium/F12 medium [10 ng/ml recombinant human
endothelial growth factor, 20 ng/ml recombinant human basic
fibroblast growth factor, 2% B27 supplement, 1% N2 supplement, 100
U/ml penicillin and 100 µg/ml streptomycin (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA)] at 37°C with 5% CO2
and 90% humidity. The RPC and BNSC populations were enriched based
on their ability to form neurospheres within 3–5 days, and the
cultures were passaged every 2–3 days by mechanical trituration to
obtain single cell suspensions that were then diluted 1:2 with
fresh culture medium. The cells were collected at the first passage
and cultured in 96-well plates at a density of 5–10×103
cells per well in 0.2 ml medium, in 24-well plates with
polylysine-coated glass slides in each well at a density of
5–10×104 cells per well in 0.5 ml medium or in 6-well
plates at a density of 5–10×105 cells per well in 2 ml
medium.
Identification of RPCs and BNSCs by
fluorescent immunocytochemistry
The RPCs and BNSCs were characterized by
immunofluorescence staining for progenitor and eye field
developmental marker paired box (Pax)6 and neural stem
cell-specific marker Nestin. Cells were fixed with 4%
paraformaldehyde, washed three times with 1X PBS, incubated in
blocking buffer (0.3% Triton X-100 in 5% goat serum (Invitrogen;
Thermo Fisher Scientific, Inc.) for 1 h at room temperature and
stained with primary antibodies [mouse monoclonal to Nestin (cat.
no. 2Q178, 1:200 dilution; Abcam, Cambridge, MA, USA) and rabbit
polyclonal to Pax6 (cat. no. ab5790; 1:100 dilution, Abcam)]
overnight at 4°C in blocking buffer. The cells were then washed
three times with 1X PBS, stained with secondary antibodies [goat
Cy3-conjugated anti-mouse or goat fluorescein isothiocyanate
(FITC)-conjugated anti-rabbit (ZF-0511, Alexa Fluor 488, 1:200
dilution and ZF-0513, Alexa Fluor 594, 1:200 dilution; Zhongshan
Goldenbridge Biotechnology, Co., Ltd., Beijing, China),] for one
hour at room temperature. Subsequent to counterstaining with DAPI
for five min, cells were washed three times with 1X PBS prior to
imaging. The fluorescent images were captured using an inverted
fluorescence microscope (DP71; Olympus Corp., Tokyo, Japan).
Grouping
The harvested rat neuronal RPCs and BNSCs were
divided into 10 groups as follows: i) NC, normal control group, in
which the cells were cultured under normal conditions for 4 h; ii)
hypoxia model (HM) group, in which cells were cultured in an
incubator at 37°C, containing 5% CO2 + 95% N2
(<1% oxygen) and saturated humidity for 4 h; iii-vi) Pre-hypoxia
groups, treated with CA (Beijing Zizhu Pharmaceutical Co., Ltd.,
Beijing, China) at various concentrations (C1, 0.126; C2, 0.252;
C3, 0.505; C4, 1.010 g/l) prior to hypoxia; vii-x) Post-hypoxia
groups, treated with CA (C1-4) after hypoxia. The Pre-hypoxia
groups were treated with CA just before the 4 h-hypoxia, and the
Post-hypoxia groups were treated with different concentrations of
CA at the start of reoxygenation following being cultured in
hypoxia for 4 h. Both groups were assessed in the subsequent assays
following 4 h of culture in normal conditions. The morphology
images were captured using an inverted phase contrast microscope
(CKX41; Olympus Corp.).
Cell viability assay
Cell viability was determined by an MTT assay
(Boster Biological Techonology Inc., Wuhan, China) in 96-well
plates at 24 h after the cells were treated. In brief, cells were
incubated with 20 µl MTT reagent in 100 µl culture media for 4 h at
37°C. After centrifugation at 398.3 × g, the culture media was
removed, 0.15 ml dimethylsulfoxide was added to each well and the
plates were agitated for 10 min. Optical density (OD) values were
measured at 490 nm using an automatic enzyme-linked immunity
analyzer (SS228BEPIII, Beijing Chinese and Western technology Co.,
Ltd., Beijing, China). The survival rate was calculated as follows:
Survival rate (%)=(ODdrug group-ODHM
group)/(ODNC group-ODHM group)
×100%.
Bromodeoxyuridine (BrdU) incorporation
assay
To assess the proliferation of the treated RPCs and
BNSCs, the cultures were incubated with 10 µM BrdU (Sigma-Aldrich;
Merck KGaG, Darmstadt, Germany; cat. no. B-5002) for 4 h and then
processed for immunofluorescence staining. The cells were fixed in
4% paraformaldehyde, permeabilized with blocking buffer and then
treated with 2 N HCl in 37°C for 40 min, followed by incubation
with 0.1 M borate buffer (pH 8.5) at room temperature for 12 min.
The cells were stained with mouse monoclonal antibody to BrdU (cat.
no. Bu20a; 1:1,000 dilution; Cell Signaling Technology, Inc.,
Danvers, MA, USA) overnight at 4°C followed by incubation with goat
FITC-conjugated anti-mouse antibody (1:200 dilution; Zhongshang
Goldenbridge Biotechnology, Co., Ltd.). In each group, ten fields
of view were observed under the microscope for the counting of
BrdU+ cells.
Cell cycle analysis by flow cytometry
(FCM)
The cells were grown in 6-well plates at a density
of 1×106 cells/well. Following incubation at 37°C for 24
h, cells were divided into four groups: Normal control group,
hypoxia model group, C4 Pre-hypoxia group and C4 post-hypoxia
group. Then, cells were cultured following hypoxia for 48 h under
normoxia condition. RPCs were collected and cell suspensions were
fixed using 75% ethanol. Fixed cells were then treated with 0.1 g/l
RNaseA (Sigma-Aldrich; Merck KGaG) and stained with 0.1 g/l
propidium iodide (Sigma-Aldrich; Merck KGaG). At least 10,000
events were acquired on a BD FACSCalibur® flow cytometer
(BD Biosciences, Franklin Lakes, NJ, USA) with an
excitation/emission wavelength of 488/630 nm in order to determine
the proportion of cell cycle distribution [(S+G2)%]. The results
were analyzed using CellQuest™ Pro Software (Version 5.1; BD
Biosciences).
Measurement of the intracellular
calcium concentration ([Ca2+]i)
[Ca2+]i was measured with the
membrane-permeant acetoxymethyl ester (AM) form of the
Ca2+-sensitive fluorescent dye Fluo 4 (Fluo 4-AM), and
the fluorescence intensity was measured via laser scanning confocal
microscopy (LSCM). The cultures were loaded with 3 µM Fluo 4-AM
(Dojindo, Kumamoto, Japan), which was first dissolved in dimethyl
sulfoxide with Pluornic F-127 (0.05%) and incubated for 45 min at
room temperature in D-Hank's solution. Following washing off any
extracellular Fluo 4-AM, the samples were incubated in D-Hank's
solution for another 25 min. Fluorescence measurement was performed
using LSCM (Q550-CW; Leica, Wetzlar, Germany) with
excitation/emission wavelengths of 494/516 nm. The images were
analyzed using the software provided with the LSCM system, with the
average fluorescence per unit randomly measured. Each experiment
was performed in triplicate and in two independent assays.
Western blot analysis
After treatment with CA for 4 h, cultures were
harvested for protein analysis by western blot by lysis in
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Shanghai, China) and the protein concentration was
determined using the bicinchoninic acid protein assay kit (BCA1 AND
B9643, Sigma-Aldrich; Merck KGaG). Protein (80 µg) was separated by
8% SDS-PAGE, transferred to a polyvinylidene difluoride membrane
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) and blocked with 8%
non-fat milk in Tris-buffered saline containing Tween 20 (TBST) for
1 h at room temperature. Subsequently, blots were stained with the
primary antibodies (rabbit anti-cyclin D1 antibody, 1:1,000,
ab134175; mouse anti-HIF-1α, 1:1,000, ab113642; rabbit anti-VEGF
receptor 1, 1:1,000, ab32152; rabbit anti-phospho-ERK
1/2Thr202/Tyr204, 1:1,000, ab214362; rabbit anti-ERK
1/2, 1:2,000, ab196883; mouse anti-β-actin, 1:20,000, ab8226;
Abcam, Cambridge, UK) at 4°C overnight. After washing with TBST,
the membrane was incubated with horseradish peroxidase-conjugated
anti-rabbit immunoglobulin G (1:100,000 dilution; Abcam) at room
temperature for 1 h. Resulting bands were imaged with ECL Plus
(Merck Millipore KGaA, Darmstadt, Germany) and CL-Xposure film
(Thermo Fisher Scientific, Inc.), and band density was measured
using ImageJ software version 1.48 (National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
Values are expressed as the mean ± standard
deviation. One-way analysis of variance and Dunnet's post hoc test
were applied to the means in order to determine statistical
differences between experimental groups in RPCs or BNSCs. Moreover,
a Student's t-test was performed to examine whether there were any
differences between RPCs and BNSCs. All statistical analyses were
performed using SPSS software, version 17.0 (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Isolation and characterization of rat
RPCs and BNSCs
Rat RPCs and BNSCs were isolated from SD rats at P0,
and appeared as mulberry-like neurospheres suspended in the medium
with strong refraction. With increasing time in culture, the size
of the neurospheres increased; within 3–4 days, the large clumps of
spheres became dark in the center, indicating that they were ready
to be passaged. If left in culture for 7–8 days at passage 2, the
irregular microspheres adhered to the wells and differentiated into
dendritic-like nerve cells. To further characterize the neural
linage differentiation capability of rat RPCs and BNSCs, cells were
subjected to immunofluorescence staining for progenitor and eye
field developmental marker Pax6 and neural stem cell-specific
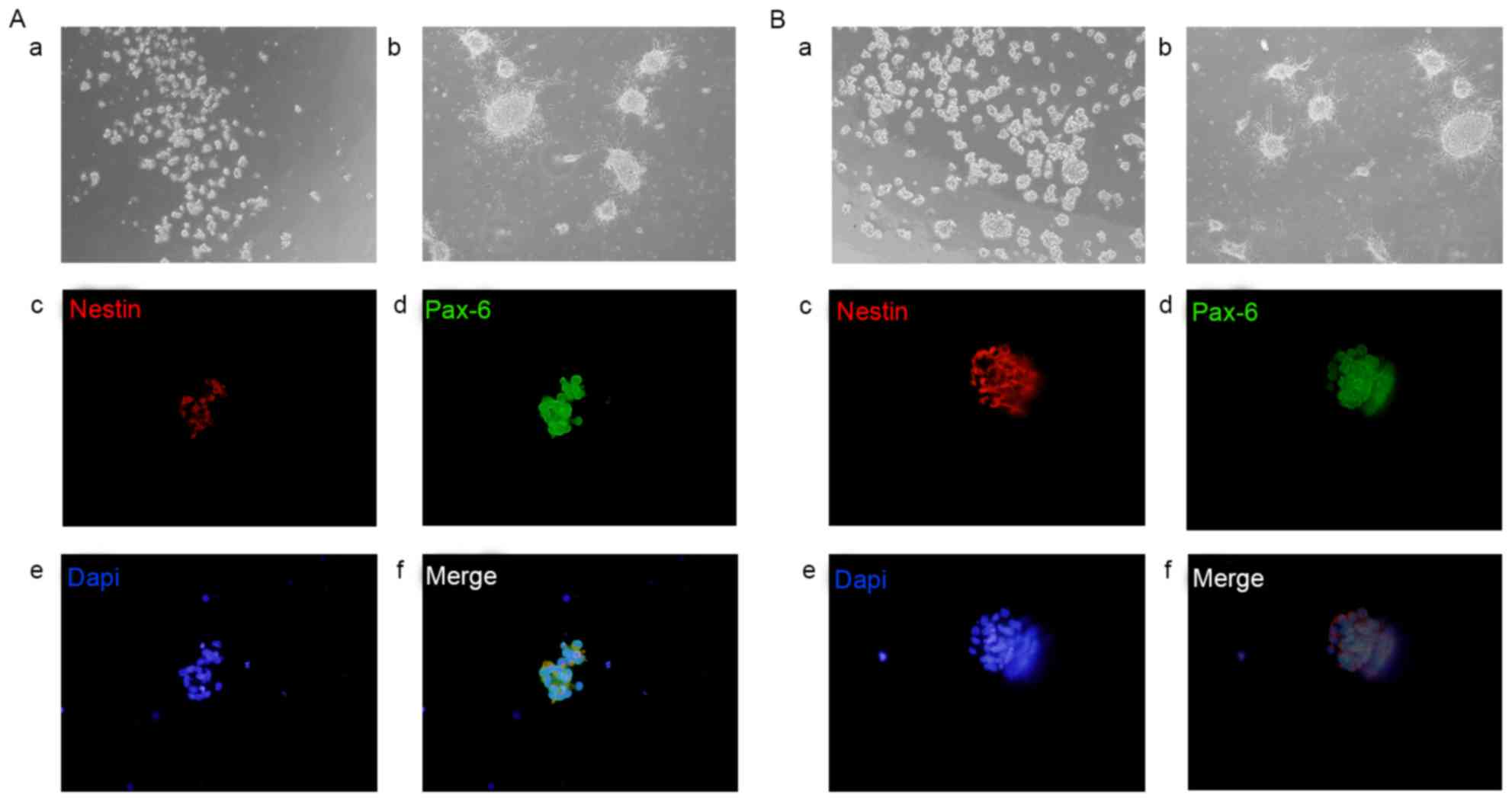
marker Nestin. Immunocytochemistry revealed that RPC and BNSC
neurospheres showed high levels of Nestin and Pax6 (Fig. 1).
CA improves the viability of RPCs and
BNSCs under hypoxia
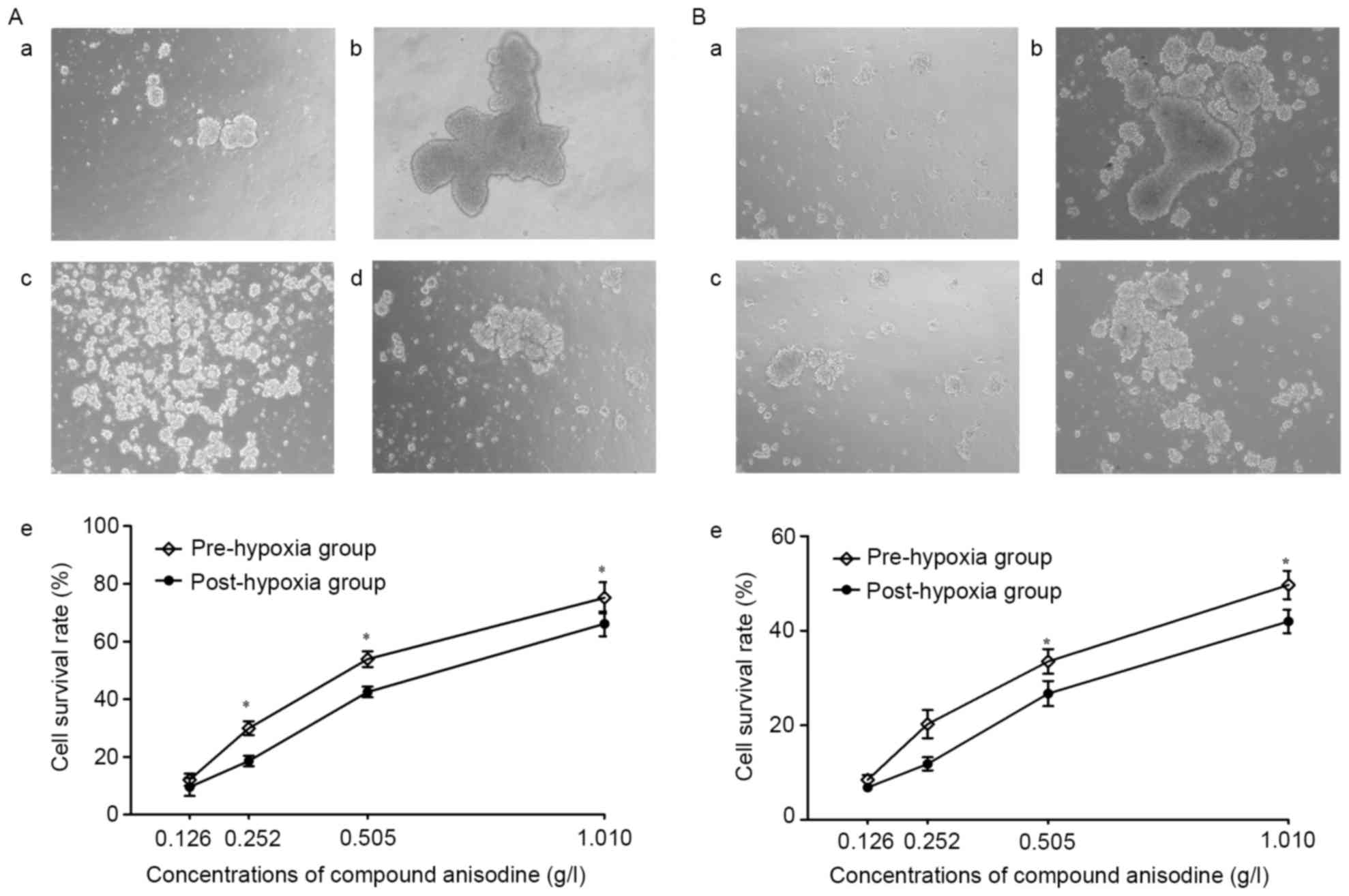
After culture in hypoxia (<1% oxygen) for 4 h,
the neurospheres aggregated to form large and irregular clusters,
which became dark in the center, indicating that an increasing
number of cells were dying; at the same time, the amount of
floating cell debris visible in the medium increased. The
morphological changes of the neurospheres were markedly improved
following treatment with 0.126–1.010 g/l CA, compared with the
hypoxia model group.
The cultured RPCs and BNSCs were treated with CA for
4 h prior to or after hypoxia, after which the cells were cultured
under normal oxygen conditions for 24 h. MTT detection demonstrated
that following treatment with 0.126–1.010 g/l CA, the cell
viability was markedly improved compared with that in the HM group.
In the C2, C3 and C4 concentration groups of RPCs and the C3 and C4
concentration groups of BNSCs, the cell viability in the
pre-hypoxia group was significantly higher as compared with that in
the post-hypoxia group. Furthermore, regardless of whether CA
treatment was performed prior to or after hypoxia, the cell
viability of RPCs was better than that of BNSCs in the C2, C3 and
C4 concentration groups (P<0.05; Fig.
2). These results suggested that 0.126–1.010 g/l CA may protect
RPCs and BNSCs against hypoxia-induced apoptosis, and treatment
prior to hypoxia showed superiority, with RPCs being more sensitive
to CA, as regardless of whether CA treatment was performed prior to
or following hypoxia, the cell viability of RPCs was higher
compared with the BNSCs in the C2, C3 and C4 concentration
groups.
CA attenuates hypoxia-induced
decreases in RPC and BNSC proliferation
BrdU incorporation of the cells was assessed after 4
h of incubation. Immunocytochemical staining indicated that after
culture in a hypoxia incubator (<1% oxygen) for 4 h, the BrdU
incorporation in RPCs (22.94±2.77%) and BNSCs (21.34±1.79%) was
significantly decreased compared with that in the normal control
groups (41.58±3.71 and 39.92±2.05%, respectively). Treatment with
1.010 g/l CA for 4 h prior to hypoxia significantly improved the
BrdU incorporation in RPCs (31.41±1.08%) and BNSCs (29.12±1.62%)
compared with that in the HM group (P<0.05; Fig. 3).
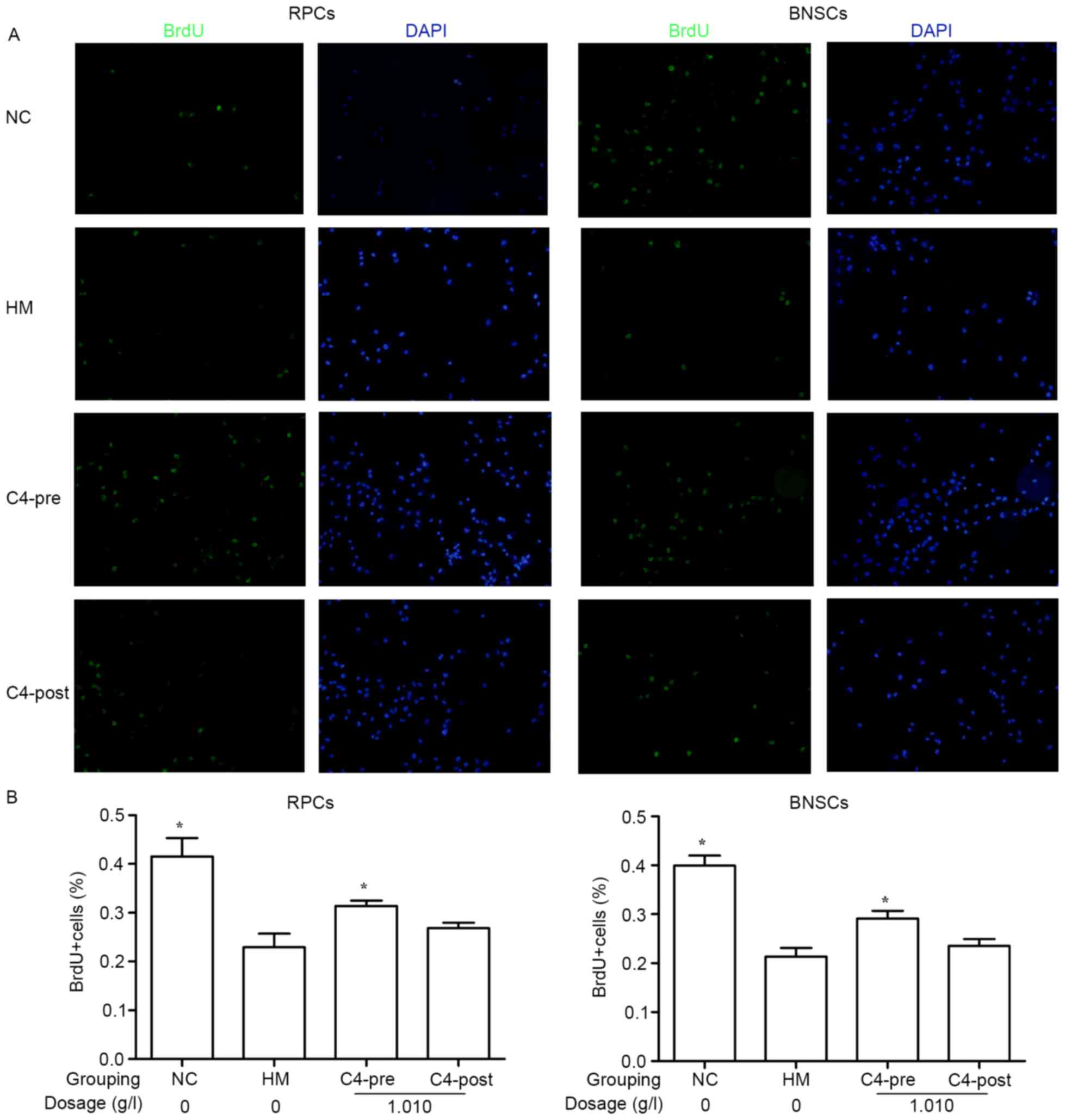 | Figure 3.Effects of 1.010 g/l CA on
hypoxia-induced RPC and BNSC proliferation. (A) Wide-field
microscopy analysis of BrdU incorporation in RPCs and BNSCs treated
with 1.010 g/l prior to and after hypoxia. BrdU immunocytochemistry
(green) was perfomed 4 h after addition of BrdU. (B) Quantification
of BrdU incorporation as representatively shown in A. Cells
displaying a clear nuclear BrdU signal were counted as positive
(magnification, ×400). *P<0.05 compared with HM group. BrdU,
bromodeoxyuridine; PRCs, retinal progenitor cells; BNSCs, brain
neural stem cells; HM, hypoxia model; NC, negative control; pre,
pre-hypoxia; post, post-hypoxia; C4, pre-treated with 1.010 g/l CA;
CA, compound anisodine. |
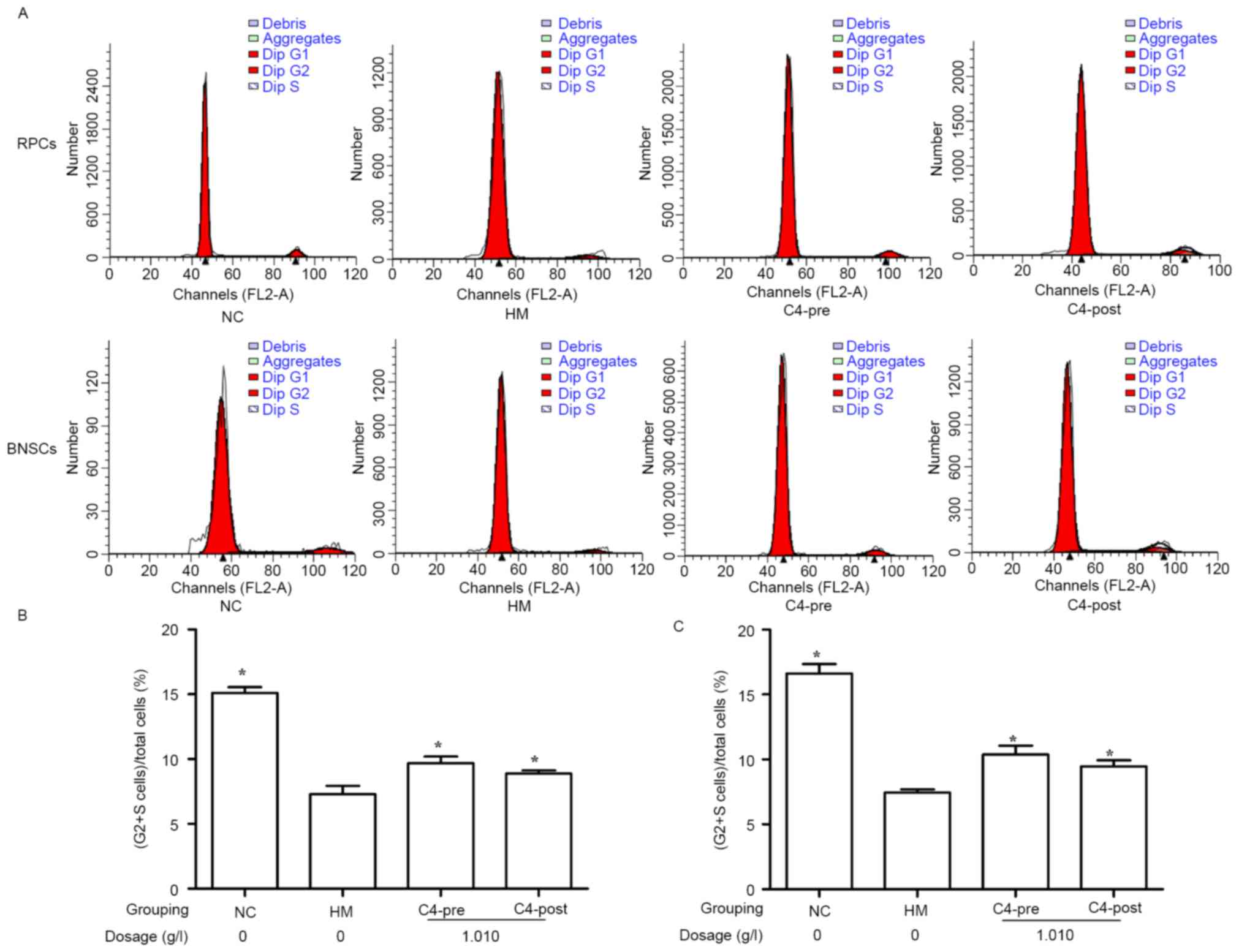
CA attenuates hypoxia-induced cell
cycle inhibition in RPCs and BNSCs
The influence of 1.010 g/l CA on the cell cycle of
hypoxia-induced RPCs and BNSCs was investigated using FCM. The
results demonstrated that after culture in a hypoxia incubator
(<1% oxygen) for 4 h, the (S+G2)% of RPCs and BNSCs was
significantly decreased as compared with that in the normal control
group (7.29±0.65 vs. 15.11±0.45% for RPCs and 7.45±0.25 vs.
16.63±0.71% for BNSCs; P<0.05). Furthermore, treatment with
1.010 g/l CA for 4 h prior to or after hypoxia significantly
increased the (S+G2)% of RPCs (9.68±0.51% in the C4 Pre-hypoxia
group; 8.88±0.24% in the C4 Post-hypoxia group) and BNSCs
(10.37±0.69% in the C4 Pre-hypoxia group; 9.48±0.47% in the C4
Post-hypoxia group) compared with that in the HM group (P<0.05;
Fig. 4).
CA inhibits hypoxia-induced
Ca2+ overload in RPCs and BNSCs
[Ca2+]i was measured using
Fluo 4-AM with detection of intracellular calcium fluorescence
intensity by LSCM. The results showed that the
[Ca2+]i in hypoxia-cultured RPCs and BNSCs
was markedly increased, and that 0.126–1.010 g/l CA elicited
concentration-dependent decreases in [Ca2+]i.
For the C2 and C4 concentration groups of RPCs and the C3 and C4
concentration groups of BNSCs, the [Ca2+]i in
the pre-hypoxia group was significantly decreased as compared with
that in the post-hypoxia group (P<0.05). Regardless of whether
CA treatment was performed prior to or after hypoxia, the
decreasing effect of CA on [Ca2+]i in RPCs
was greater than that in BNSCs in the C1 and C2 concentration
groups (P<0.05; Fig. 5).
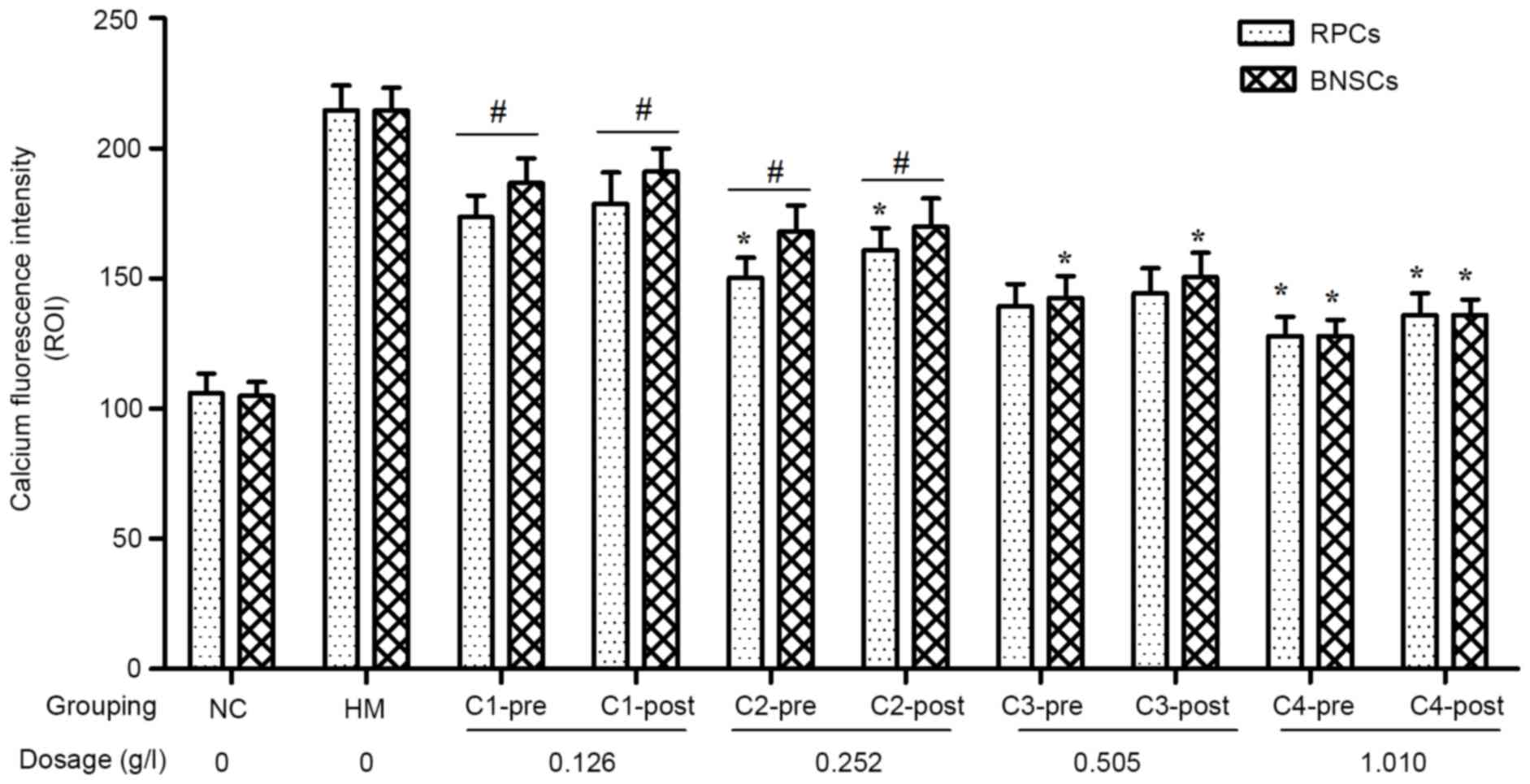 | Figure 5.Effect of various concentrations of CA
on intracellular calcium ion fluorescence intensity of RPCs and
BNSCs before and after hypoxia. *P<0.05 pre-hypoxia vs.
post-hypoxia groups; #P<0.05 RPCs vs. BNSCs. PRCs,
retinal progenitor cells; BNSCs, brain neural stem cells; HM,
hypoxia model; NC, negative control; pre, pre-hypoxia; post,
post-hypoxia; C1-4, pre-treated with 0.126, 0.252, 0.505 or 1.010
g/l CA, respectively; CA, compound anisodine. |
CA attenuates hypoxia-induced changes
in Cyclin D1, HIF-1α, VEGF and p-ERK in RPCs and BNSCs
Western blot analysis demonstrated that HIF-1α and
VEGF protein levels in the hypoxia-induced RPCs and BNSCs were
upregulated, whereas Cyclin D1 expression levels and p-ERK were
downregulated (P<0.05). After treatment with 1.010 g/l CA, the
expression of HIF-1α and VEGF was downregulated, whereas Cyclin D1
and p-ERK were upregulated in both cell types compared with those
in the HM group (P<0.05; Figs. 6
and 7). However, t-ERK was not
significantly affected by any of the treatments.
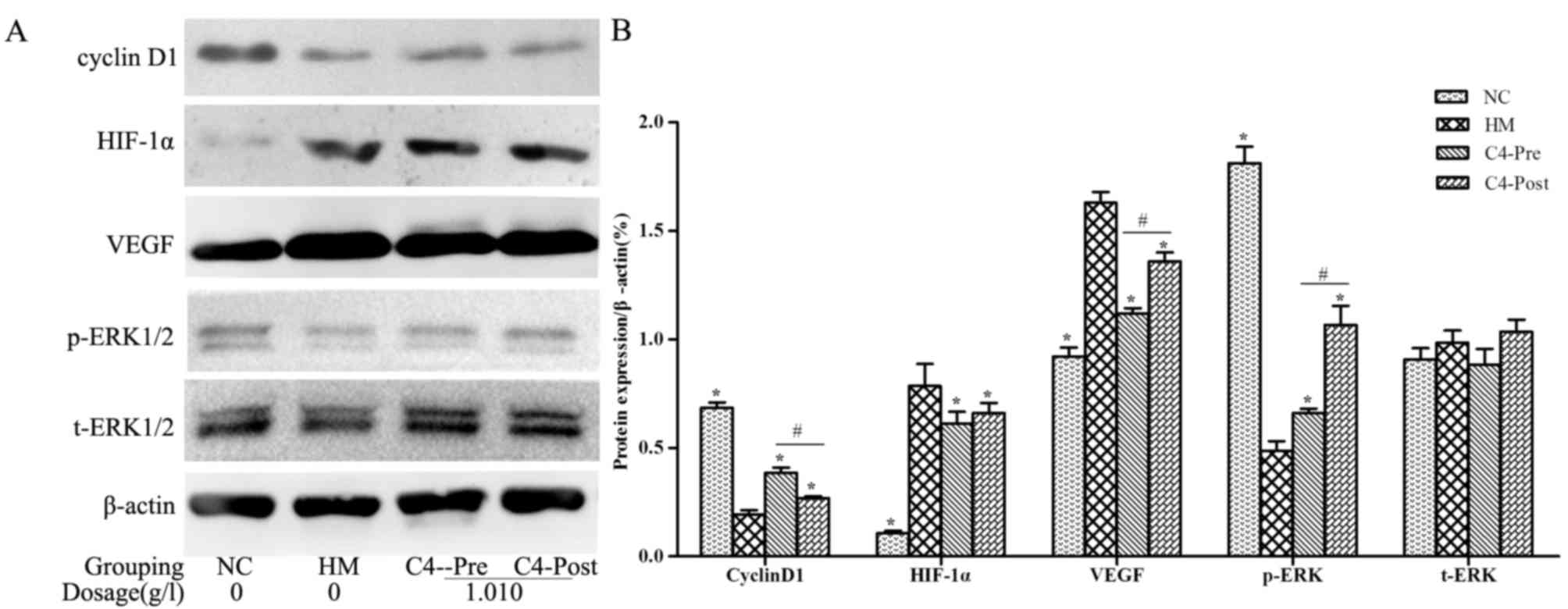 | Figure 6.CA affects the expression of Cyclin
D1, HIF-1α, VEGF, p-ERK and ERK in hypoxia-induced RPCs. (A)
Representative western blots showing the protein expression levels
of Cyclin D1, HIF-1α, VEGF, p-ERK and ERK in hypoxia-induced RPCs;
(B) Quantified results of the levels of Cyclin D1, HIF-1α, VEGF,
p-ERK and ERK protein in the NC, HM, C4-Pre and C4-Post groups of
RPCs. Data were derived from three independent experiments and are
expressed as the mean ± standard deviation. *P<0.05 vs. HM
group; #P<0.05 C4-pre vs. C4-post group. HIF,
hypoxia-inducible factor; p-ERK, phosphorylated extracellular
signal-regulated kinase; t, total; VEGF, vascular endothelial
growth factor; PRCs, retinal progenitor cells; HM, hypoxia model;
NC, negative control; pre, pre-hypoxia; post, post-hypoxia; C4,
pre-treated with 1.010 g/l CA; CA, compound anisodine. |
 | Figure 7.CA affects the expression of Cyclin
D1, HIF-1α, VEGF, p-ERK and ERK in hypoxia-induced BNSCs. (A)
Representative western blots showing the protein expression levels
of Cyclin D1, HIF-1α, VEGF, p-ERK and ERK in hypoxia-induced BNSCs.
(B) Quantified results of the levels of Cyclin D1, HIF-1α, VEGF,
p-ERK and ERK protein in the NC, HM, C4-Pre and C4-Post groups of
BNSCs. Data were derived from three independent experiments and are
expressed as the mean ± standard deviation. *P<0.05 vs. hypoxia
model group; #P<0.05 C4-Pre vs. C4-Post groups. HIF,
hypoxia-inducible factor; p-ERK, phosphorylated extracellular
signal-regulated kinase; t, total; VEGF, vascular endothelial
growth factor; BNSCs, brain neural stem cells; HM, hypoxia model;
NC, negative control; pre, pre-hypoxia; post, post-hypoxia; C4,
pre-treated with 1.010 g/l CA; CA, compound anisodine. |
Discussion
A variety of ocular diseases, including diabetic
retinopathy, retinopathy of prematurity, glaucoma and
age-associated macular degeneration, which are often caused by
ischemia, hypoxia and ischemic reperfusion of retinal lesions, lead
to a hypoxic microenvironment in damaged regions of the retina,
accompanied with changes of a series of cytokines and chemical
substances. When transplanted, it is inevitable that the
capabilities of stem cells to proliferate, differentiate, migrate
and integrate are affected by these substances (16). Numerous studies have confirmed that
severe hypoxia caused the increase of intracellular reactive oxygen
species (ROS), mitochondrial damage, and calcium overload,
eventually leading to cell apoptosis. However, it has become
obvious that hypoxia has a fundamental role in the maintenance of
the stem cell niche. Emerging evidence suggested that low oxygen
benefits the self-renewal of human embryonic, mesenchymal,
hematopoietic, retinal and brain neural stem cells, as well as
improving the efficiency of genetic reprogramming to induced
pluripotency (17).
The oxygen concentration in mammalian tissues varies
from 0.5% (retina) to 19% (upper airway epithelia), while it is
maintained at 20% during routine cell culture. In adult retina, the
oxygen concentration varies from 0.5% (inner nuclear layer) to 7%
(outer segments) (18–20). Over the last decade it has become
increasingly evident that the physiological condition of mild
hypoxia (2.5–5.0% O2) typical of neural tissues promotes
the self-renewal of NSC (21); it
also favors the success of engraftment when in
vitro-expanded NSCs are transplanted into the brain of
experimental animals. On the other hand, the cell death of NSCs
increases under lower oxygen conditions (<1% O2) and
peaks at anoxia (0% O2). In the present study, an oxygen
concentration of <1% was selected for the experiments and the
aim of the study was to investigate the neuroprotective effects of
CA on the proliferation and calcium overload of hypoxia-induced
RPCs and BNSCs. In the present study, observation showed that after
culture in a hypoxia incubator (<1% oxygen) for 4 h, the
neurospheres clumped together into large and irregular clusters,
which eventually became dark in the center; at the same time,
increasing amounts of floating cell debris were visible in the
medium, indicating that an increased number of cells were dying.
Furthermore, the results showed a reduction in cell proliferation
and viability as well as an increase in the
[Ca2+]i, in the hypoxia-induced RPCs and
BNSCs.
In a previous clinical study (12–15), CA
showed a favorable neuroprotective efficacy in various types of
chemic optic neuropathy and choroidoretinopathy, particularly in
the protection of the optic nerve in glaucoma and optic nerve
contusion, which indicated that CA may have a protective effect on
neurons and neural stem cells. Studies have shown that CA is
capable to relieve angiospasm and increasing ocular blood flow, and
to have anti-oxidant effects (22,23).
Moreover, Liu et al (24)
suggested that oral administration of CA protects the function of
retinal ganglion cell bodies and axons by increasing their survival
rates in a mouse model with high intraocular pressure (24). In the present study, 0.126–1.010 g/l
CA improved the viability and proliferation of hypoxia-induced RPCs
and BNSCs, and protected the two cell types against hypoxia-induced
calcium overload.
ERK1/2/HIF-1α/VEGF signaling is a critical pathway
of physiological responses to acute and chronic hypoxia. HIF-1α is
a heterodimeric transcription factor, composed of two subunits, the
HIF-1α (or its analogs HIF-2α and −3α) and HIF-1β subunits. HIF-1α
is an oxygen-sensitive subunit and its expression is induced under
hypoxic conditions (25). In the
present study, after culture in a hypoxia incubator (<1% oxygen)
for 4 h, the protein levels of HIF-1α and VEGF were increased in
RPCs and BNSCs. The ERK1/2 pathway is involved in hypoxia-induced
HIF-1α protein expression. Cyclin D1, as the most significant
positive regulatory factor of the cell cycle, has a key role in G1
phase regulation and G1/S phase transition. In the HM groups, the
(S+G2) % was decreased, indicating that cell mitosis was
suppressed, and Cyclin D1 expression levels were downregulated.
However, after treatment with 1.010 g/l CA, the expression of
HIF-1α and VEGF was downregulated, whereas p-ERK and Cyclin D1
expression levels were upregulated in both cell types compared with
those in the HM groups.
In conclusion, the results suggested that
0.126–1.010 g/l CA attenuated the hypoxia-induced inhibition of
proliferation of RPCs and BNSCs and protected against
hypoxia-induced calcium overload by altering the protein expression
levels of Cyclin D1 as well as hypoxia-associated proteins: HIF-1α,
VEGF and p-ERK. However, assessing the influence of CA on the
hypoxia tolerance of RPCs and BNSCs based on the experimental
results in vitro may not be representative of the true
effect of the complex microenvironment during retinal stem cell
transplantation. Future experiments will involve detailed analysis
of cellular and molecular events contributing to long-term effects
of CA, including the association between HIF-1α and intracellular
calcium ions.
While studies in the retinal stem cell field have
led to the identification of various cell sources and methods to
enhance transplant cell migration and integration, a large amount
of research is required for the implementation of cell-based
therapies for treating human retinal disease.
References
|
1
|
Garcia JM, Mendonça L, Brant R, Abud M,
Regatieri C and Diniz B: Stem cell therapy for retinal diseases.
World J Stem Cells. 7:160–164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Balmer J, Stanzel BV and Fischer MD: Stem
cell therapy for retinal diseases. Ophthalmologe. 112:728–737.
2015.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ng TK, Fortino VR, Pelaez D and Cheung HS:
Progress of mesenchymal stem cell therapy for neural and retinal
diseases. World J Stem Cells. 6:111–119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pellegrini G, De Luca M and Arsenijevic Y:
Towards therapeutic application of ocular stem cells. Semin Cell
Dev Biol. 18:805–818. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu D and Silva GA: Stem cell sources and
therapeutic approaches for central nervous system and neural
retinal disorders. Neurosurg Focus. 24:E112008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dunn-Thomas TE, Dobbs DL, Sakaguchi DS,
Young MJ, Honovar VG and Greenlee MH: Proteomic differentiation
between murine retinal and brain-derived progenitor cells. Stem
Cells Dev. 17:119–131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qu Z, Guan Y, Cui L, Song J, Gu J, Zhao H,
Xu L, Lu L, Jin Y and Xu GT: Transplantation of rat embryonic stem
cell-derived retinal progenitor cells preserves the retinal
structure and function in rat retinal degeneration. Stem Cell Res
Ther. 6:2192015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chacko DM, Das AV, Zhao X, James J,
Bhattacharya S and Ahmad I: Transplantation of ocular stem cells:
The role of injury in incorporation and differentiation of grafted
cells in the retina. Vision Res. 43:937–946. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Francis PJ, Wang S, Zhang Y, Brown A,
Hwang T, McFarland TJ, Jeffrey BG, Lu B, Wright L, Appukuttan B, et
al: Subretinal transplantation of forebrain progenitor cells in
nonhuman primates: Survival and intact retinal function. Invest
Ophthalmol Vis Sci. 50:3425–3431. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo Y, Saloupis P, Shaw SJ and Rickman DW:
Engraftment of adult neural progenitor cells transplanted to rat
retina injured by transient ischemia. Invest Ophthalmol Vis Sci.
44:3194–3201. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nishida A, Takahashi M, Tanihara H, Nakano
I, Takahashi JB, Mizoguchi A, Ide C and Honda Y: Incorporation and
differentiation of hippocampus-derived neural stem cells
transplanted in injured adult rat retina. Invest Ophthalmol Vis
Sci. 41:4268–4274. 2000.PubMed/NCBI
|
|
12
|
Chen YH and Wu LQ: Therapeutic effect of
compound anisodine for primary open angle glaucoma. Zhejiang Da Xue
Xue Bao Yi Xue Ban. 40:659–662. 2011.(In Chinese). PubMed/NCBI
|
|
13
|
Wang W, Huang Y, Zhang J, Jiang J and
Huang J: Efficacy of cytidine-5′-diphosp-bocholine combined with
compound anisodine in the treatment of early optic nerve contusion.
Eye science. 27:37–40. 2012.PubMed/NCBI
|
|
14
|
Wang Z: Observation on clinical
application of compound Anisodine for patients with optic atrophy
disease. Huli Yanjiu. 24:16572010.
|
|
15
|
Yi CM, Yu HY, Zhang L, Mfng W, Peng HY,
Liu LB and Yang CY: Clinical observation of compound anisodine
treating for ischemic ophthalmopathy. Inner Mongolia Med J. 1:011,
31–34. 2008.
|
|
16
|
Kelley MW, Turner JK and Reh TA:
Regulation of proliferation and photoreceptor differentiation in
fetal human retinal cell cultures. Invest Ophthalmol Vis Sci.
36:1280–1289. 1995.PubMed/NCBI
|
|
17
|
Clarke L and van der Kooy D: Low oxygen
enhances primitive and definitive neural stem cell colony formation
by inhibiting distinct cell death pathways. Stem Cells.
27:1879–1886. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baranov PY, Tucker BA and Young MJ:
Low-oxygen culture conditions extend the multipotent properties of
human retinal progenitor cells. Tissue Eng Part A. 20:1465–1475.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu DY and Cringle SJ: Oxygen distribution
in the mouse retina. Invest Ophthalmol Vis Sci. 47:1109–1112. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cringle SJ and Yu DY: Oxygen supply and
consumption in the retina: Implications for studies of retinopathy
of prematurity. Doc Ophthalmol. 120:99–109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo Z, Shi F, Zhang L, Zhang H, Yang J, Li
B, Jia J and Wang X and Wang X: Critical role of L-type
voltage-dependent Ca2+ channels in neural progenitor cell
proliferation induced by hypoxia. Neurosci Lett. 478:156–160. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song C, Shen W and He Q: An experimental
study on compound anisodine III for softening scar of mouse skin
after burn. Zhonghua Yan Ke Za Zhi. 32:176–178. 1996.(In Chinese).
PubMed/NCBI
|
|
23
|
Zhu Y, Song C and Wang S: The changes of
hemodynamics in ocular trauma and treatment with compound
anisodine. Zhonghua Yan Ke Za Zhi. 32:110–113. 1996.(In Chinese).
PubMed/NCBI
|
|
24
|
Liu WD, Chen LL, Shen CY and Jiang LB:
Neuroprotective effect of compound anisodine in a mouse model with
chronic ocular hypertension. Chin Med J (Engl). 128:2652–2657.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ejtehadifar M, Shamsasenjan K,
Movassaghpour A, Akbarzadehlaleh P, Dehdilani N, Abbasi P,
Molaeipour Z and Saleh M: The effect of hypoxia on mesenchymal stem
cell biology. Adv Pharm Bull. 5:141–149. 2015. View Article : Google Scholar : PubMed/NCBI
|