Introduction
Cardiovascular diseases remain a leading cause for
morbidity and mortality worldwide, although there have been a
number of in-depth basic and clinical studies (1,2).
Diabetes (DM) is a risk factor for coronary heart disease and
significantly increases the incidence of cardiovascular diseases.
In addition, mortality resulting from ischemic cardiovascular
complications account for 75% of all mortality associated with
diabetes (3).
Cardiovascular complications, such as accelerated
atherosclerosis are associated with a number of cellular and
subcellular changes in the vessels (4). It is generally accepted that vessels
affected by DM exhibit altered vasomotor function (5). In particular, vascular smooth muscle
cells (VSMCs) in patients with DM serve a critical role in numerous
cardiovascular pathologies (6).
Oxidative stress serves an important role in the
remodeling of arterial vascular tissue in patients with diabetes by
affecting the proliferation and apoptotic balance of VSMCs, in
addition to extracellular matrix synthesis (7). Following high-glucose stimulation,
intracellular oxygen free radicals accumulate and act as
intracellular messengers that activate various cellular pathways,
inhibit nitric oxide synthase activity and ultimately induce the
proliferation of smooth muscle cells (8). The exact mechanism of this process
remains unclear, although it is may be associated with glucose
auto-oxidation, over-activation of nicotinamide adenine
dinucleotide phosphate (NADPH) oxidase (9), activation of sarcoma viral oncogene
(10), non-enzymatic glycosylation
of proteins, increased activity of the polyol pathway, protein
kinase C activation (11) and
weakened scavenging activities of antioxidant systems. A previous
study demonstrated that the addition of antioxidants or free
radical scavengers reduced oxygen radical levels under high-glucose
conditions, thereby inhibiting the proliferation of VSMCs (12).
In mammalian cells, mitochondria act to produce
energy, regulate cell signaling pathways and cell growth, generate
reactive oxygen species (ROS) and apoptosis. The mitochondrion is a
highly dynamic organelle maintaining the homeostasis in a cell by
constant fusion and fission, a process called mitochondrial
dynamics. The relative speed of mitochondrial fusion-fission
determines organelle shape, number and distribution. This process
is precisely regulated by a series of dyneins associated with
guanosine triphosphate enzymes (13). These enzymes include mitochondrial
fusion-related protein mitofusin 1 (Mfn1), mitofusin 2 (Mfn2) and
optic atrophy 1 (Opa1) protein, mitochondrial fission-related
protein dynamin-related protein (Drp1) and fission 1 (Fis1)
protein. An increasing number of studies (14,15) have
identified a variety of proteins involved in mitochondrial dynamics
that are associated with VSMC proliferation, which therefore
implicates these proteins as potential drug targets. However, no
direct evidence has been provided to support the hypothesis that
mitochondrial dynamics participate in high-glucose induced VSCM
proliferation, and the mechanism remains to be elucidated.
In addition to produce adenosine triphosphate (ATP)
for the cell, mitochondria are also the primary site of ROS
production. A mutual regulation between mitochondrial dynamic
changes and oxidative stress levels has been identified. When
arterial VSMCs were isolated and exposed to high oxygen levels,
excessive fission of mitochondria was observed, in addition to
damage to the mitochondrial network structure (16), indicating that ROS levels may affect
mitochondrial fission. However, the direct impact of high oxidative
stress levels on mitochondrial dynamic changes in the diabetic
state remains to be elucidated.
Salidroside (p-hydroxyphenethyl-bD-glucoside) is the
primary active ingredient in Rhodiola rosea, a plant used in
traditional Chinese medicine (17).
Previous studies have revealed that Salidroside has protective
effects on the nerve, heart, liver and vascular system and may also
have beneficial effects on glucose metabolism (18–22).
Furthermore, it has already been demonstrated that Salidroside may
counteract the severe oxidative damage induced in vitro by
hypochlorous acid in human red blood cells (23,24).
However, extensive evidence of the role of Salidroside in the
prevention and treatment of cardiovascular diseases, the mechanism
and precise association between Salidroside and its protective
effects on VSMCs in diabetes are not completely understood.
The present study hypothesized that Salidroside may
inhibit high glucose (HG)-induced excessive proliferation of VSMCs,
resulting from changes to mitochondrial dynamics and ROS levels. In
addition, these metabolic changes were compared with alterations in
the level of the mitochondrial inhibition of Mdivi-1 at the same
time. The present study attempts to investigate the molecular
mechanisms underlying the potential protective effect of
Salidroside on VSMCs.
Materials and methods
Cell culture and reagents
The present study was conducted using vascular
smooth muscle cells purchased from the Shanghai RanTai Biological
Company (Shanghai, China). The vascular smooth muscle cells were
isolated from the aorta of 6–8 week-old male Sprague Dawley rats
using an enzymatic digestion method prior to purchase. Cells were
cultured in growth medium containing Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), 1% minimum essential medium
containing nonessential amino acids (25-025-CI; Corning Life
Sciences, Corning, NY, USA) and 500 µg/ml penicillin/streptomycin
(Gibco; Thermo Fisher Scientific, Inc.) under 5% CO2 at
37°C. The VSMCs were then treated with 10 and 25 µM Mdivi-1
(Sigma-Aldrich; Merck KGaA) or 0.3 and 0.5 mM Salidroside (Abcam,
Cambridge, UK) for 24 h either in low-glucose (5 mM) or
high-glucose (25 mM) conditions. In addition, two subgroups of
VSMCs treated with Salidroside under high-glucose conditions were
treated with H2O2 (25 mM) for 2 h at 5%
CO2 and 37°C to increase levels of ROS, or with
Mfn2-siRNA.
RNA interference and transfection
Small double-stranded RNAs (siRNAs) against rat Mfn2
and control sequences were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). The primer sequences for targeting of MFN2
were as follows: Forward, 5′-CAAGCACUUUGUCACUGCCAAGAAA-3′ and
reverse, 5′-UUUCUUCGGCAGUGACAAAGUGCUUG-3′. The negative control
scramble siRNA primer sequences were as follows: Forward,
5′-CCUCUUACCUCAGUUACAAUUUAUA-3′ and reverse,
5′-UAUAAAUUGUAACUGAGGUAAGAGG-3′. After 2 days of culture as above,
1×106 VSMCs were seeded into 6-well plates (Corning,
USA). After 1 day, cells at ~90% confluence were co-transfected
with siRNA and a EGFP pCDNA-plasmid, as a control to determine
transfection efficiency, using Lipofectamine™ 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Medium was refreshed 1 day after
transfection and cells were grown for a further 2 days at 5%
CO2 in a 37°C incubator.
Measurement of cell proliferation
A bromodeoxyuridine (BrdU) assay was used to
evaluate cell proliferation. The cells were treated with BrdU
(Sigma-Aldrich; Merck KGaA) for 2 h and incubated with an anti-BrdU
antibody (1:100; B8434; Sigma-Aldrich; Merck KGaA) overnight at
4°C, followed by incubation with Alexa Fluor® dyes for 2
min at room temperature (1:200; Invitrogen; Thermo Fisher
Scientific, Inc.). ELISA reader (Carl Zeiss AG, Oberkochen,
Germany) with a 450 nm filter was used to analyze the cellular
incorporation of BrdU.
Confocal laser scanning fluorescence
microscope
Confocal fluorescence microscopy was performed using
a Laser Scanning Confocal Microscope (TCS SP5; Leica Microsystems,
Inc., Buffalo Grove, IL, USA). Following drug treatment, the cells
were incubated for 30 min at room temperature with 100 nM
Mito-Tracker Red (Beyotime Institute of Biotechnology, Haimen,
China) to stain the inner mitochondrial membrane and the cells were
imaged at ×400 magnification. Image J software (V 2.1.4.7; National
Institutes of Health, Bethesda, MA, USA) was used to analyze the
mitochondrial fragmentation count (MFC).
Western blot analysis
Following treatment with Salidroside and Mdivi-1 for
24 h, 1×106 cells were harvested and lysed in
radio-immunoprecipitation assay buffer with 1 mM
phenylmethylsulfonyl fluoride at 4°C, then centrifuged at 12,000 ×
g for 10 min at 4°C. Total protein was quantified using the
bicinchoninic acid method (Beijing CoWin Biotech Co., Ltd.,
Beijing, China) and lysates (200 µl) containing 2 mg total protein
were boiled for 8 min in 800 µl 5X SDS Loading sample buffer, after
which 10 µl of each sample was collected. Samples were then
separated by 12% SDS-PAGE and transferred to polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA) using
standard procedures (25). Membranes
were then blocked with 5% non-fat dry skimmed milk (Sigma-Aldrich;
Merck KGaA) in a 0.05% tris-buffered saline with Tween-20 solution
(containing 20 mM/L Tris, pH 7.6, 137 mM/L NaCl and 0.05% Tween)
for 1 h at room temperature. Subsequently, the blots were probed
with the following primary antibodies at 4°C overnight: Mouse
anti-Drp 1 (ab56788; 1:500; Abcam), mouse anti-Mfn2 (ab56889;
1:500; Abcam) and Rabbit anti-GAPDH (sc-47724; 1:1,000; EPR16891;
Santa Cruz Biotechnology, Inc.). The blot was then washed with
Tris-buffered saline with Tween-20 (150 mM NaCl, 10 mM Tris, 0.05%
Tween-20; pH 8.0) and incubated with the secondary goat anti-mouse
antibodies (sc-2039; 1:5,000; Santa Cruz Biotechnology, Inc.) for 1
h at room temperature. Finally an enhanced chemiluminescence kit
(345818; EMD Millipore) was used for signal detection. Three
independent experiments were performed.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from VSMCs using a total RNA
extraction kit (Takara MiniBEST Plant RNA Extraction kit; Takara
Bio, Inc., Otsu, Japan). Reverse transcription of 1,000 ng total
RNA in a 20 ul system was performed using a cDNA Synthesis Reverse
Transcriptase kit (PrimeScriptTM reagent kit; Takara Bio, Inc.)
according to the manufacturer's protocol. Primer sequences were
designed with Beacon Designer™ Software (Premier
Biosoft, Palo Alto, CA, USA; Table
I). RT-PCR was carried out in a 20 µl total reaction volume
containing 6 µl H2O, 10 µl SYBR Premix Ex Taq II (Takara
Bio, Inc.), 0.4 µl ROX Reference Dye, 0.8 µl of each primer (10 µM
each), and 2 µl cDNA using a ABI PRISM 7500 Sequence Detection
System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
expression levels of mRNA for target genes were normalized using
GAPDH as a reference gene. Finally, relative mRNA expression ratios
were calculated with a mathematical model:
Ratio=(Etarget)ΔCqtarget(control-sample)/(ERef)ΔCqref(control-sample)
(26). Three independent experiments
were performed.
 | Table I.Sequences of primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Sequences of primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Sequence
(5′-3′) | Product length,
bp | Tm, °C |
|---|
| GAPDH |
F-GGCATCGTGGAAGGGCTCATG | 186 | 63.70 |
|
|
R-GCCAGTGAGCTTCCCGTTCAG |
| 63.54 |
| Drp1 |
F-GTGGTCAGGAACCGACAACAG | 160 | 61.14 |
|
|
R-GCAACTGGAACTGGCACATATAG |
| 61.72 |
| Mfn2 |
F-GCAGTGGGCTGGAGACTCATC | 118 | 62.78 |
|
|
R-CCACAAACATGGCGCTTGAAGG |
| 62.60 |
Measurement of ROS levels and NADPH
oxidase activity (ELISA assay)
The concentration of ROS in VSMCs was measured using
a dichloro-dihydro-fluorescein diacetate-ELISA assay (ROS activity
assay kit; Nanjing JianCheng Bioengineering Institute, Nanjing,
China), according to the manufacturer's instructions. NADPH oxidase
activity was measured using NADPH oxidase activity assay kit
(Genmed Scientifics, Inc., Wilmington, DE, USA) according to the
manufacturer's protocol.
Statistical analysis
All quantitative data and experiments described in
the present study were repeated at least three times. Results were
expressed as mean ± standard deviation. Differences between groups
were analyzed statistically by student's T-test (two groups) or
one-way analysis of variance (multiple groups) followed by Tukey
post hoc test using SPSS, version 15.0 (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to represent statistically
significant differences.
Results
Salidroside inhibits vascular smooth
muscle cell proliferation induced by high glucose
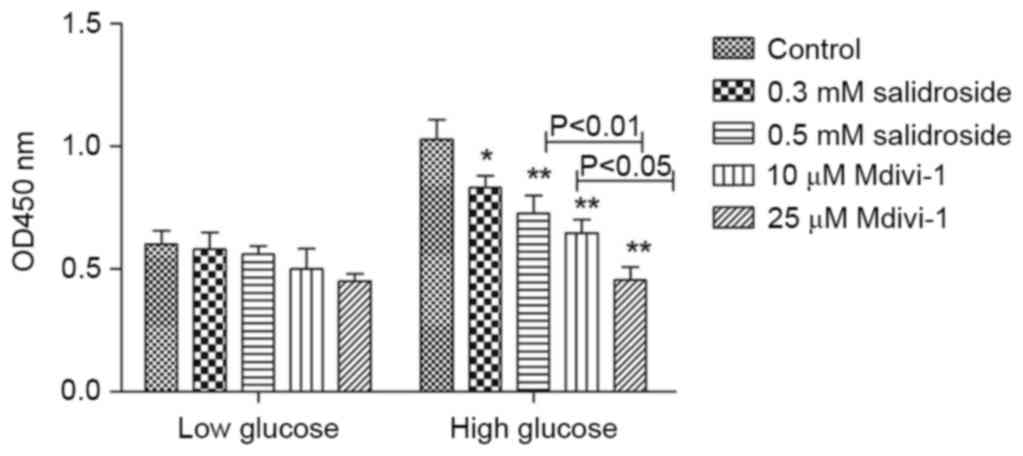
Under high glucose conditions, Salidroside
significantly inhibited the hyper-proliferation of VSMCs in a
concentration-dependent manner (P<0.05; Fig. 1). A similar effect was observed in
Mdivi-1. The effect of 0.5 mM Salidroside had no statistical
difference with that of 10 µM Mdivi-1 (Fig. 1). Previous results have demonstrated
that proteins involved in mitochondrial dynamics are associated
with VSMC proliferation (27).
Collectively, these results suggest that Salidroside may
effectively inhibit excessive VSMCs proliferation induced by high
glucose, possibly via regulating mitochondrial dynamics.
Salidroside inhibits mitochondrial
fission in VSMCs cultured in high glucose conditions
The results of the present study demonstrated that
high glucose may induce increases in globular mitochondria and
mitochondrial gathering. Furthermore, Salidroside and Mdivi-1 may
inhibit mitochondrial fission and promote mitochondrial fusion,
which is indicated by a significantly increased proportion of
filamentous mitochondria (P<0.05; Figs. 2 and 3; with mitochondrial fusion and fission
defined as globular mitochondria representing <35% or >65% of
total mitochondria). As Salidroside is able to inhibit both VSMCs
proliferation and mitochondrial fission, it is indicated the
inhibitive effect of Salidroside on VSMCs proliferation under high
glucose condition was through inhibiting mitochondrial fission.
Salidroside may inhibit mitochondrial
fission in VSMCs by downregulating Drp1 and upregulating Mfn2
expression
It is well accepted that Mdivi-1 is a specific
inhibitor of Drp-1, but there is not enough evidence on the effect
of it on Drp-1 in VSMCs cultured under high-glucose conditions.
Salidroside and Mdivi-1 may downregulate Drp1 and upregulate Mfn2
protein and mRNA expression in a dose-dependent manner (P<0.05;
Fig. 4). Based on the aforementioned
results of confocal fluorescence microscopy, it is indicated that
the inhibitive effect of Salidroside on VSMCs proliferation under
high glucose condition is exerted via regulation of mitochondrial
dynamics, specifically the proteins associated with mitochondrial
fission.
Salidroside inhibits oxidative stress
in VSMCs induced by high glucose
Intracellular ROS production and NADPH oxidase
activity in VSMCs was significantly increased in the presence of
high-glucose (P<0.05; Fig. 5A and
B). However, Salidroside treatment resulted in a significant
decrease in ROS level and NADPH oxidase activity (P<0.05;
Fig. 5A and B). In VSMCs treated
with different concentrations of Salidroside (0.3 and 0.5 mM) and
Mdivi-1 (10 and 25 µM), the fluorescence intensity was reduced
accordingly (P<0.05; Fig. 5A). In
addition, Salidroside and Mdivi-1 may also dose-dependently inhibit
NADPH oxidase activity (P<0.05; Fig.
5B). It is indicated that Salidroside, like Mdivi-1, may
inhibit high-glucose induced oxidative stress, potentially through
inhibition of mitochondria fission.
Salidroside inhibits high-glucose
induced VSMCs proliferation by inhibiting ROS generation
To identify the association between the effect of
ROS level and Salidroside on excessive proliferation of VSMCs
induced by high glucose, the present study added 25 µM
H2O2 to increase the ROS level in cultured
VSMCs. Results indicate that exogenous ROS may stimulate VSMCs
proliferation after being inhibited by Salidroside. Results
demonstrated that the inhibitive effect of Salidroside on
high-glucose induced hyper-proliferation of VSMCs was by
significantly reducing the ROS level in the cells (P<0.01;
Fig. 6).
Salidroside inhibits mitochondrial
fission in VSMCs cultured in high glucose by directly inhibiting
the sensitivity of Drp1 to ROS
In order to identify whether ROS levels were the
regulative target of Salidroside during its inhibitory effects on
mitochondrial fission in VSMCs, the present study added 25 µM
H2O2 to cells cultured under high glucose
conditions following treatment with Salidroside.
H2O2 partially reversed the inhibitive effect
of Salidroside on Drp1 protein expression and the stimulatory
effect of Salidroside on Mfn2 protein expression, though these
changes were not significant (P>0.05, Fig 7A and 7B). Similarly, no marked alterations were
observed in the gene expression of Drp1 and Mfn2 between the HG +
Salidroside groups with and without H2O2
(P>0.05, Fig. 7C and 7D). Based
on the aforementioned results that Salidroside may downregulate
Drp1 and upregulate Mfn2 expression, it is possible that
Salidroside inhibits the sensitivity of Drp1 to ROS, which means it
has a direct effect on mitochondria.
 | Figure 7.Salidroside may directly suppress the
sensitivity of Drp1 to ROS in VSMCs under high-glucose condition.
(A) Western blot analysis and (B) quantification of VSMCs divided
into four subgroups as low-glucose control, high-glucose control,
high-glucose + Salidroside and high-glucose + Salidroside +
H2O2. The western blot analysis indicates
that exogenous ROS was not able to reverse the effect of
Salidroside on Drp1 and Mfn2 protein expression. Relative mRNA
expression of (C) Drp1 and (D) Mfn2 following reverse
transcription-quantitative polymerase chain reaction indicates that
exogenous ROS was not able to reverse the effect of Salidroside on
Drp1 and Mfn2 mRNA expression. *P<0.05 and **P<0.01 vs. low
glucose group, n=3 per treatment subgroup. Drp1, dynamin-related
protein; ROS, reactive oxygen species; VSMC, vascular smooth muscle
cells; Mfn2, mitofusin 2; GAPDH, glyceraldehyde-3-phosphate
dehydrogenase; LG, low glucose; HG, high glucose. |
Salidroside inhibits oxidative stress
in VSMCs cultured in high glucose by upregulating the expression of
Mfn2
To further elucidate the association between ROS,
Drp1 and Mfn2 in the process of Salidroside inhibiting high-glucose
induced proliferation of VSMCs. Mfn2-siRNA was added to silence
Mfn2 expression following intervention with Salidroside. The
results indicate that the inhibition of Mfn2 expression may in part
reverse the antioxidative effect of Salidroside. The NADPH oxidase
activity demonstrated a statistical difference between Mfn2-siRNA
subgroup and high-glucose control group (Fig. 8A; P<0.05) while ROS level did not
(Fig. 8B). Therefore, it is
indicated that Salidroside may upregulate Mfn2 expression to reduce
the level of ROS, partly by inhibiting NADPH oxidase activity.
Discussion
The primary aim of the present study was to
investigate whether Salidroside was able to protect VSMCs against
high glucose-induced proliferation, ROS generation and
mitochondrial dynamic imbalance in vitro. The potential
mechanisms were also investigated. The data suggested that
Salidroside may have therapeutic potential in preventing diabetes
associated vascular diseases.
Diabetes-related vascular diseases, including
coronary artery disease and cerebrovascular and peripheral vascular
diseases, are the leading causes of mortality and morbidity in
developed countries (28). VSMCs
contribute to the pathogenesis of vascular lesions, since their
proliferation is a critical event for progressive intima thickening
and development of arterial wall sclerosis. It is widely understood
that hyperglycemia directly leads to detrimental changes in VSMCs
phenotype and function, and may accelerate cardiovascular
complications (29). Therefore, the
inhibition of VSMC proliferation may have a beneficial effect in
retarding the development of atherosclerotic disease.
Salidroside is a phenylpropanoid glycoside extracted
from the raw plant Rhodiola rosea and previous results have
indicated its efficacy in improving endothelial function and
delaying atherosclerosis (20). In a
Goto-Kakizaki diabetes rat model, Salidroside was demonstrated to
perform beneficial effects on dilating vessels (21), in addition to endothelial cells and
VSMCs. Focusing on the effect of Salidroside on VSMCs cultured in
high glucose, the current study demonstrated that Salidroside
inhibits the excessive proliferation of VSMCs, along with altering
the level of mitochondrial fission and oxidase stress.
Mitochondrial dynamics are regulated by a host of
proteins, including Drp1 and Mfn2 (30). This process is necessary in the
maintenance of mitochondrial homeostasis and its dysfunction may
lead to a variety of diseases (31).
It has been reported that Mdivi-1 may have a therapeutic effect on
pulmonary arterial hypertension by effectively inhibiting pulmonary
vascular cell proliferation and downregulating Drp1 expression
(32). In addition, Mdivi-1 was also
demonstrated to reverse both the upregulation of Drp-1 and
downregulation of Mfn2 in an overloaded heart-failure mouse model,
thus restoring mitochondrial homeostasis (32). Following carotid artery stenting in
spontaneously hypertensive rat and Apo-E knockout mice, it was
identified that thickening artery walls and hyper-proliferation of
VSCMs are associated with decreased Mfn2 levels (33). Notably, stenosis following stenting
may be significantly reduced by inducing Mfn2 overexpression via
viral transcription. Collectively these results indicate a close
association between mitochondrial dynamic related proteins and
VSMCs proliferation. However, to the best of our knowledge, there
is no study concerning the therapeutic effect of anti-mitofission
on VSMCs proliferation under high glucose condition. The current
study demonstrated that Salidroside, like Mdivi-1 (a specific
inhibitor of Drp-1), reduced high glucose-induced excessive
proliferation of VSMCs. It also inhibited mitochondrial fission and
rebuilt mitochondrial network homeostasis by downregulating Drp1
and upregulating Mfn2 expression at gene and protein levels with a
concentration dependent manner specifically for Mfn2 expression.
This means Salidroside may exert a VSMCs anti-proliferation effect
under high glucose conditions via its effect on mitochondrial
dynamics.
Oxidative stress is known to serve an important role
in diabetic vascular injury. At high levels of glucose and/or
insulin, VSMC proliferation is triggered, while the synthesis and
clearance of intracellular oxygen free radicals is interrupted,
resulting in ROS accumulation and increased NADPH oxidase activity
(34). This leads to oxidative
stress, which may result in increased insulin resistance, while
also promoting the occurrence and development of diabetes.
Therefore, reduction of ROS levels by the addition of an
antioxidant may significantly reduce NADPH oxidase activity and
inhibit hyper-proliferation of VSMCs (35). In conclusion, ROS levels are able to
regulate the high-glucose-induced proliferation of VSMCs, but the
specific mechanism has yet to be elucidated. ROS is an important
product of cell metabolism in the mitochondrial respiratory chain.
A number of studies have suggested that mitochondrial dynamic
proteins may be important regulatory factors of ROS levels
(16,36). In addition, previous studies have
also indicated that increased mitochondrial fission was associated
with ROS levels (37). In the
present study, changes in ROS levels were directly associated with
the proliferation of VSMCs and related to mitochondrial dynamics.
The effect of Salidroside to downregulate Drp1 and upregulate Mfn2
at a protein and gene level was not easily reversed by exogenous
ROS. However, suppressing Mfn2 expression may reverse the
inhibitive effect of Salidroside on ROS synthesis. Thus,
Salidroside may directly impose an effect on mitochondria and also
reduce ROS levels during the inhibition of VSMCs proliferation.
To the best of our knowledge, there is no
information regarding the effect of mitochondrial dynamics on VSMC
proliferation under high-glucose conditions. Therefore, the present
study is the first to demonstrate that Salidroside may inhibit the
excessive proliferation of VSMCs under high glucose conditions and
its inhibitive effect is via inhibition of mitochondrial fission
(downregulation of Drp1 and upregulation of Mfn2) and oxidative
stress. In addition, the present study demonstrated that
Salidroside is able to suppress the sensitivity of Drp1 to ROS
levels. Although the exact inhibitory mechanism requires further
investigation, the present study indicates that Salidroside may
complete its therapeutic effect on the excessive proliferation of
high glucose induced VSMCs via maintaining mitochondrial dynamic
homeostasis.
The present study included a number of limitations.
A major limitation was the absence of a subsequent animal
experiment to observe the effect of Salidroside on vascular smooth
muscle cells in vivo. In addition, while the present results
indicated that Salidroside was associated with reduced VSMC
proliferation under high glucose conditions, the underlying
molecular mechanism remains unclear. Thus, the alleviative effects
of Salidroside on a molecular level remain poorly understood and
warrant further investigation. Furthermore, future clinical trials
involving the administration of Salidroside to patients with DM may
provide greater insight into the disease pathophysiology, and
further elucidate the therapeutic potential of Salidroside.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81573710), the
Chinese Medicine Science Foundation of Shanghai Health and Family
Planning Committee (grant no. 2014JZ006A) and the Natural Science
Foundation of Shanghai (grant no. 13ZR1404900).
References
|
1
|
Mendis S, Davis S and Norrving B:
Organizational update: The world health organization global status
report on noncommunicable diseases 2014; one more landmark step in
the combat against stroke and vascular disease. Stroke.
46:e121–e122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
GBD 2013 DALYs and HALE Collaborators, ;
Murray CJ, Barber RM, Foreman KJ, Ozgoren A Abbasoglu, Abd-Allah F,
Abera SF, Aboyans V, Abraham JP, Abubakar I, et al: Global,
regional and national disability-adjusted life years (DALYs) for
306 diseases and injuries and healthy life expectancy (HALE) for
188 countries, 1990–2013: Quantifying the epidemiological
transition. Lancet. 386:2145–2191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Novack V, Tsyvine D, Cohen DJ, Pencina M,
Dubin J, Dehghani H, Kleiman NS and Cutlip DE: Multivessel
drug-eluting stenting and impact of diabetes mellitus-a report from
the EVENT registry. Catheter Cardiovasc Interv. 73:874–880. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Costa PZ and Soares R: Neovascularization
in diabetes and its complications. Unraveling the angiogenic
paradox. Life Sci. 92:1037–1045. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Woodman RJ, Chew GT and Watts GF:
Mechanisms, significance and treatment of vascular dysfunction in
type 2 diabetes mellitus: Focus on lipid-regulating therapy. Drugs.
65:31–74. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Williams SB, Cusco JA, Roddy MA, Johnstone
MT and Creager MA: Impaired nitric oxide-mediated vasodilation in
patients with non-insulin-dependent diabetes mellitus. J Am Coll
Cardiol. 27:567–574. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fiorentino TV, Prioletta A, Zuo P and
Folli F: Hyperglycemia-induced oxidative stress and its role in
diabetes mellitus related cardiovascular diseases. Curr Pharm Des.
19:5695–5703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bellin C, De Wiza DH, Wiernsperger NF and
Rösen P: Generation of reactive oxygen species by endothelial and
smooth muscle cells: Influence of hyperglycemia and metformin. Horm
Metab Res. 38:732–739. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Basuroy S, Bhattacharya S, Leffler CW and
Parfenova H: Nox4 NADPH oxidase mediates oxidative stress and
apoptosis caused by TNF-alpha in cerebral vascular endothelial
cells. Am J Physiol Cell Physiol. 296:C422–C432. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xi G, Shen X, Maile LA, Wai C, Gollahon K
and Clemmons DR: Hyperglycemia enhances IGF-I-stimulated Src
activation via increasing Nox4-derived reactive oxygen species in a
PKCζ-dependent manner in vascular smooth muscle cells. Diabetes.
61:104–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xia L, Wang H, Goldberg HJ, Munk S, Fantus
IG and Whiteside CI: Mesangial cell NADPH oxidase upregulation in
high glucose is protein kinase C dependent and required for
collagen IV expression. Am J Physiol Renal Physiol. 290:F345–F356.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nishikawa T, Edelstein D, Du XL, Yamagishi
S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP,
et al: Normalizing mitochondrial superoxide production blocks three
pathways of hyperglycaemic damage. Nature. 404:787–790. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ishihara N, Nomura M, Jofuku A, Kato H,
Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y, et al:
Mitochondrial fission factor Drp1 is essential for embryonic
development and synapse formation in mice. Nat Cell Biol.
11:958–966. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu
X, Fang YH, Thenappan T, Piao L, Zhang HJ, Pogoriler J, et al:
Dynamin-related protein 1-mediated mitochondrial mitotic fission
permits hyperproliferation of vascular smooth muscle cells and
offers a novel therapeutic target in pulmonary hypertension. Circ
Res. 110:1484–1497. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen KH, Guo X, Ma D, Guo Y, Li Q, Yang D,
Li P, Qiu X, Wen S, Xiao RP and Tang J: Dysregulation of HSG
triggers vascular proliferative disorders. Nat Cell Biol.
6:872–883. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hong Z, Kutty S, Toth PT, Marsboom G,
Hammel JM, Chamberlain C, Ryan JJ, Zhang HJ, Sharp WW, Morrow E, et
al: Role of dynamin-related protein 1 (Drp1)-mediated mitochondrial
fission in oxygen sensing and constriction of the ductus
arteriosus. Circ Res. 112:802–815. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang JK, Yang L, Meng GL, Yuan Z, Fan J,
Li D, Chen JZ, Shi TY, Hu HM, Wei BY, et al: Protection by
salidroside against bone loss via inhibition of oxidative stress
and bone-resorbing mediators. PLoS One. 8:e572512013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu L, Wei T, Gao J, Chang X, He H, Luo F,
Zhou R, Ma C, Liu Y and Yan T: The cardioprotective effect of
salidroside against myocardial ischemia reperfusion injury in rats
by inhibiting apoptosis and inflammation. Apoptosis. 20:1433–1443.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu L, Wei T, Gao J, Chang X, He H, Miao M
and Yan T: Salidroside attenuates lipopolysaccharide (LPS) induced
serum cytokines and depressive-like behavior in mice. Neurosci
Lett. 606:1–6. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xing SS, Yang XY, Zheng T, Li WJ, Wu D,
Chi JY, Bian F, Bai XL, Wu GJ, Zhang YZ, et al: Salidroside
improves endothelial function and alleviates atherosclerosis by
activating a mitochondria-related AMPK/PI3K/Akt/eNOS pathway.
Vascul Pharmacol. 72:141–152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alameddine A, Fajloun Z, Bourreau J,
Gauquelin-Koch G, Yuan M, Gauguier D, Derbre S, Ayer A, Custaud MA
and Navasiolava N: The cardiovascular effects of salidroside in the
Goto-Kakizaki diabetic rat model. J Physiol Pharmacol. 66:249–257.
2015.PubMed/NCBI
|
|
22
|
Zou H, Liu X, Han T, Hu D, Wang Y, Yuan Y,
Gu J, Bian J, Zhu J and Liu ZP: Salidroside protects against
cadmium-induced hepatotoxicity in rats via GJIC and MAPK pathways.
PLoS One. 10:e01297882015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Battistelli M, De Sanctis R, De Bellis R,
Cucchiarini L, Dachà M and Gobbi P: Rhodiola rosea as antioxidant
in red blood cells: Ultrastructural and hemolytic behaviour. Eur J
Histochem. 49:243–254. 2005.PubMed/NCBI
|
|
24
|
de Sanctis R, De Bellis R, Scesa C,
Mancini U, Cucchiarini L and Dachà M: In vitro protective effect of
Rhodiola rosea extract against hypochlorous acid-induced oxidative
damage in human erythrocytes. Biofactors. 20:147–159. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ness TL, Robinson RL, Mojadedi W, Peavy L
and Weiland MH: A streamlined Western blot exercise: An efficient
and greener approach in the laboratory classroom. Biochem Mol Biol
Educ. 43:358–365. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ruijter JM, Lefever S, Anckaert J,
Hellemans J, Pfaffl MW, Benes V, Bustin SA, Vandesompele J and
Untergasser A: RDML consortium: RDML-Ninja and RDMLdb for
standardized exchange of qPCR data. BMC Bioinformatics. 16:1972015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maimaitijiang A, Zhuang X, Jiang X and Li
Y: Dynamin-related protein inhibitor downregulates reactive oxygen
species levels to indirectly suppress high glucose-induced
hyperproliferation of vascular smooth muscle cells. Biochem Biophys
Res Commun. 471:474–478. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Desilles JP, Meseguer E, Labreuche J,
Lapergue B, Sirimarco G, Gonzalez-Valcarcel J, Lavallée P, Cabrejo
L, Guidoux C, Klein I, et al: Diabetes mellitus, admission glucose,
and outcomes after stroke thrombolysis: A registry and systematic
review. Stroke. 44:1915–1923. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Porter KE and Riches K: The vascular
smooth muscle cell: A therapeutic target in Type 2 diabetes? Clin
Sci (Lond). 125:167–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang CR and Blackstone C: Cyclic
AMP-dependent protein kinase phosphorylation of Drp1 regulates its
GTPase activity and mitochondrial morphology. J Biol Chem.
282:21583–21587. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hom J and Sheu SS: Morphological dynamics
of mitochondria-a special emphasis on cardiac muscle cells. J Mol
Cell Cardiol. 46:811–820. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Givvimani S, Pushpakumar S, Veeranki S and
Tyagi SC: Dysregulation of Mfn2 and Drp-1 proteins in heart
failure. Can J Physiol Pharmacol. 92:583–591. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou W, Chen KH, Cao W, Zeng J, Liao H,
Zhao L and Guo X: Mutation of the protein kinase A phosphorylation
site influences the anti-proliferative activity of mitofusin 2.
Atherosclerosis. 211:216–223. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mori J, Zhang L, Oudit GY and Lopaschuk
GD: Impact of the renin-angiotensin system on cardiac energy
metabolism in heart failure. J Mol Cell Cardiol. 63:98–106. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu LH, Wang L, Wang D, Jiang H, Tang QZ,
Yan L, Bian ZY, Wang XA and Li H: Puerarin attenuates
high-glucose-and diabetes-induced vascular smooth muscle cell
proliferation by blocking PKCbeta2/Rac1-dependent signaling. Free
Radic Biol Med. 48:471–482. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu T, Sheu SS, Robotham JL and Yoon Y:
Mitochondrial fission mediates high glucose-induced cell death
through elevated production of reactive oxygen species. Cardiovasc
Res. 79:341–351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shen T, Zheng M, Cao C, Chen C, Tang J,
Zhang W, Cheng H, Chen KH and Xiao RP: Mitofusin-2 is a major
determinant of oxidative stress-mediated heart muscle cell
apoptosis. J Biol Chem. 282:23354–23361. 2007. View Article : Google Scholar : PubMed/NCBI
|