Introduction
Epilepsy is a chronic neurological disease,
characterized by recurrent epileptic seizures, and causes
impairments in neurobiology, cognition, psychology and social
behavior (1,2). Multiple antiepileptic drugs are
available; however, ~30% of patients with epilepsy experience
undesirable adverse reactions and develop resistance to these drugs
(3,4).
The ketogenic diet (KD) is a high-fat,
low-carbohydrate and moderate-protein diet. It has anticonvulsant
and anti-epileptogenic effects, including epileptogenesis
inhibition and neuronal loss prevention, in amygdala-kindling
seizures (5) and is considered to be
an effective treatment for medically refractory epilepsy (6,7).
However, the mechanisms underlying its clinical efficacy have not
been elucidated. Increasing evidence supports that
β-hydroxybutyrate (BHB) induced by KD may increase the
concentration of γ-aminobutyric acid (GABA) in the epileptic brain
by inhibiting astrocytic GABA degradation (6). Additionally, it has been reported that
levels of BHB in the blood are positively correlated with seizure
resistance (8). Abdelmalik et
al (9) observed that
pretreatment with BHB reduced the frequency of seizures induced by
acute hypoglycemia. Furthermore, exogenous BHB administration may
prolong the onset time of seizure in an epilepsy model induced by
pilocarpine and flurothyl (10,11).
However, the dose of BHB administered varies in different studies
(9–11), suggesting that the dose of exogenous
BHB may be a key factor for epilepsy therapy. Additionally, the
association between blood BHB levels and the dose of BHB
administered remains unclear. A path analysis demonstrated that the
seizure threshold is significantly elevated with increasing
ketogenic ratios, but not with increasing BHB levels in rats
(12). Therefore, determining the
optimal dose of BHB administration that is close to the BHB levels
in rats following KD may optimize the anticonvulsant and
anti-epileptogenic effects of BHB in epilepsy.
The aim of the present study was to investigate the
association between BHB levels in the blood and the BHB exogenous
dosage, and to investigate the anticonvulsant effects of exogenous
BHB on the rat seizure model induced by kainic acid (KA). In
addition, Nissl and Timm staining were used to evaluate the
histological changes in KA-induced seizure models. The results of
the present study may facilitate the development of novel
therapeutic strategies to treat epilepsy.
Materials and methods
Animals
The present study was approved the Ethics Committee
of Shandong University School of Medicine (Shandong, China). All
experimental procedures were conducted according to the National
Institute of Health Guidelines (13).
A total of 102 male Wistar rats on postnatal 21 days
were obtained from Shandong University Animal Center (Jinan,
China), weighing 60±10 g. Rats had free access to food and tap
water and housed at a standard temperature (22±1°C) and humidity
(50±5%) under a 12-h light/dark cycle (lights on from 07:00 a.m. to
07:00 p.m.). Rats continued to be kept in the standard housing
conditions until the time of the experiment. To detect the
concentration of BHB, 32 rats were divided into the following
groups: BHB treatment (2, 4 and 8 mmol/kg; n=9 in each) and normal
saline (NS) control (n=5). A total of 20 rats were used to detect
the glucose concentration, treated with either 4 mmol/kg of BHB or
4 ml/kg NS (n=10 in each group). To explore the anticonvulsant
effect of BHB on KA-induced seizure model, a total of 50 rats were
divided into BHB+KA and NS+KA groups (n=25 in each group).
BHB and glucose concentration
detection
DL-BHB (cat. no. H6501, Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was dissolved in sterile 0.9% NS at a
concentration of 1 mol/l. Wistar rats on postnatal day 21were
intraperitoneally administered with different concentrations of BHB
(2, 4 and 8 mmol/kg, respectively; n=9 in each BHB group)
immediately. Rats (n=5) used as a control were administered with NS
intraperitoneally at a dose of 4 ml/kg. The serum obtained from
these rats was used for detecting the concentration of BHB. In
addition, another 20 Wistar rats on postnatal day 21 were
intraperitoneally administered with 4 mmol/kg of BHB and 4 ml/kg NS
(n=10 in each group) and their serum was used for detecting the
concentration of glucose concentration.
At 0, 15, 45, 75 and 90 min after BHB
administration, blood was collected from angular veins of the rats
in above groups under ether anesthesia (Sigma-Aldrich; Merck KGaA).
After the last time of blood collection, the rats were sacrificed
immediately by decapitation under ether anesthesia. A total of 2 h
after collection at room temperature, the blood was centrifuged at
3,000 × g for 15 min at 4°C to obtain serum.
The concentration of BHB in the serum was then
detected using a BHB assay kit (cat. no. MAK041, Sigma-Aldrich;
Merck KGaA) according to the manufacturer's protocol. Glucose
concentration in the serum was tested using a glucose meter (Roche
Diagnostics, Basel, Switzerland).
Establishment of KA-induced rat
seizure model
To induce seizure, 50 rats received intraperitoneal
injection of KA [10 mg/kg in 0.9% NaCl, (pH 7.0), cat. no. K0250,
Sigma-Aldrich; Merck KGaA] on postnatal day 21. The seizure
behavior of animals was then analyzed 1 h after KA injection for 2
h according to the scale devised by Racine (13): Stage I, facial clonus; Stage II, head
nodding and wet dog shaking; Stage III, forelimb clonus; Stage IV,
forelimb with rearing; Stage V, rearing, jumping and falling. If 3
consecutive behaviors at each stage appeared, rats were scored.
Then 10% chloral hydrate (400 mg/kg; Sigma-Aldrich; Merck KGaA) was
administered intraperitoneally to stop seizure behavior if the
status epilepticus continued over 90 min. All rats presented with
seizure behavior above stage IV and were considered to be a
successful epileptic model.
Anticonvulsant effect of BHB on
KA-induced seizure model
Another 50 Wistar rats were randomly selected and
divided into BHB+KA and NS+KA groups (n=25 each group). Rats in the
BHB+KA group were administered with 4 mmol/kg BHB intraperitoneally
30 min prior to KA injection. Rats in the NS+KA group were
administered with NS intraperitoneally prior to KA injection, and
this group was used as a control. The onset time of stage IV or V
and the degree of seizure behavior were recorded for 2 h following
KA administration. Seizure behaviors of the rats in each group were
observed and evaluated by an observer blind to the grouping.
Histological examination
Rats were anesthetized with 10% chloral hydrate (400
mg/kg) and their skulls were then immediately cut open for
obtaining brains at 3 or 14 days following KA administration (n=5
at each time point). Brains were fixed in 4% paraformaldehyde for
24 h at 4°C and then embedded in paraffin. Coronal paraffin
sections 4-µm thick were prepared for staining. The remaining rats
were housed under the conditions outlined above for use in future
studies.
Nissl staining was performed to observe neuronal
loss and damage in the CA1 and CA3 regions of the hippocampus in
NS- and BHB-treated rats 3 days following KA administration.
Coronal sections on day 3 were dewaxed, rinsed in crystal violet
stain for 1 h at 56°C and heated for 10 min. Sections were then
immediately rinsed in distilled water, immersed in Nissl staining
solution (Arcturus Bioscience, Inc., Mountain View, CA) for 3 min,
dehydrated in absolute ethyl alcohol, cleared in xylene and mounted
with neutral gum solution. Typical neuronal loss and damage in the
CA1 and CA3 region following treatment were observed under a
microscope (Nikon 80i, Nikon Corporation, Tokyo, Japan) at a
magnification of ×50 and ×400.
Timm staining was performed to observe mossy fiber
sprouting (MFS) in NS- and BHB-treated rats 14 days following KA
administration. Coronal sections on day 14 were stained in Timm
staining solution (120 ml 50% arabic gum, 20 ml citric acid buffer,
30 ml 57% hydroquinone and 30 ml 0.73% silver lactate; all
Sigma-Aldrich; Merck KGaA) in the dark at 26°C for 90 min. Sections
were then washed with distilled water, dehydrated and mounted with
neutral gum solution. MFS was evaluated by rating the granule
distribution in the dentate gyrus and CA3 region in Timm staining.
Timm staining scale ranged between 0 and 5 following these
criteria: 0, No granules; 1, sporadic granules in a patchy
distribution; 2, more granules in patchy distributions; 3, granules
in a continuous distribution with occasional patches; 4, dense
granules in a near-continuous laminar band and 5, dense granules in
a continuous laminar band. Coronal sections were observed under a
microscope (Nikon 80i, Nikon Corporation) at a magnification of
×100 and ×200.
Statistical analysis
Statistical analyses were performed using SPSS
software 20.0 (IBM SPSS, Armonk, NY, USA). All data were expressed
as the mean ± standard error of the mean, obtained from at least
three independent experiments. The differences of the BHB
concentration, glucose concentration and average Timm staining
score were evaluated using one-way analysis of variance and further
comparison between groups was performed by post hoc Tukey test.
Differences in the onset time and degree of seizure behavior were
evaluated using the Student's t-test and P<0.05 was considered
to indicate a significant difference.
Results
Exogenous BHB administration
significantly increases the concentration of BHB in the blood
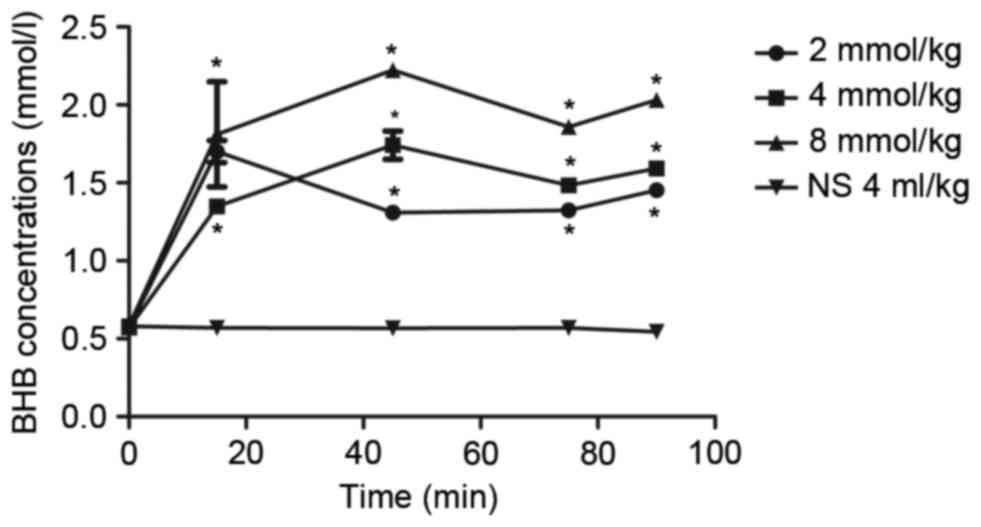
Prior to BHB administration, the concentration of
BHB in the blood was ~0.57±0.01 mmol/l. The concentration of BHB in
the blood increased to 1.35–2.37 mmol/l 15 min after BHB
administration, and this level was maintained for the next 75 min
(Fig. 1). Compared with the NC
group, BHB concentrations were significantly higher in BHB groups
(P<0.05) at all time points; however, there was no significant
difference in the blood BHB concentration between the rats
administrated with 2, 4, or 8 mmol/kg BHB. Notably, it was observed
that the BHB concentration in the blood was relatively stable at
1–2 mmol/l after the rats were administrated 4 mmol/kg BHB
(Fig. 1), which was most similar to
the BHB levels after rats had been on KD (14). There were significant differences
among different times in 4 mmol/kg BHB group compared with the
control group (P=0.020 at 15 min and P<0.001 at 45, 75 and 90
min). Thus, 4 mmol/kg BHB was used to treat rats in the subsequent
analyses.
Exogenous BHB administration has no
significant effect on blood glucose
The concentration of glucose in the blood initially
increased over 15 min following BHB administration and then
decreased in the subsequent next 75 min (Fig. 2). However, there were no significant
differences in glucose concentrations compared with the control
group (P=0.702, 0.398, 0.350, 0.891, 0.838 at 0, 15, 45, 75, 90
min, respectively; Fig. 2).
Additionally, there were also no significant differences in the
glucose concentration among different times in each group (P=0.246
in the BHB and P=0.333 in the NS groups; Fig. 2).
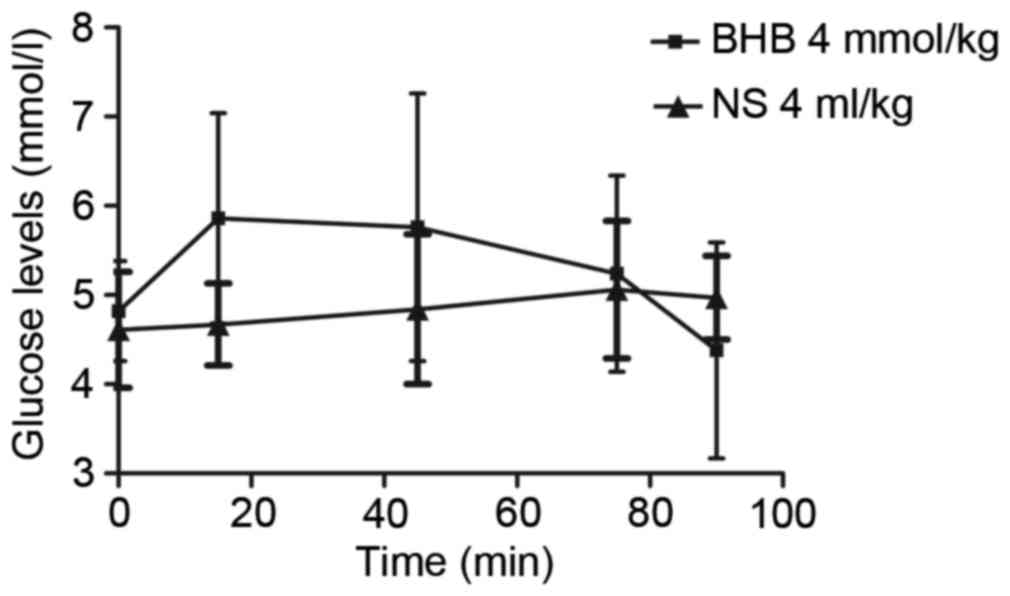 | Figure 2.Exogenous BHB administration had no
significant effect on blood glucose levels. Following exogenous BHB
administration, the glucose level gradually increased over 15 min
and then decreased in the next 75 min. However, there was no
significant difference between the two groups (P=0.702, 0.398,
0.350, 0.891 and 0.838 at 0, 15, 45, 75 and 90 min, respectively).
There was also no significant difference in the glucose
concentration among the different times in the same group (P=0.246
in the BHB and P=0.333 in the NS group). BHB, β-hydroxybutyrate;
NS, normal saline. |
Onset time of seizure is prolonged in
a KA-induced seizure model following BHB pretreatment
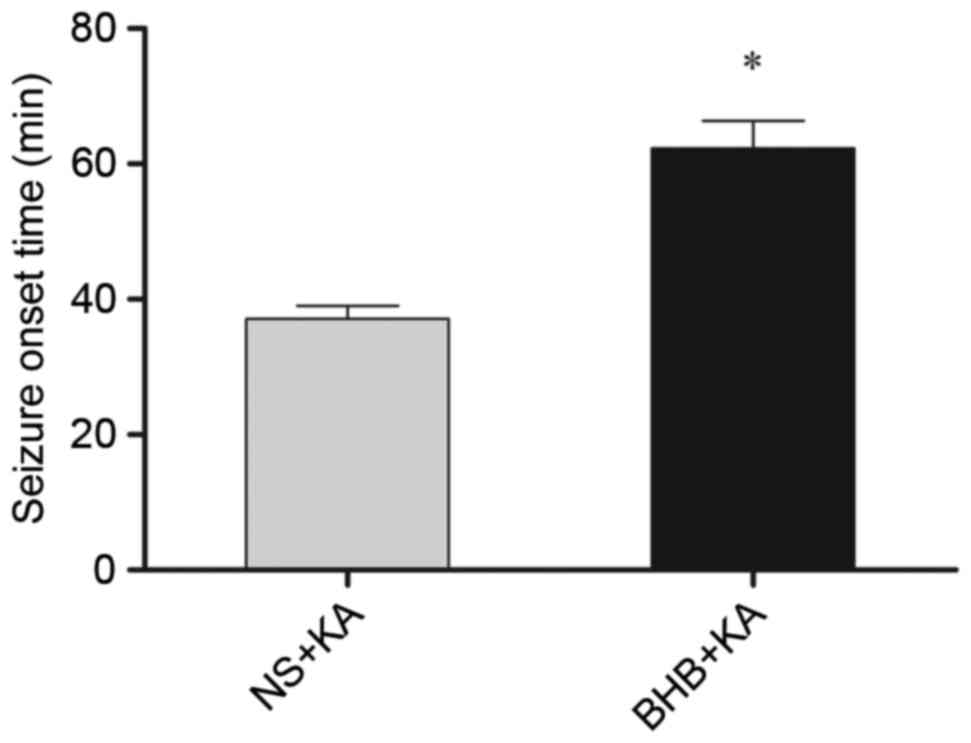
The onset time of seizure in the BHB+KA group was
63.31±4.050 min, which was significantly longer (P=0.039) than that
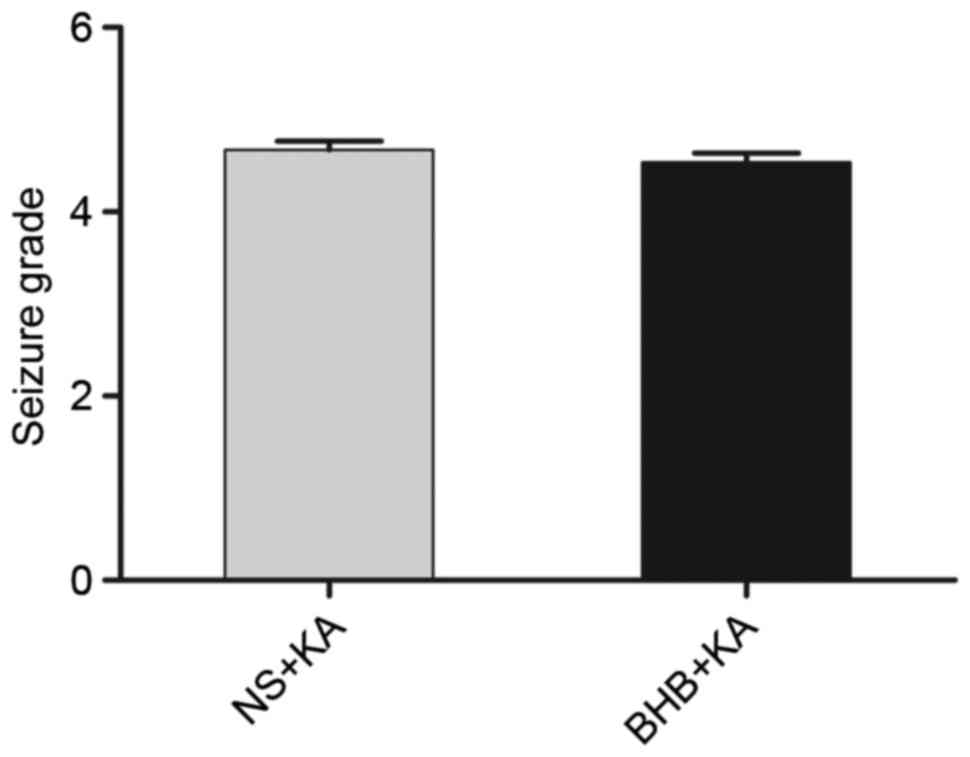
of the NS+KA group (37.08±1.958 min; Fig. 3). Furthermore, the average degree of
seizure behavior in the BHB+KA group was 4.54±0.100, which was
slightly lower than that in the NS+KA group (4.67±0.098), however,
this difference was not significant (P=0.069; Fig. 4).
Neuronal loss in the hippocampus and
MFS is alleviated in a BHB-pretreated KA-induced seizure model
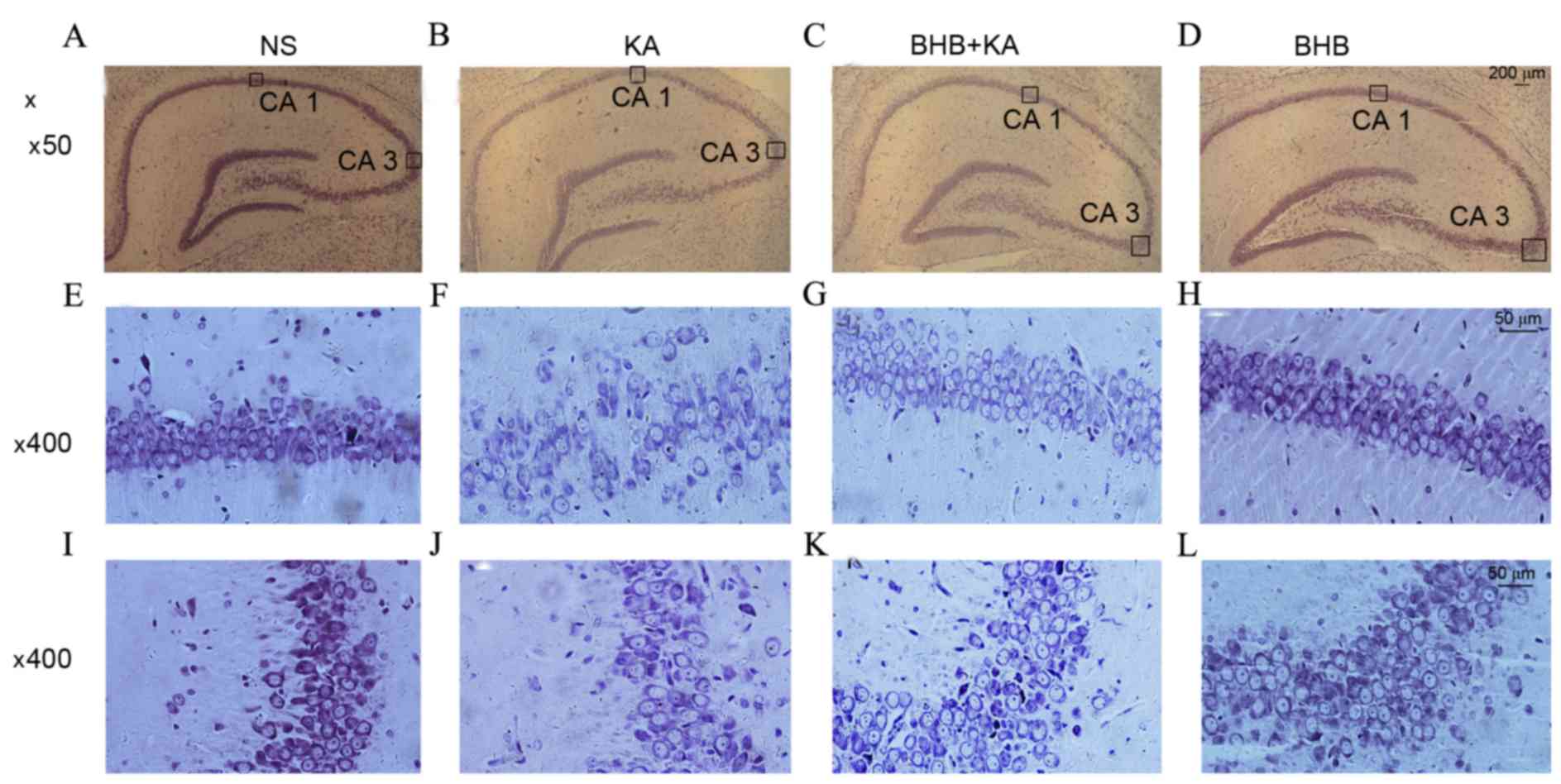
The results of Nissl staining demonstrated that
there was no neuronal loss in the hippocampus in NS- and
BHB-treated rats on day 3. However, typical neuronal loss and
damage in the CA1 and CA3 region were found in the KA-induced
seizure model that did not receive BHB pretreatment. Furthermore,
neuronal loss was attenuated in the BHB+KA group compared with the
NS+KA group (Fig. 5).
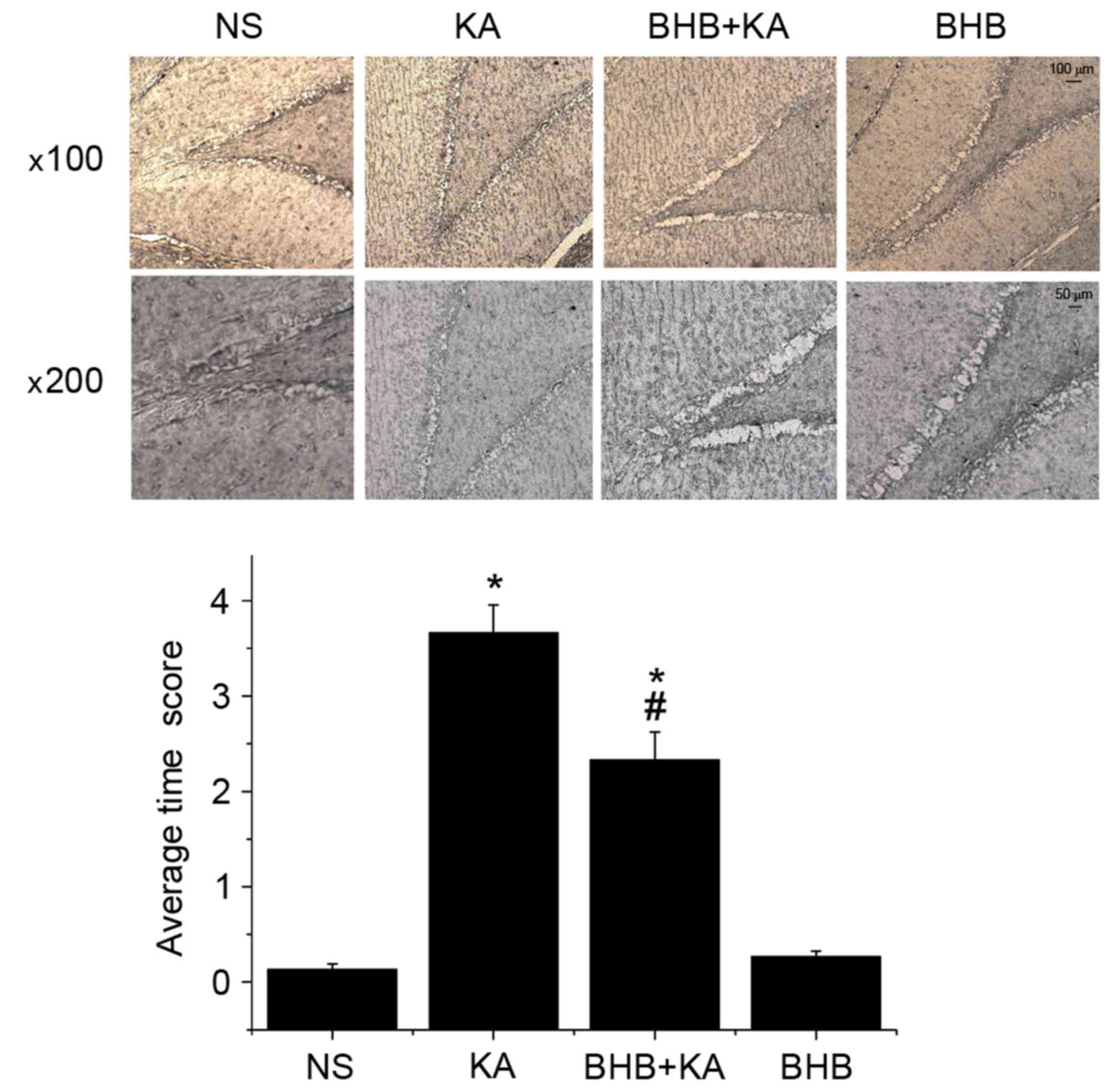
MFS in each group on day 14 was observed using Timm
staining. The results demonstrated that the average Timm score in
KA-induced seizure rats was significantly higher than that in the
NS- and BHB-treated rats (P=0.005) Furthermore, there was no
significant difference in the average Timm score between the NS
(0.13±0.06) and BHB groups (0.23±0.06; P=0.183; Fig. 6). Compared with the NS group
(3.67±0.15), the average Timm score of the BHB+KA group (1.5±0.50)
was significantly decreased (P=0.021; Fig. 6), indicating that MFS was alleviated
in the KA-induced seizure model group receiving BHB
pretreatment.
Discussion
Epilepsy is a chronic illness and ~30% of patients
with epilepsy are refractory to current pharmacotherapies (15). Thus, it is important to identify more
effective therapies to treat patients with epilepsy. Studies have
determined that exogenous BHB is neuroprotective and acts as an
anticonvulsant in vitro and in vivo (16,17).
Therefore, the present study investigated the anticonvulsant effect
of BHB and the results demonstrated that exogenous BHB could
increase blood BHB concentration, but had no evident effect on
blood glucose. Exogenous BHB administration could also increase the
concentration of BHB in the blood to a similar level observed in
rats treated with KD (14) and this
concentration could be maintained for 90 min. Furthermore, the
onset time of seizure was significantly prolonged while neuronal
loss and MFS were attenuated in BHB-pretreated rats with a
KA-induced seizure.
KD is an established and effective therapy in the
management of refractory epilepsy (18,19).
In vivo, KD can be metabolized into ketone bodies, including
acetoacetic acid, BHB and acetone. Compared with acetoacetic acid
and acetone, BHB has some advantages in that it is stable at
physiological temperatures and can easily pass the blood-brain
barrier (16). Additionally, the
level of BHB in the blood can be altered by exogenous
administration and BHB is considered to be preferable to treat
patients with epilepsy, particularly for those with related
metabolic abnormalities as it is a simple and safe method to induce
elevated plasma levels of ketone bodies (16). It has also been reported that
seizures occur more frequently if the blood glucose level is
elevated during KD treatment (11).
Meidenbauer and Roberts (14)
demonstrated that acute glucose utilization would increase aberrant
synchronous neuronal discharges, thus leading to seizure burst. The
results of the present study determined that exogenous
administration of 4 mmol/kg BHB could increase blood BHB
concentration, but had no effects on glucose levels, despite the
fact that rats were fed a normal, unrestricted diet. This suggests
that exogenous BHB administration does not cause an acute increase
in glucose levels.
Furthermore, KD can increase the BHB level
significantly and rats on KD had a significantly increased
threshold for seizure induction (20). Furthermore, KD is not antiepileptic
until BHB levels in the blood reach an efficacious level (1–2
mmol/l in rats) (14). In the
present study, the concentration of BHB in the blood increased to
an efficacious level just 15 min following exogenous BHB
administration and this level was maintained for 90 min, indicating
that exogenous BHB administration may be a convenient and efficient
way to elevate its concentration in the blood. If the BHB
concentration in the cerebrospinal fluid was also tested at the
corresponding time, the process of BHB utilization and metabolism
following exogenous administration would be elucidated in more
detail. Additionally, the onset time of seizures was prolonged in a
KA-induced seizure model following BHB pretreatment in the present
study, even though the degree of seizure behavior was not
significantly decreased. This is consistent with previous findings
demonstrating that KD increased the seizure threshold in rats but
did not alleviate seizure severity (20). More experiments are still needed to
verify the findings of the present study.
The present study also showed that neuronal loss and
MFS were markedly diminished in the BHB-pretreated group compared
with rats that did not undergo BHB pretreatment. MFS is thought to
be epileptogenic and strongly associated with the occurrence of
spontaneous recurrent seizure (21).
Altogether, the similarity in the effects of BHB and KD on seizure
susceptibility suggests that exogenous BHB may be an anticonvulsant
alternative to KD. Patients on KD are only allowed a narrow range
of foods, thus limiting patient food choices and permitted dishes
may require long, complex preparation. Furthermore, adverse
reactions such as nausea, vomit and diarrhea, experienced by some
patients on KD have inhibited its application (22). Therefore, exogenous BHB preparations
may be preferable to KD to treat epilepsy. Further studies are
necessary to verify whether exogenous BHB administration may have a
better therapeutic effect than KD in epilepsy treatment.
In conclusion, exogenous BHB administration at a
dose of 4 mmol/kg could increase the BHB concentration in the blood
without affecting blood glucose levels and increase the threshold
of seizures, although it does not significantly the grades of
seizure behavior. Additionally, exogenous BHB administration may
attenuate the neuronal loss and MFS that occur in the hippocampus
following convulsions. Exogenous BHB may be an alternative to KD to
provide a protective effect in the epileptic model induced by KA.
Therefore, the results of the present study may allow novel
therapeutic techniques to be developed to treat epilepsy.
Acknowledgements
The present study was supported by a project of the
Shandong Province Science and Technology Program (grant no.
2014GSF118179) and the Special Foundation for Taishan Scholars
(grant no. ts20110814).
References
|
1
|
Banerjee PN, Filippi D and Hauser WA: The
descriptive epidemiology of epilepsy-a review. Epilepsy Res.
85:31–45. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luan G, Zhao Y, Zhai F, Chen Y and Li T:
Ketogenic diet reduces Smac/Diablo and cytochrome c release and
attenuates neuronal death in a mouse model of limbic epilepsy.
Brain Res Bull. 89:79–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kwan P and Brodie MJ: Early identification
of refractory epilepsy. N Engl J Med. 342:314–319. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wei C-X, Bian M and Gong GH: Current
research on antiepileptic compounds. Molecules. 20:20741–20776.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang Y, Yang Y, Wang S, Ding Y, Guo Y,
Zhang MM, Wen SQ and Ding MP: Ketogenic diet protects against
epileptogenesis as well as neuronal loss in amygdaloid-kindling
seizures. Neurosci Lett. 508:22–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suzuki Y, Takahashi H, Fukuda M, Hino H,
Kobayashi K, Tanaka J and Ishii E: β-hydroxybutyrate alters
GABA-transaminase activity in cultured astrocytes. Brain Res.
1268:17–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gama IR, Trindade-Filho EM, Oliveira SL,
Bueno NB, Melo IT, Cabral-Junior CR, Barros EM, Galvão JA, Pereira
WS, Ferreira RC, et al: Effects of ketogenic diets on the
occurrence of pilocarpine-induced status epilepticus of rats. Metab
Brain Dis. 30:93–98. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van Delft R, Lambrechts D, Verschuure P,
Hulsman J and Majoie M: Blood beta-hydroxybutyrate correlates
better with seizure reduction due to ketogenic diet than do ketones
in the urine. Seizure. 19:36–39. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abdelmalik PA, Shannon P, Yiu A, Liang P,
Adamchik Y, Weisspapir M, Samoilova M, Burnham WM and Carlen PL:
Hypoglycemic seizures during transient hypoglycemia exacerbate
hippocampal dysfunction. Neurobiol Dis. 26:646–660. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yum MS, Ko TS and Kim DW:
β-Hydroxybutyrate increases the pilocarpine-induced seizure
threshold in young mice. Brain Dev. 34:181–184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Minlebaev M and Khazipov R: Antiepileptic
effects of endogenous beta-hydroxybutyrate in suckling infant rats.
Epilepsy Res. 95:100–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bough KJ, Chen RS and Eagles DA: Path
analysis shows that increasing ketogenic ratio, but not
β-hydroxybutyrate, elevates seizure threshold in the rat. Dev
Neurosci. 21:400–406. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
National Research Council, . Guide for the
Care and Use of Laboratory Animals. 8. Washington (DC): National
Academies Press (US); 103. pp. 1072–1073. 2011
|
|
14
|
Meidenbauer JJ and Roberts MF: Reduced
glucose utilization underlies seizure protection with dietary
therapy in epileptic EL mice. Epilepsy Behav. 39:48–54. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Löscher W: Current status and future
directions in the pharmacotherapy of epilepsy. Trends Pharmacol
Sci. 23:113–118. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Samoilova M, Weisspapir M, Abdelmalik P,
Velumian AA and Carlen PL: Chronic in vitro ketosis is
neuroprotective but not anti-convulsant. J Neurochem. 113:826–835.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie G, Tian W, Wei T and Liu F: The
neuroprotective effects of β-hydroxybutyrate on Aβ-injected rat
hippocampus in vivo and in Aβ-treated PC-12 cells in vitro. Free
Radic Res. 49:139–150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Neal EG, Chaffe H, Schwartz RH, Lawson MS,
Edwards N, Fitzsimmons G, Whitney A and Cross JH: The ketogenic
diet for the treatment of childhood epilepsy: A randomised
controlled trial. Lancet Neurol. 7:500–506. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Henderson CB, Filloux FM, Alder SC, Lyon
JL and Caplin DA: Efficacy of the ketogenic diet as a treatment
option for epilepsy: Meta-analysis. J Child Neurol. 21:193–198.
2006.PubMed/NCBI
|
|
20
|
Bough KJ and Eagles DA: A ketogenic diet
increases the resistance to pentylenetetrazole-induced seizures in
the rat. Epilepsia. 40:138–143. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Buckmaster PS, Zhang GF and Yamawaki R:
Axon sprouting in a model of temporal lobe epilepsy creates a
predominantly excitatory feedback circuit. J Neurosci.
22:6650–6658. 2002.PubMed/NCBI
|
|
22
|
Giordano C, Marchiò M, Timofeeva E and
Biagini G: Neuroactive peptides as putative mediators of
antiepileptic ketogenic diets. Front Neurol. 5:632014. View Article : Google Scholar : PubMed/NCBI
|