Introduction
Gastric cancer is one of the most common cancer
types diagnosed in humans with a poor prognosis (1,2).
Although significant progress has been achieved, the clinical
outcomes for gastric cancer are not yet satisfactory (1,2).
Dysfunctions of oncogenes or tumor suppressors, including microRNAs
(miRs) and human epidermal growth factor receptor 2 (HER2), have
been implicated in the development and drug resistance of gastric
cancer (3,4). Thus, an understanding of the molecular
mechanisms is urgently required for the development of more
effective treatments for patients with gastric cancer.
miRs are non-coding RNAs, 18–25 nucleotides in
length, that typically have a suppressive role in regulating gene
expression by directly binding to the 3′-untranslational region of
their target mRNAs and leading to mRNA degradation or translation
inhibition (5,6). Deregulation of specific miRs,
associated with tumor development and progression, has been
observed in gastric cancer along with drug resistance (7,8).
Therefore, investigating the roles of these miRs may help improve
the diagnosis and treatment of gastric cancer.
miR-125b is located at chromosome 11q24 and
chromosome 21q21, the so-called fragile sites that are frequently
deleted (9). It has been reported to
be significantly downregulated in osteosarcoma (10), breast cancer (11), ovarian cancer (12), and hepatocellular carcinoma (13), suggesting a tumor suppressive role.
However, miR-125b is upregulated in colorectal cancer (14), prostate cancer (15) and non-small-cell lung cancer
(16), suggesting that miR-125b has
dual roles depending on the type of cancer. miR-125b has been
implicated in gastric cancer; two studies reported that miR-125b
was among the most upregulated miRs in gastric cancer tissues
compared with non-tumour gastric tissues (17,18). In
addition, miR-125b was identified to be upregulated in gastric
cancer tissues and cell lines where it significantly promoted
proliferation, migration and invasion by downregulating the
expression of protein phosphatase 1 catalytic subunit-α (PPP1CA)
and upregulating retinoblastoma (Rb) phosphorylation (19). Furthermore, it was demonstrated in a
previous study that miR-125b is involved in the malignant
progression of gastric cancer and increased miR-125b expression
predicated a poor prognosis for patients with gastric cancer
(19). These results suggest that
miR-125b has an oncogenic role in gastric cancer. By contrast,
Fassan et al (20)
demonstrated that miR-125a-5p and miR-125b were significantly
downregulated in intestinal-type gastric cancer. Accordingly, the
exact role of miR-125b in gastric cancer, specifically in some
subtypes, is yet to be fully uncovered. Moreover, trastuzumab is an
important target drug for patients with human epidermal growth
factor receptor 2 (HER2)-positive breast and gastric cancers
(21). However, the role of miR-125b
in trastuzumab resistance in the HER2-positive gastric remains
unclear.
The present study aimed to investigate the
expression of miR-125b in gastric cancer and analyzed the
association between miR-125b expression and clinicopathological
characteristics, prognosis and trastuzumab resistance in the
HER2-positive subtype.
Materials and methods
Tissue samples and clinical data
Gastric tissues from a total of 132 cases of gastric
cancer and 38 non-cancerous cases (21 male and 17 female; mean age,
47.5±8.5) were collected at the Yuhuangding Hospital (Yantai,
China) between November 2008 and September 2012 who were admitted
due to gastrohelcosis. The mean age of patients with gastric cancer
was 54.5±10.8 years and of the 132 patients, 75 were male and 57
were female. Among patients with HER2-positive gastric cancer, 28
cases received trastuzumab (6 mg/kg, once every 3 weeks, for 6
months; Roche Diagnostics, Basel, Switzerland) until the time of
tissue collection. Clinicopathological characteristics of the
patients with gastric cancer were evaluated according to the TNM
system (22), as summarized in
Table I. Overall survival (OS) was
defined as the time from diagnosis to mortality or the date last
known alive. Informed consent was obtained from all patients or
their dependents involved. The present study was approved by the
Committee on the Ethics of Yuhuangding Hospital (Yantai,
China).
 | Table I.Clinicopathological characteristics
of patients with gastric cancer (n=132). |
Table I.
Clinicopathological characteristics
of patients with gastric cancer (n=132).
| Characteristic | Value |
|---|
| Age, mean ± SD | 54.5±10.8 |
| Sex |
|
|
Male | 56.8 (75/132) |
|
Female | 43.2 (57/132) |
| T stage |
|
|
T1–2 | 53.8 (71/132) |
|
T3–4 | 46.2 (61/132) |
| Lymph node
metastasis |
|
|
Present | 60.6 (80/132) |
|
Absent | 39.4 (52/132) |
| Distant
metastasis |
|
|
Present | 10.6 (14/132) |
|
Absent | 89.4 (195/132) |
| TNM stage |
|
| I | 6.1 (8/132) |
| II | 57.6 (76/132) |
|
III | 25.0 (33/132) |
| IV | 11.4 (15/132) |
In situ hybridization
The probe for in situ hybridization of
miR-125b was synthesized by Sangon Biotech, Co., Ltd. (Shanghai,
China). An enhanced Sensitive in situ hybridization
detection kit I (POD; Wuhan Boster Biological Technology, Ltd.,
Wuhan, China) was used to perform in situ hybridization,
according to manufacturer's protocol. Slides were deparaffinized,
dehydrated with xylene, put through an ethanol gradient (100, 95,
90, 80 and 70%, each for 5 min), and rinsed with dH2O.
Following treatment with 3% H2O2 for 15 min
and washing twice with dH2O, the slides were incubated
with pepsin solution (enhanced Sensitive in situ
hybridization detection kit I; Wuhan Boster Biological Technology,
Ltd.) for 10 min at 37°C and washed three times with
phosphate-buffered saline (PBS) and once with dH2O at
room temperature. Following this, the slides were incubated with
prehybridization solution (enhanced Sensitive in situ
hybridization detection kit I; Wuhan Boster Biological Technology,
Ltd.) at 37°C for 3 h and then with the miR-125b probe (2 µg/ml;
Yearthbio, Changsha, China) overnight at 55°C. Subsequently, the
slides were incubated in 2X saline sodium citrate (SSC) for 30 min
at 37°C, washed once with 0.5X SSC for 15 min and washed three
times with 0.2X SSC for 10 min. Slides were incubated with normal
goat serum (1:50; Yearthbio) at 37°C for 30 min and then with
biotin-antidigoxin IgG (1:200; 21563; Yearthbio) for 1.5 h at 37°C.
Subsequently, the slides were washed three times with PBS, followed
incubation with Streptavidin-Biotin-Complex (1:100; 21511;
Yearthbio) and biotin-peroxidase (1:100; 21435; Yearthbio) for 30
min at 37°C. Slides were visualized with 3,3′-diaminobenzidine
(DAB; Fuzhou Maixin Biotech, Co., Ltd., Fuzhou, China) for 5 min
and counterstained with hematoxylin for 2 min. Slides were dried
and observed using a CX23 microscope (magnification, ×200; Olympus
Corp., Tokyo, Japan).
Immunohistochemical staining
Expression of HER2 was evaluated using
immunohistochemical staining (22).
Sections with a thickness of 4 µm were deparaffinized and subjected
to heat-induced antigen retrieval using citrate buffer for 22 min
using a microwave oven. The sections were incubated at 37°C for 2 h
with primary anti-HER2 antibody (1:100; ab16901; Abcam, Cambridge,
MA, USA). Sections were washed with PBS for 10 min and incubated
with the secondary antibody (1:10,000; ab150116; Abcam) for 60 min
at room temperature. The reaction was developed using substrate
DAB, counterstained with hematoxylin, and observed under a CX23
microscope (magnification, ×200).
Evaluation of staining data
All tissue sections were scored independently by two
experienced pathologists, and the average score was calculated. For
evaluating the expression of miR-125b and HER2 in gastric cancer
tissues, the staining extent and intensity were evaluated. The
scoring system was as follows: Percentage of positively stained
cells was scored as 0 (negative, -), 1 (>0 and ≤25% of cells
positive, weak, +), 2 (>25 and ≤75% of cells positive, moderate,
++) and 3 (>75% of cells positive, strong, +++). Scores of 0 or
1 were considered as low expression, whereas scores of 2 or 3 were
considered as high expression.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA (10 µl) was extracted using TRIzol reagent
(Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to
the manufacturer's protocol. For miR-125b expression analysis, PCR
was performed using a PrimeScript miRNA RT-PCR kit (Takara
Biotechnology, Co., Ltd., Dalian, China) with an ABI PRISM 7500
Fast Real-Time PCR system (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Primer sequences for
miR-125b were as follows: Forward, 5′-TGCGCTCCTCTCAGTCCCTGAGA-3′
and reverse, 5-′TGCGCTCCTCTCAGTCCCTGAG-3. U6 (sequences not
provided by the manufacturer) was used as the internal reference.
qPCR was performed using SYBR-Green (Invitrogen; Thermo Fisher
Scientific, Inc.). Reaction conditions were 95°C for 10 min,
followed by 45 cycles of 95°C for 15 sec and 60°C for 15 sec.
Relative expression was analyzed by the
2−ΔΔCq method (23). Experiment were performed in
triplicate.
Statistical analysis
Data are presented as the mean ± standard deviation.
GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA,
USA) was used for statistical analysis. Differences in miR-125b
expression between gastric cancer and non-cancerous gastric tissue
were analyzed via Student's t-test. Contingency data were analyzed
via χ2, χ2 test for trend or Fisher's exact
tests. OS estimates over time were calculated using the
Kaplan-Meier method with log-rank test. P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-125b is significantly upregulated
in gastric cancer
To elucidate the role of miR-125b in gastric cancer,
the present study examined the level of miR-125b expression in
gastric cancer tissues and non-cancerous gastric tissues using
in situ hybridization and RT-qPCR. As presented in Fig. 1, positive staining of miR-125b was
observed in the cytoplasm and nuclei of gastric cancer cells.
Positive expression of miR-125b was observed in 81.8% (108/132) of
gastric cancer tissues. However, only 26.3% (10/38) of non-tumor
gastric tissues were miR-125b-positive, indicating that miR-125b is
more frequently expressed in gastric cancer tissues compared with
non-tumor gastric tissues.
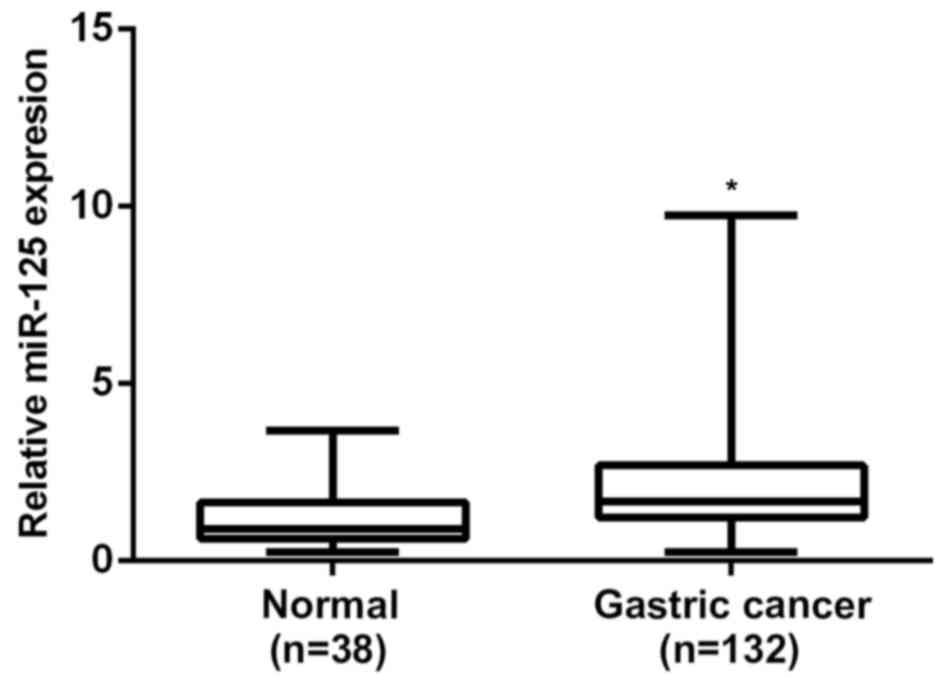
RT-qPCR was performed to determine the expression
levels of miR-125b in gastric cancer tissues and non-tumor gastric
tissues, respectively. The results of the present study indicate
that miR-125b levels were significantly increased in gastric cancer
tissues, when compared with non-cancerous gastric tissues (P=0.001;
Fig. 2). According to the results of
the present study, miR-125b is significantly upregulated in gastric
cancer.
Association between miR-125b levels
and clinicopathological features of gastric cancer
The association between the level of miR-125b and
clinicopathological characteristics of gastric cancer was analyzed
using a univariate χ2 test, which included age, sex, T
stage, lymph node metastasis, distant metastasis and TNM stage
data. No significant difference was observed between miR-125b
expression and the age, sex or distant metastasis of gastric cancer
(P>0.05; Table II). However,
miR-125b expression was significantly associated with T stage
(P<0.001), lymph node metastasis (P=0.001) and clinical TNM
stage (P=0.009), suggesting that the upregulation of miR-125b is
associated with the malignant progression of gastric cancer
(Table II).
 | Table II.Association between low (n=79) and
high (n=29) miR-125b expression and clinicopathological
characteristics in patients with breast cancer. |
Table II.
Association between low (n=79) and
high (n=29) miR-125b expression and clinicopathological
characteristics in patients with breast cancer.
| Variable | High expression
(score ≥1) | Low expression
(score <1) | χ2
P-value |
|---|
| Age, mean ± SD | 52.3±7.2 | 53.7±8.8 | 0.796 |
| Sex |
|
| 0.652 |
|
Male | 48 (60.8) | 19 (65.5) |
|
|
Female | 31 (39.2) | 10 (34.5) |
|
| T stage |
|
|
<0.001a |
|
T1–2 | 32 (40.5) | 23 (79.3) |
|
|
T3–4 | 47 (59.5) | 6 (20.7) |
|
| Lymph node
metastasis |
|
| 0.001a |
|
Present | 60 (75.9) | 12 (41.4) |
|
|
Absent | 19 (24.1) | 17 (58.6) |
|
| Distant
metastasis |
|
| 0.416 |
|
Present | 12 (15.2) | 2 (6.9) |
|
|
Absent | 67 (84.8) | 27 (93.1) |
|
| TNM stage |
|
| 0.009a |
|
I–II | 47 (59.5) | 25 (86.2) |
|
|
III–IV | 32 (40.5) | 4 (13.8) |
|
The association between miR-125b level and the
prognosis of patients with gastric cancer was analyzed. OS duration
ranged from 3 to 65 months. Data from the current study indicated
that the OS rate of patients with gastric cancer exhibiting high
miR-125b expression was significantly lower than those with low
miR-125b expression (P=0.044; Fig.
3A). Multivariate cox regression analysis was completed and
indicated that the miR-125b expression (P=0.001), lymph node
metastasis (P=0.004), distant metastasis (P=0.001) and TNM stage
(P=0.005) may be independent prognostic factors for gastric cancer
(data not shown). However, no significant differences were observed
in the OS rate of patients with gastric cancer regarding the age,
sex and tumor stage (P>0.05).
Association between miR-125b levels
and clinicopathological characteristics in HER2-positive gastric
cancer
HER2 has been identified as a direct target of
miR-125b and is involved in the development and progression of
gastric cancer (20). Therefore, the
association between miR-125b levels and clinicopathological
characteristics in HER2-positive gastric cancer was assessed. As
presented in Table III, among the
82 cases of HER2-positive gastric cancer patients, miR-125b
expression was significantly associated with the T stage (P=0.015),
lymph node metastasis (P=0.014) and TNM stage (P=0.002). However,
no significant differences were observed between miR-125b levels
and the age, sex or distant metastasis of patients with
HER2-positive gastric cancer (P>0.05; Table III).
 | Table III.Association between miR-125b
expression and clinicopathological characteristics in patients with
HER2-positive breast cancer. |
Table III.
Association between miR-125b
expression and clinicopathological characteristics in patients with
HER2-positive breast cancer.
|
| miR-125b
expression |
|
|---|
|
|
|
|
|---|
| Variables | High (score≥1)
n=58 | Low (score<1)
n=24 | χ2 test
P-value |
|---|
| Age, mean ± SD | 52.7±6.2 | 53.3±7.1 |
|
| Sex |
|
| 0.639 |
|
Male | 33 (56.9) | 15 (62.5) |
|
|
Female | 25 (43.1) | 9 (37.5) |
|
| T stage |
|
| 0.015a |
|
T1–2 | 24 (41.4) | 17 (70.8) |
|
|
T3–4 | 34 (58.6) | 7 (29.2) |
|
| Lymph node
metastasis |
|
| 0.014a |
|
Present | 41 (70.7) | 10 (41.7) |
|
|
Absent | 17 (29.3) | 14 (58.3) |
|
| Distant
metastasis |
|
| 1.000 |
|
Present | 8 (13.8) | 3 (12.5) |
|
|
Absent | 50 (86.2) | 21 (87.5) |
|
| TNM stage |
|
| 0.002a |
|
I–II | 27 (46.6) | 20 (83.3) |
|
|
III–IV | 31 (53.4) | 4 (16.7) |
|
Furthermore, the OS rate of patients with
HER2-positive gastric cancer and higher miR-125b expression was
significantly reduced compared with those with lower miR-125b
expression (P=0.034; Fig. 3B). This
suggests that the upregulation of miR-125b expression predicts a
poor prognosis in patients with HER2-positive gastric cancer.
Upregulation of miR-125b expression
predicts poor prognosis in patients with HER2-positive gastric
cancer who received trastuzumab treatment
Trastuzumab is a targeted drug used for
HER2-positive cancer (21). In the
current study, the effect of miR-125b expression on the clinical
outcome of trastuzumab treatment in HER2-positive gastric cancer
was examined. Among patients with HER2-positive gastric cancer, 28
cases received trastuzumab treatment. Our data indicated that the
OS rate of patients with HER-2-positive gastric cancer and higher
miR-125b expression was significantly reduced, when compared with
those with low miR-125b expression (P=0.047; Fig. 3C). This suggests that low expression
of miR-125b is beneficial for the prognosis of patients with
HER-2-positive gastric cancer, while a higher miR-125b level may
predicate poor prognosis.
Discussion
miR-125b has been reported to be deregulated in
various types of human cancer. It is significantly downregulated in
osteosarcoma (10), breast cancer
(11), ovarian cancer (12), and hepatocellular carcinoma (13), but upregulated in colorectal cancer
(14), prostate cancer (15) and non-small-cell lung cancer
(16), suggesting dual roles of
miR-125 in different cancer types. In the present study, miR-125b
expression was demonstrated to be significantly increased in
gastric cancer tissues compared with non-cancerous gastric tissues,
which is consistent with previous studies (17,19).
However, the underlying mechanism by which miR-125b expression is
upregulated in gastric cancer remains unclear. Bousquet et
al (24) reported that the
translocation t(2;11)(p21;q23) led to increased expression of
miR-125b in myelodysplastic syndrome and acute myeloid leukemia.
Similarly, the translocation t(11;14)(q24;q32) led to the
upregulation of miR-125b in B-cell progenitor acute lymphoblastic
leukemia (25). Therefore, it is
reasonable that chromosomal translocation may also result in higher
expression of miR-125b expression in gastric cancer, and future
studies should aim to verify this speculation.
Data from the current study indicated that, although
the higher miR-125b expression was not associated with sex or
distant metastasis of gastric tumors, it was significantly
associated with the T stage, lymph node metastasis and clinical TNM
stage. A previous study also indicated that miR-125b expression was
positively associated with the tumor size, depth of invasion, lymph
node metastasis and clinical TNM stage of gastric tumors (19). In addition, data from their study and
the present study indicated that increased miR-125b expression
predicted poor prognosis in gastric cancer patients (19). Based on these results, the
upregulation of miR-125b is potentially involved in the malignant
progression of gastric cancer.
Song et al (26) performed a consensus clustering
analysis of miR profiles for 90 different gastric cancer tissues
and identified a key miR regulatory network potentially driving the
poor-prognosis gastric cancer subtype, which was characterized by
the overexpression of epithelial-to-mesenchymal transition markers.
Within this regulatory network, miR-125b was observed to target
genes that were significantly associated with survival. These data
are consistent with the results of the current study, in that a
high miR-125 expression is associated with a poor prognosis in
patients with gastric cancer. Furthermore, Wu et al
(19) conducted an in vitro
investigation and demonstrated that miR-125b overexpression
significantly promoted the proliferation, migration and invasion of
gastric cancer cells by inhibiting the expression of PPP1CA and
upregulating Rb phosphorylation. This revealed an important
molecular mechanism by which miR-125b has an oncogenic role in
gastric cancer.
HER2 is a member of the epidermal growth factor
receptor family of receptor tyrosine kinases (27–30).
Although HER2 has no ligand binding domain of its own and therefore
cannot bind growth factors, it is able to bind to other
ligand-bound EGF receptor family members to form a heterodimer.
This heterodimer is able to stabilize ligand binding and enhance
kinase-mediated activation of downstream signaling pathways, such
as mitogen-activated protein kinase and phosphatidylinositol-3
kinase, the aberrant activation of which has key roles in the
development and progression of human cancer (27–30).
Furthermore, amplification and/or overexpression of HER2 has been
identified in common human cancer types, including breast, ovarian
and gastric cancer, and has been developed into an important
therapeutic target (31–33). Fassan et al (20) reported that HER2 status correlated
inversely with miR-125 expression and dysregulation of
miR-125a-5p/125b. Also indicating that HER2 is an early event in
intestinal-type gastric cancer (16). However, to the best of our knowledge,
no previous study has focused on the association between miR-125b
expression and the clinicopathological characteristics in
HER2-positive gastric tumors. In the present study, high miR-125b
expression was demonstrated to be significantly associated with T
stage, lymph node metastasis, and TNM stage, although no
significant association was observed between miR-125b expression
and the age, sex or distant metastasis of HER2-positive gastric
tumors. Data from the current study suggests that miR-125b should
be considered among the therapeutic targets in HER2-positive
gastric cancer.
In addition, accumulating evidence has suggested
that miR-125b participates in the regulation of drug resistance in
human cancer types. Vilquin et al (34) demonstrated that increased miR-125b
expression predicted a poor prognosis in letrozole resistant breast
cancer and overexpression of miR-125b conferred the resistance of
breast cancer MCF-7 cells to letrozole and anastrozole. As
trastuzumab has been commonly used as a targeted drug for
HER2-positive cancer, the present study speculated that miR-125b
may also affect the clinical outcomes of patients with
HER2-positive gastric cancer who received trastuzumab treatment.
Indeed, the results of the present study indicate that high
miR-125b expression is significantly associated with a poor
prognosis of patients with HER2-positive gastric cancer that are
treated with trastuzumab, suggesting that miR-125b is involved in
the regulation of drug resistance. Therefore, targeting miR-125b
may be beneficial for improving the treatment of trastuzumab in
patients with HER2-positive gastric cancer. Yagishita et al
(35) also reported that cytotoxic
drugs, such as cisplatin, induced significant upregulation of HER2
through the downregulation of miR-125b, which in turn acted as a
novel therapeutic target for trastuzumab-mediated cellular
cytotoxicity in small cell lung cancer.
In conclusion, the present study demonstrates that
the expression of miR-125b is significantly increased in gastric
cancer, and its upregulation is associated with the malignant
progression and poor prognosis of patients with gastric cancer,
including HER2-postive gastric cancer. In addition, upregulation of
miR-125b expression predicts poor prognosis in patients with
HER2-positive gastric cancer who received trastuzumab treatment.
Therefore, the miR-125b/HER2 axis may become a potential
therapeutic target for gastric cancer.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Z, Lei H, Luo M, Wang Y, Dong L, Ma Y,
Liu C, Song W, Wang F, Zhang J, et al: DNA methylation
downregulated mir-10b acts as a tumor suppressor in gastric cancer.
Gastric Cancer. 18:43–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qiu T, Zhou X, Wang J, Du Y, Xu J, Huang
Z, Zhu W, Shu Y and Liu P: miR-145, miR-133a and miR-133b inhibit
proliferation, migration, invasion and cell cycle progression via
targeting transcription factor Sp1 in gastric cancer. FEBS Lett.
588:1168–1177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
John B, Enright AJ, Aravin A, Tuschl T,
Sander C and Marks DS: Human MicroRNA targets. PLoS Biol.
2:e3632004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu C, Shan Z, Li C and Yang L: miR-129
regulates cisplatin-resistance in human gastric cancer cells by
targeting P-gp. Biomed Pharmacother. 86:450–456. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Xu W, Ni P, Li A, Zhou J and Xu
S: miR-99a and miR-491 regulate cisplatin resistance in human
gastric cancer cells by targeting CAPNS1. Int J Biol Sci.
12:1437–1447. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M
and Croce CM: Human microRNA genes are frequently located at
fragile sites and genomic regions involved in cancers. Proc Natl
Acad Sci USA. 101:pp. 2999–3004. 2004; View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karbasy SH, Taheriazam A, Mirghasemi A,
Sedaghati F, Shakeri M, Yahaghi E and Bahador R: RETRACTED ARTICLE:
Upregulation of miR-300 and downregulation of miR-125b act as
potential predictor biomarkers in progression, metastasis, and poor
prognosis of osteosarcoma. Tumour Biol. 2015.(Epub ahead of print).
PubMed/NCBI
|
|
11
|
Zhang Y, Yan LX, Wu QN, Du ZM, Chen J,
Liao DZ, Huang MY, Hou JH, Wu QL, Zeng MS, et al: miR-125b is
methylated and functions as a tumor suppressor by regulating the
ETS1 proto-oncogene in human invasive breast cancer. Cancer Res.
71:3552–3562. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guan Y, Yao H, Zheng Z, Qiu G and Sun K:
miR-125b targets BCL3 and suppresses ovarian cancer proliferation.
Int J Cancer. 128:2274–2283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pan S, Cheng X, Chen H, Castro PD, Ittmann
MM, Hutson AW, Zapata SK and Sifers RN: ERManI is a target of
miR-125b and promotes transformation phenotypes in hepatocellular
carcinoma (HCC). PLoS One. 8:e728292013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nishida N, Yokobori T, Mimori K, Sudo T,
Tanaka F, Shibata K, Ishii H, Doki Y, Kuwano H and Mori M: MicroRNA
miR-125b is a prognostic marker in human colorectal cancer. Int J
Oncol. 38:1437–1443. 2011.PubMed/NCBI
|
|
15
|
Amir S, Ma AH, Shi XB, Xue L, Kung HJ and
White RW Devere: Oncomir miR-125b suppresses p14(ARF) to modulate
p53-dependent and p53-independent apoptosis in prostate cancer.
PLoS One. 8:e610642013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuxia M, Zhennan T and Wei Z: Circulating
miR-125b is a novel biomarker for screening non-small-cell lung
cancer and predicts poor prognosis. J Cancer Res Clin Oncol.
138:2045–2050. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ueda T, Volinia S, Okumura H, Shimizu M,
Taccioli C, Rossi S, Alder H, Liu CG, Oue N, Yasui W, et al:
Relation between microRNA expression and progression and prognosis
of gastric cancer: A microRNA expression analysis. Lancet Oncol.
11:136–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, Zhang Y, Zhang H, Liu X, Gong T, Li
M, Sun L, Ji G, Shi Y, Han Z, et al: miRNA-223 promotes gastric
cancer invasion and metastasis by targeting tumor suppressor
EPB41L3. Mol Cancer Res. 9:824–833. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu JG, Wang JJ, Jiang X, Lan JP, He XJ,
Wang HJ, Ma YY, Xia YJ, Ru GQ, Ma J, et al: miR-125b promotes cell
migration and invasion by targeting PPP1CA-Rb signal pathways in
gastric cancer, resulting in a poor prognosis. Gastric Cancer.
18:729–739. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fassan M, Pizzi M, Realdon S, Balistreri
M, Guzzardo V, Zagonel V, Castoro C, Mastracci L, Farinati F, Nitti
D, et al: The HER2-miR125a5p/miR125b loop in gastric and esophageal
carcinogenesis. Hum Pathol. 44:1804–1810. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin MH, Nam AR, Park JE, Bang JH, Bang YJ
and Oh DY: Resistance mechanism against trastuzumab in
HER2-positive cancer cells and its negation by Src inhibition. Mol
Cancer Ther. pii:molcanther.0669.2016. 2017.
|
|
22
|
Sun R, Shen J, Gao Y, Zhou Y, Yu Z,
Hornicek F, Kan Q and Duan Z: Overexpression of EZH2 is associated
with the poor prognosis in osteosarcoma and function analysis
indicates a therapeutic potential. Oncotarget. 7:38333–38346.
2016.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bousquet M, Quelen C, Rosati R, Mansat-de
Mas V, La Starza R, Bastard C, Lippert E, Talmant P,
Lafage-Pochitaloff M, Leroux D, et al: Myeloid cell differentiation
arrest by miR-125b-1 in myelodysplastic syndrome and acute myeloid
leukemia with the t(2;11)(p21;q23) translocation. J Exp Med.
205:2499–2506. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chapiro E, Russell LJ, Struski S, Cavé H,
Radford-Weiss I, Valle VD, Lachenaud J, Brousset P, Bernard OA,
Harrison CJ and Nguyen-Khac F: A new recurrent translocation
t(11;14)(q24;q32) involving IGH@ and miR-125b-1 in B-cell
progenitor acute lymphoblastic leukemia. Leukemia. 24:1362–1364.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song F, Yang D, Liu B, Guo Y, Zheng H, Li
L, Wang T, Yu J, Zhao Y, Niu R, et al: Integrated microRNA network
analyses identify a poor-prognosis subtype of gastric cancer
characterized by the miR-200 family. Clin Cancer Res. 20:878–889.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Berg M and Soreide K: EGFR and downstream
genetic alterations in KRAS/BRAF and PI3K/AKT pathways in
colorectal cancer: Implications for targeted therapy. Discov Med.
14:207–214. 2012.PubMed/NCBI
|
|
28
|
Ching CB and Hansel DE: Expanding
therapeutic targets in bladder cancer: The PI3K/Akt/mTOR pathway.
Lab Invest. 90:1406–1414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Siegfried Z, Bonomi S, Ghigna C and Karni
R: Regulation of the Ras-MAPK and PI3K-mTOR signalling pathways by
alternative splicing in cancer. Int J Cell Biol. 2013:5689312013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schneider MR and Yarden Y: The EGFR-HER2
module: A stem cell approach to understanding a prime target and
driver of solid tumors. Oncogene. 35:2949–2960. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thiel A and Ristimäki A: Targeted therapy
in gastric cancer. APMIS. 123:365–372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Teplinsky E and Muggia F: Targeting HER2
in ovarian and uterine cancers: Challenges and future directions.
Gynecol Oncol. 135:364–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
D'Amato V, Raimondo L, Formisano L,
Giuliano M, De Placido S, Rosa R and Bianco R: Mechanisms of
lapatinib resistance in HER2-driven breast cancer. Cancer Treat
Rev. 41:877–883. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vilquin P, Donini CF, Villedieu M, Grisard
E, Corbo L, Bachelot T, Vendrell JA and Cohen PA: MicroRNA-125b
upregulation confers aromatase inhibitor resistance and is a novel
marker of poor prognosis in breast cancer. Breast Cancer Res.
17:132015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yagishita S, Fujita Y, Kitazono S, Ko R,
Nakadate Y, Sawada T, Kitamura Y, Shimoyama T, Maeda Y, Takahashi
F, et al: Chemotherapy-regulated microRNA-125-HER2 pathway as a
novel therapeutic target for trastuzumab-mediated cellular
cytotoxicity in small cell lung cancer. Mol Cancer Ther.
14:1414–1423. 2015. View Article : Google Scholar : PubMed/NCBI
|