Introduction
Idiopathic pulmonary fibrosis (IPF) is an
unexplained chronic fibrotic interstitial pneumonia, as well as the
main type of idiopathic interstitial pneumonia (IIP). In recent
years, its prevalence and annual incidence rate has significantly
increased (1). The lung histology
and/or chest high-resolution computed tomography (HRCT) of IPF has
been characterized by usual interstitial pneumonia (UIP) in
previous studies (2,3). IPF exhibits unknown etiology and poor
prognosis, with a median survival period of only 2 to 3 years
following diagnosis and there is a current lack of satisfactory
treatment available (4,5). The combination of prednisone,
azathioprine and N-acetylcysteine has been demonstrated to have
little effect on hindering disease progression in patients with
IPF; however, multiple side effects may occur (6). Furthermore, N-acetylcysteine
monotherapy is not able to suppress the acute exacerbation and
reduce the mortality of patients with IPF (7). Pirfenidone possesses anti-inflammatory,
anti-fibrotic and anti-oxidant properties and has been indicated to
impede the declining rate of lung functions, but it has little
benefit on the mortality of the disease (8). Therefore, considering more effective
agents for the treatment of IPF should be a predominant focus. As a
synthetic ligand, rosiglitazone (ROS) is the representative agonist
of peroxisome proliferator-activated receptor γ (PPARγ). PPARγ and
its ligand participate in lipid and sugar metabolism, immune and
inflammatory responses and exert anti-fibrosis effects in multiple
organs (9–11). Retinoin (RET) is the product of
oxidative metabolism of vitamin A in vivo and exhibits
regulatory roles for a variety of immune and inflammatory
responses. RET is able to promote the repair of alveolar epithelial
cells (12,13). To date, few reports have indicated
the anti-pulmonary fibrosis roles of ROS (5). The present study observed the in
vivo intervention effects and efficacies of ROS and RET on
bleomycin-induced pulmonary fibrosis in rats.
Materials and methods
Animal grouping and model
preparation
A total of 48 healthy male Wistar rats, 2 months
old, weighing 220±23 g, were purchased from Qingdao Institute for
Food and Drug Control (Qingdao, China), had free access to food and
water and were maintained in an environment at a temperature of
22°C. Rats were randomly divided into control (group C), model
(group M), dexamethasone (group D), ROS (group R), RET (group W)
and ROS + RET (group L) groups. Group C received 0.3–0.4 ml of
saline which was slowly intratracheally instilled, while 0.3–0.4 ml
bleomycin A5 saline solution (5 mg/kg; CLEA Japan, Inc., Tokyo,
Japan) was slowly intratracheally instilled to prepare the model of
bleomycin-induced pulmonary fibrosis. All groups received
consecutive 31-day oral administration and intraperitoneal
injection 24 h following modeling. Group C was intraperitoneally
injected with an 5 mg/kg saline + oral administration of saline;
group M was intraperitoneally injected the equal amount of saline +
oral administration of saline; group D was intraperitoneally
injected with 5 mg/kg dexamethasone (Jiangsu Lianshui
Pharmaceutical Co., Ltd., Lianshui, China) + an equal amount of
oral saline; group R was orally administered 4 mg/kg ROS +
intraperitoneally injected saline (equal amount); group W was
orally administered 20 mg/kg RET (Jiangsu Lianshui Pharmaceutical
Co., Ltd.) + intraperitoneally injected saline (equal amount); and
group L was orally administered 4 mg/kg ROS (Chongqing Fuling
Pharm., Taiji Group, China) + 20 mg/kg RET + intraperitoneally
injected saline (equal amount). The present study was performed in
accordance with the recommendations of the Guide for the Care and
Use of Laboratory Animals of the National Institutes of Health. The
animal use protocol was reviewed and approved by the Institutional
Animal Care and Use Committee of Qingdao University (Qingdao,
China).
Specimen collection
Rats from each group were inspected using HRCT on
days 7, 21 and 31, respectively, followed by bone algorithm and
standard algorithms for the reconstruction. Two rats from each
group were randomly selected and sacrificed on day 7, while the
rest rats were all sacrificed on day 31.
Hematoxylin and eosin (H&E)
staining and Masson staining
Lung tissue sections from sacrificed rats were fixed
with 4% formalin for 24 h, paraffin embedded, sectioned (5-µm
thick) and exposed to H&E staining and Masson staining and the
morphological changes of lung tissues were observed using a light
microscope (magnification, ×200). The degrees of alveolitis and
pulmonary fibrosis were determined as outlined by Szapiel et
al (14).
Alkaline hydrolysis method
The alkaline hydrolysis method was used to detect
the hydroxyproline (Hyp) content in lung tissues. A
spectrophotometer (Shanghai Analytical Instrument Co., Shanghai,
China) was used to measure the absorbance of each sample tube. Hyp
content was subsequently calculated according to the following
formula: Hyp content=(absorbance of sample tube-absorbance of blank
tube)/(absorbance of standard tube-absorbance of blank tube) ×
content of standard tube (5 µg/ml) × total volume of hydrolyzate (5
ml)/tissue wet weight (mg).
Detection of serum transforming growth
factor-β1 (TGF-β1)
ELISA assay (48 t; Wuhan Boster biotechnology Co.,
Wuhan, China) was performed to detect serum TGF-β1 concentration,
according to the manufacturer's instructions.
Statistical analysis
Experimental data were processed using SPSS 17.0
statistical software (SPSS, Inc., Chicago, IL, USA). Data were
expressed as the mean ± standard deviation. Grade data were
transformed into the measurement data: 0 point for grade 0, 1 point
for grade 1, 2 points for grade 2, 3 points for grade 3, and so on.
Intergroup comparison was performed using Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Appearance
Rats in group C were generally in good condition;
however, rats in group M were listless and tired, and suffered from
mild dyspnea and cyanosis of their peripheral limbs and lips, and
the rats' activities decreased: The overall mental status, food
intake, body weight, colors of extremities and lips and greater
levels of motility were observed in R, W and L groups when compared
with group M; while rats in group D were emaciated and their
abilities of disease-resistance were weak.
Pathological observation
H&E staining revealed that rats in group C
exhibited normal lung tissue structures at each observation time
point. However, the alveolar space of the rats in group M exhibited
marked inflammatory exudation and inflammatory cell infiltration on
day 7 following modeling. Furthermore, the alveolar septum was
widened and rare fibroblasts were observed. The inflammatory
response in each intervention group was mild (Fig. 1). The alveolitis scores and
performance of each group are indicated in Table I. On day 31, no evidence of
inflammatory cell infiltration was apparent in group M; however,
the alveolar structures were disordered, the alveolar wall and
alveolar septum were thickened and an increased number of mature
fibroblasts were accumulated inside. Furthermore, the red-stained
fibrous tissues were proliferated and the number of matrix
components was significantly increased. All intervention groups (R,
W and L groups) exhibited essentially identical characteristics of
mild inflammation and fibrosis as group M at each time point.
Masson staining revealed that the lung tissue structures in group C
were clear and complete at each time point; however, at different
time points (on day and 31) an increased amount of blue collagen
was deposited in the alveolar septum, bronchi and perivascular
areas in group M. Over time, the collagen content increased.
However, blue collagen deposition in the alveolar septum, bronchi
and perivascular areas in group R, W and L groups were mild.
Fibrosis scores of all intervention groups were significantly
decreased when compared with group M (P<0.01). Fibrosis scores
of groups L, R and W were significantly decreased when compared
with group D (P<0.05). No significant difference in fibrosis
scores was observed between groups R and W; however, the score of
group L was significantly decreased when compared with groups R and
W (P<0.05; Fig. 2). The pulmonary
fibrosis score and performance of each group on day 31 is indicated
in Table II.
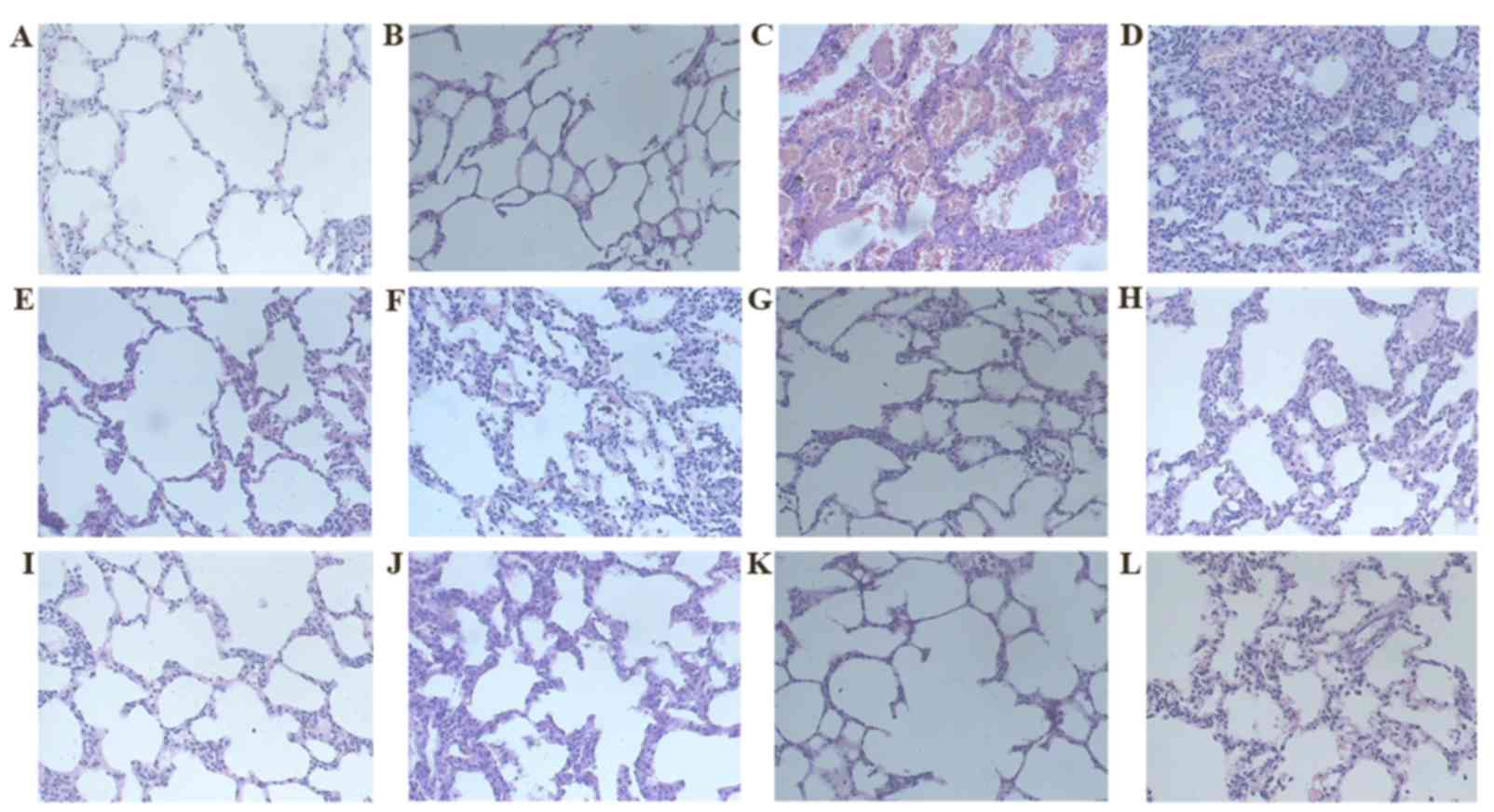 | Figure 1.H&E staining (magnification,
×200). Group C on days (A) 7 and (B) 31, respectively. Group M on
days (C) 7 and (D) 31, respectively. Group D on days (E) 7 and (F)
31, respectively. Group R on days (G) 7 and (H) 31, respectively.
Group W on days (I) 7 and (J) 31, respectively. Group L on days (K)
7 and (L) 31, respectively. H&E staining revealed that rats in
Group C exhibited normal lung tissue structures at each observation
time point; while the alveolar space of the rats in group M
exhibited markedly increased inflammatory exudation and
inflammatory cell infiltration on day 7 following modeling. The
alveolar septum was widened, while rare fibroblasts were seen; the
inflammation situation in groups L, R and W was mild. On day 31, no
inflammatory cell infiltration in group M was apparent and the
alveolar structures were disordered; the alveolar wall and alveolar
septum were thickened and a large number of mature fibroblasts had
accumulated inside. The red-stained fibrous tissues proliferated,
and the number of matrix components had markedly increased. All
intervention groups exhibited essentially identical characteristics
of mild inflammation and fibrosis as group M at each time point.
H&E, hematoxylin and eosin; ROS, rosiglitazone; RET, retinoin;
group C, the control group; group M, the model group; group D, the
dexamethasone group; group R, the ROS group; group W, the RET
group; group L, the ROS + RET group. |
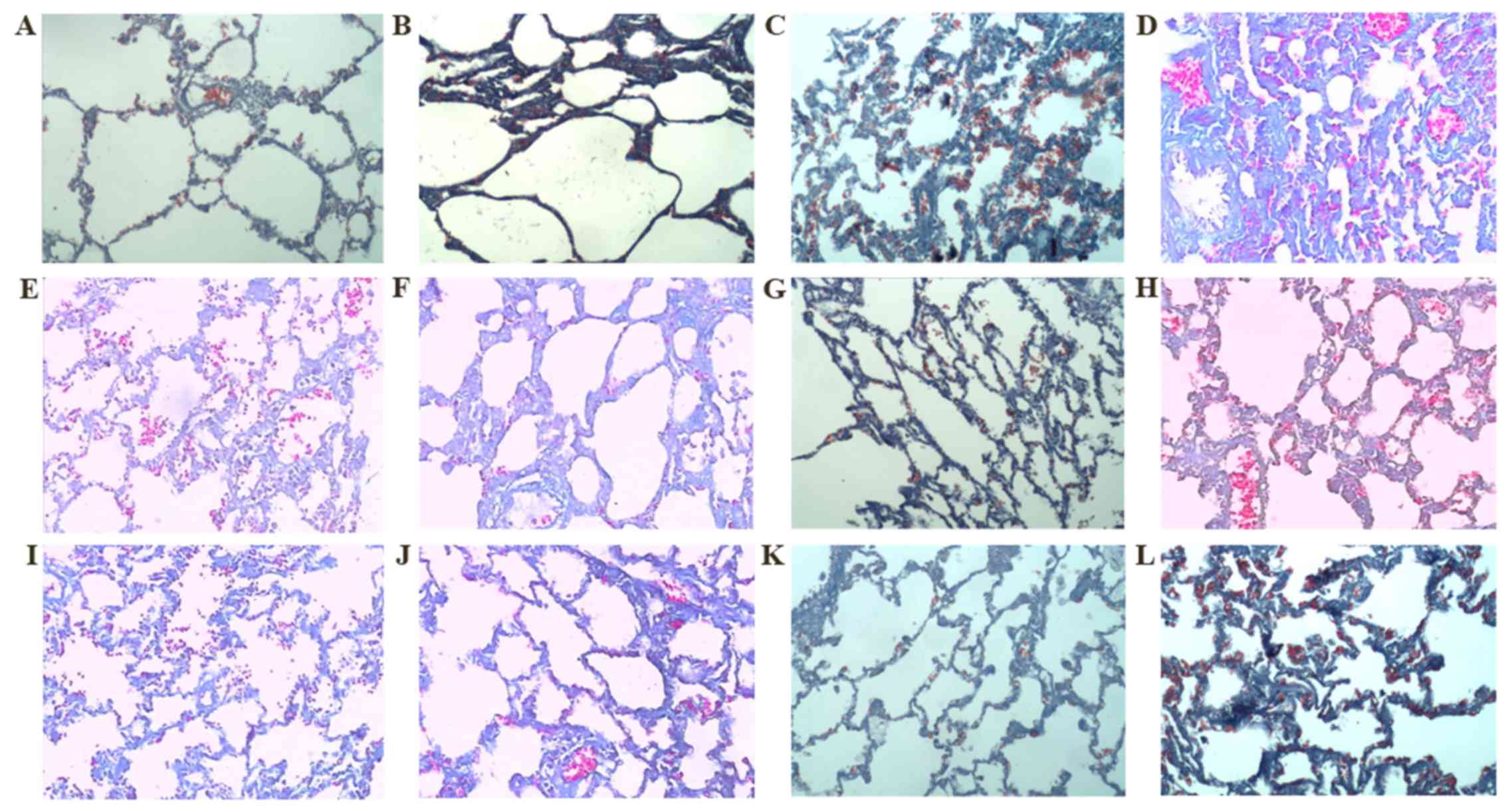 | Figure 2.Masson staining (magnification, ×200).
Group C on days (A) 7 and (B) 31, respectively. Group M on days (C)
7 and (D) 31, respectively. Group D on days (E) 7 and (F) 31,
respectively. Group R on days (G) 7 and (H) 31, respectively. Group
W on days (I) 7 and (J) 31, respectively. Group L on days (K) 7 and
(L) 31, respectively. Masson staining revealed that the lung tissue
structures in group C were clear and complete at each time point,
while at different time points, an abundance of blue collagens were
deposited in the alveolar septum, bronchi and perivascular areas in
group M suggesting that over time, the quantity of collagens
increased. As outlined in Table II,
the fibrosis scores of all intervention groups were significantly
decreased when compared with group M (P<0.01). Group L, R and W
exhibited decreased fibrosis scores when compared with group D
(P<0.05). No significant difference in fibrosis score was
observed between groups R and W, while the score of group L was
significantly decreased when compared with groups R and W
(P<0.05). ROS, rosiglitazone; RET, retinoin; group C, the
control group; group M, the model group; group D, the dexamethasone
group; group R, the ROS group; group W, the RET group; group L, the
ROS + RET group. |
 | Table I.Alveolitis score of each group on day
7 (mean ± standard deviation). |
Table I.
Alveolitis score of each group on day
7 (mean ± standard deviation).
| Group | Alveolitis score |
|---|
| C | 0±0.10 |
| M | 2.5±0.52a |
| D | 1.5±0.22a,b |
| R | 1.0±0.16a–c |
| W | 1.0±0.14a-c |
| L | 0.5±0.13a,b,d |
 | Table II.Fibrosis score of each group on day 31
(mean ± standard deviation). |
Table II.
Fibrosis score of each group on day 31
(mean ± standard deviation).
| Group | Fibrosis score |
|---|
| C | 0.167±0.230 |
| M |
2.833±1.213a |
| D |
2.500±0.921a,b |
| R |
1.400±0.412a–c |
| W |
1.200±0.458a–c |
| L |
0.833±0.331a–d |
Hyp content and serum TGF-β1
concentration
The present study identified that the Hyp content
and serum TGF-β1 concentration in the lung tissues of group M were
significantly increased when compared with group C (P<0.01). Hyp
content and serum TGF-β1 concentration in each intervention group
were significantly decreased when compared with group M
(P<0.01). Compared with group D, the Hyp content and serum
TGF-β1 concentration in the lung tissues of group L, R and W were
significantly decreased (P<0.05); however, Hyp content and serum
TGF-β1 concentration of groups R and W exhibited no significant
difference (P>0.05). Furthermore, the Hyp content and serum
TGF-β1L concentration in the lung tissues of group L were
significantly increased when compared with group R and W
(P<0.05; Table III).
 | Table III.Hyp content and serum TGF-β1 of lung
tissues from different group (mean ± standard deviation). |
Table III.
Hyp content and serum TGF-β1 of lung
tissues from different group (mean ± standard deviation).
| Group | N (rats) | Hyp (µg/g) | TGF-β1 (pg/ml) |
|---|
| C | 6 | 1853.6±297 | 9.78±4.96 |
| M | 6 |
4863.2±546a |
37.82±9.89a |
| D | 4 |
3962.5±298a,b |
17.2l±3.10a,b |
| R | 5 |
3122.9±316a–c |
15.32±2.83a–c |
| W | 5 |
3232.1±293a–c |
14.36±3.22a–c |
| L | 6 |
2530.9±316a–d |
12.71±4.38a–d |
HRCT signs
Imaging results of rats in group C exhibited a clear
outline of the whole lung with uniformly distributed lung markings
and a regular interface; the alveolar septal thickness was normal
and the lung septal and lobular structures were normal. Group M
exhibited the typical changes of alveolitis-pulmonary fibrosis over
a period of time, including ground glass opacity and consolidation
on day 7, which may prompt the phase of alveolitis; additionally,
honeycombing was observed on day 31. Improved radiological signs of
varying degrees were observed in the intervention groups when
compared with group M at the same time point. The degree of
fibrosis exhibited in the images of groups L, W and R was decreased
when compared with group D, indicating that RET and ROS may improve
bleomycin-induced pulmonary fibrosis to some extent (Fig. 3). Radiographic scores are outlined in
Table IV and the HRCT images are
indicated in Fig. 3.
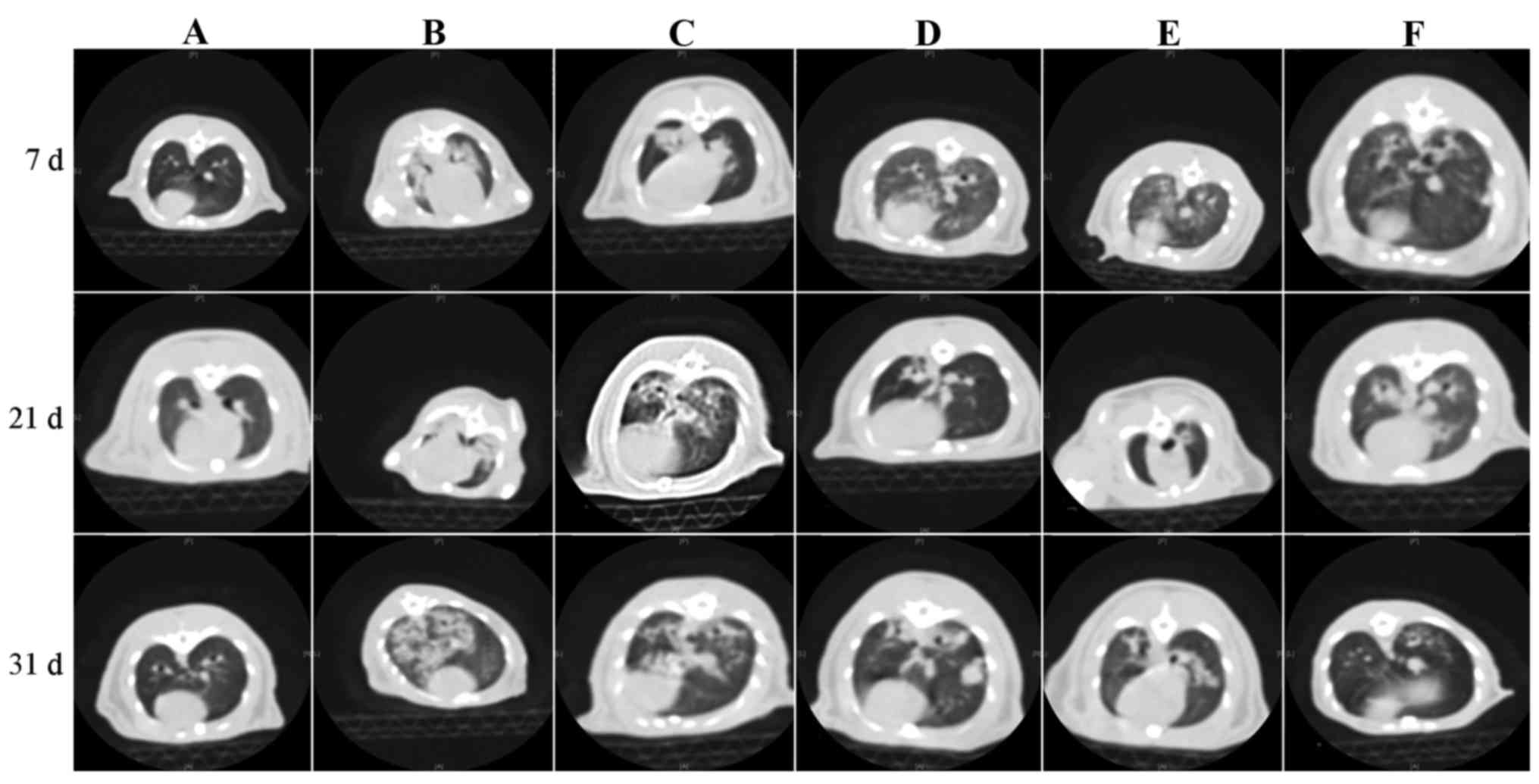 | Figure 3.Histology and/or chest high-resolution
computed tomography on day 7, 21 and 31. Day 7: (A) Lungs of normal
rat; (B) bronchial vascular bundle of group M was thickened, and
exhibited consolidated shadows; (C) group D exhibited decreased
pulmonary transmittance and consolidated shadows; (D) group R
exhibited ground glass-like shadows in two lungs; and (E) group W
exhibited ground glass-like shadows in two lungs; (F) group L
exhibited reduced pulmonary transmittance and small patchy shadows
under the pleura. Day 21: (A) Lungs of normal rat; (B) group M
exhibited large area of consolidated shadows in bilateral lungs,
which was bigger than week 1; (C) group D exhibited thickened
bronchial vascular bundle, with shallow pale ground-glass shadows
inside the lungs; (D) group R exhibited thickened pulmonary
bronchial vascular shadows and blurred edges; (E) group W exhibited
pulmonary consolidated shadows plus cavities; and (F) group L
exhibited thickened bronchial vascular bundle and blurred edges.
Day 31: (A) Lungs of normal rat; (B) group M exhibited pulmonary
increased density shadows, with small cavities similar to
honeycomb-like changes inside; (C) group D exhibited rough and
messy bronchial vascular bundles and multiple nodular shadows
inside the lungs; (D) group R exhibited thickened bronchial
vascular bundle and nodular shadows; (E) group W exhibited
thickened bronchial vascular bundle and patchy shadows; and (F)
group L exhibited pale patchy shadows. ROS, rosiglitazone; RET,
retinoin; group C, the control group (group C); group M, the model
group; group D, the dexamethasone group; group R, the ROS group;
group W, the RET group; group L, the ROS + RET group. |
 | Table IV.Changes and counts of radiological
signs in each group. |
Table IV.
Changes and counts of radiological
signs in each group.
| Group | Day | N | LC | VBB | II | PT | GGO | N | H | Sum |
|---|
| C | 7 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
| 21 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
| 31 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M | 7 | 2 | 2 | 0 | 0 | 0 | 2 | 1 | 0 | 3 |
|
| 21 | 2 | 0 | 1 | 1 | 1 | 0 | 2 | 2 | 7 |
|
| 31 | 6 | 1 | 3 | 4 | 3 | 2 | 3 | 4 | 20 |
| D | 7 | 2 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 3 |
|
| 21 | 2 | 1 | 1 | 0 | 1 | 1 | 2 | 0 | 6 |
|
| 31 | 4 | 1 | 2 | 3 | 2 | 2 | 2 | 2 | 16 |
| R | 7 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 2 |
|
| 21 | 2 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 3 |
|
| 31 | 5 | 2 | 3 | 1 | 1 | 1 | 3 | 0 | 11 |
| W | 7 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
|
| 21 | 2 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 3 |
|
| 31 | 5 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 7 |
| L | 7 | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
|
| 21 | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 |
|
| 31 | 6 | 1 | 2 | 1 | 0 | 0 | 2 | 0 | 6 |
Discussion
The method of intratracheally injecting bleomycin to
construct a pulmonary fibrosis model in rats is relatively simple
and its pathophysiological changes and processes are the most
similar to those in human pulmonary fibrosis (15). Following preparation of the bleomycin
model, the pathology of the lung tissues exhibited severe
alveolitis on day 7 and, according to the Szapiel classification
(14), the majority of the rats
exhibited grade 3 alveolitis, while the majority of inflammatory
cells were no longer observed on day 31. Furthermore, collagen
deposition was apparent, indicating that the states of fibrosis
were predominantly distributed at grades 3–4. These pathological
changes identified in the present study are consistent with those
stated in the literature (16),
indicating that the animal model was successfully established. In
order to objectively evaluate the interventional effects of each
pharmacological agent, light microscopic histological observation
and imaging HRCT observation were conducted. The contents of lung
tissue Hyp and serum TGF-β1 were also detected. Histological and
imaging observations were intuitive and allowed for lesions to be
located by the unique amino acid of collagen fibers. Detected Hyp
content in lung tissues was converted to calculate the content of
collagen and was recognized as the gold standard for pulmonary
fibrosis. TGF-β1 has been indicated to promote and stimulate the
release of a variety of pro-fibrotic cytokines from macrophages,
thus promoting the collagen deposition inside lung tissues
(17,18). The combination of various methods may
ensure the objectivity and accuracy of experimental results.
PPARγ is a member of the PPAR family, which is part
of the nuclear receptor superfamily (9). Previous studies have indicated that
PPARγ and its ligand may participate in the metabolism of lipids
and sugar; furthermore, they have been implicated in growth,
reproduction, immunity and inflammation-related reactions and
exhibit anti-fibrotic effects on multiple organs, such as the
kidney, liver and heart (9–11). As a synthetic ligand, ROS is a
typical PPARγ agonist and is a well-established treatment for type
2 diabetes (9). In vitro
experiments have indicated that PPARγ agonists may inhibit the
chemotaxis of inflammatory cells, such as macrophages. PPARγ
agonists have also been suggested to inhibit the production of
inflammatory cytokines, reduce inflammation and block the
occurrence of inflammatory responses (10). A previous study revealed that PPARγ
was expressed in bronchial and alveolar epithelial cells, vascular
endothelial cells, fibroblasts and alveolar macrophages (19), suggesting that this receptor is
closely associated with the development of lung diseases.
Furthermore, PPARγ has been indicated to elicit anti-inflammatory
and anti-fibrosis effects in lung diseases. Therefore, PPARγ
agonists may not only regulate inflammatory responses and
anti-fibrotic effects but may also inhibit the expression of
TGF-β1, thereby inhibiting the proliferation of fibroblasts and
their conversion to myofibroblasts and the synthesis of collagens,
thus reducing the occurrence of fibrosis (10,12).
Burgess et al (20) and
Genovese et al (21) have
demonstrated this phenomenon through in vitro experiments.
Additionally, Milam et al (22) demonstrated that the administration of
PPARγ agonists following the formation of pulmonary fibrosis in
rats may provoke anti-pulmonary fibrosis effects.
The morphologies of groups R and D on day 7 showed
that the states of alveolitis in lung tissues were significantly
decreased when compared with group M, suggesting that ROS and
dexamethasone are able to decrease the effects of inflammation
early on. In addition, the fibrosis score of group R on day 31 was
decreased when compared with groups M and D, and HRCT revealed the
rats exhibited mild fibrosis, which was an improvement compared
with the other two groups, suggesting that ROS had an inhibitory
effect on advanced fibrosis. The Hyp content and serum TGF-β1
concentration in the lung tissues of group R was significantly
decreased in group R compared with groups M and D, suggesting that
ROS may activate the PPARγ pathway, thus inhibiting the
transcription and expression of TGF-β1 and subsequently reducing
the transformation of fibroblasts to myofibroblasts and reducing
collagen deposition.
RET is the most active derivative of vitamin A,
which acts as a gene expression regulatory factor that is able to
bind with specific receptors, thus regulating the expression of
related target genes and controlling matrix metabolism, cell growth
and differentiation (23). RET
receptors are present in almost all cells (23). Chinese and Western researchers have
identified that RET has positive roles in fighting against fibrosis
in bone marrow, liver, kidney and other organ systems, in which RET
mediates interstitial fibrosis by predominantly reducing the
expression of TGF-β1 inside the interstitial tissues, thus reducing
the accumulation of extracellular collagen (13,24,25). RET
may inhibit the collagen synthesis process in human lung
fibroblasts, both in a steady state and following TGF-β1
stimulation (26). A prior report
revealed that, following the activation of RET receptors, alveolar
damage in rats was reduced and normal lung volume, number of
receptors and surface area was maintained, which resulted in the
inhibition of excessive alveolar differentiation (27). The repair of alveolar epithelium may
be promoted thus treating the fibrosis (27). In addition, another study has
suggested that nuclear transcription factor activator protein 1
(AP-1) may promote the excessive secretion of type I collagen and
due to the antagonism between retinoic acid (RA) and the AP-1
receptor, RA may decrease the activity of AP-1 (28).
In the present study, rats in group W exhibited
significantly decreased fibrosis scores, indicated by Masson
staining on day 31, when compared with groups M and D. HRCT
revealed that only slight fibrotic changes were observed in the
middle and late phases; when compared with group D. The Hyp content
and serum TGF-β1 concentration in the lung tissues of groups L, W
and R were notably decreased. The reasons were presumed as that
while RET and ROS elicit receptor interactions through which the
cell growth and immune functions were regulated and the expression
of TGF-β1 was inhibited, RET receptors may maintain the alveolar
volume and quantities and exhibit certain repairing effects, which
may inhibit lung fibroblast activation and collagen synthesis.
The present study investigated the effects of ROS
and RET in bleomycin-induced pulmonary fibrosis in rats. On day 31
following modeling, the pathological HRCT changes in the lungs of
each intervention group were markedly attenuated when compared with
group M. Furthermore, the pulmonary fibrosis markers, Hyp and serum
TGF-β1, were decreased in the intervention groups when compared
with group M. Additionally, during the entire treatment period, the
overall conditions, including appetite and weight of the rats were
good in group C. However, significant differences in the condition
of rats were observed in the treatment groups, which appeared as
the early anti-inflammatory effects of ROS and RET showed no
significant difference when compared with dexamethasone. The late
fibrosis degrees of groups R and W were mild and the mildest degree
of fibrosis was observed in group L, which suggested that the
treatment groups exhibited an improved condition and the
combination of ROS and RET was more effective than the application
of a single agent. No significant difference was indicated between
ROS and RET.
To conclude, the present findings suggest that ROS
and RET have preventive effects against pulmonary fibrosis in rats
and the side effects were relatively reduced when compared with
glucocorticoids. Whether one or several steps were involved inside
the specific anti-fibrotic mechanism still remains to be
elucidated. Further investigation is required into the specific
mechanisms and targets involved in the inhibition of pulmonary
fibrosis, whether these are associated with early anti-inflammatory
effects and if these factors may reverse advanced pulmonary
fibrosis. Further investigation into the pathogenesis of pulmonary
fibrosis may aid the development of novel anti-fibrotic
pharmacological agents to treat pulmonary fibrosis.
References
|
1
|
Ley B and Collard HR: Epidemiology of
idiopathic pulmonary fibrosis. Clin Epidemiol. 5:483–492. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Raghu G, Collard HR, Egan JJ, Martinez FJ,
Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et
al: An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary
fibrosis: Evidence-based guidelines for diagnosis and management.
Am J Respir Crit Care Med. 183:788–824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Travis WD, Costabel U, Hansell DM, King TE
Jr, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU,
et al: An official American Thoracic Society/European Respiratory
Society statement: Update of the international multidisciplinary
classification of the idiopathic interstitial pneumonias. Am J
Respir Crit Care Med. 188:733–748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moua T, Martinez AC Zamora, Baqir M,
Vassallo R, Limper AH and Ryu JH: Predictors of diagnosis and
survival in idiopathic pulmonary fibrosis and connective tissue
disease-related usual interstitial pneumonia. Respir Res.
15:1542014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rafii R, Juarez MM, Albertson TE and Chan
AL: A review of current and novel therapies for idiopathic
pulmonary fibrosis. J Thorac Dis. 5:48–73. 2013.PubMed/NCBI
|
|
6
|
Idiopathic Pulmonary Fibrosis Clinical
Research Network, ; Raghu G, Anstrom KJ, King TE Jr, Lasky JA and
Martinez FJ: Prednisone, azathioprine, and N-acetylcysteine for
pulmonary fibrosis. N Engl J Med. 366:1968–1977. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Idiopathic Pulmonary Fibrosis Clinical
Research Network, ; Martinez FJ, De Andrade JA, Anstrom KJ, King TE
Jr and Raghu G: Randomized trial of acetylcysteine in idiopathic
pulmonary fibrosis. N Engl J Med. 370:2093–2101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
King TE Jr, Bradford WZ, Castro-Bernardini
S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM,
Kardatzke D, Lancaster L, et al: A phase 3 trial of pirfenidone in
patients with idiopathic pulmonary fibrosis. N Engl J Med.
370:2083–2092. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calkin AC, Giunti S, Jandeleit-Dahm KA,
Allen TJ, Cooper ME and Thomas MC: PPAR-alpha and -gamma agonists
attenuate diabetic kidney disease in the apolipoprotein E knockout
mouse. Nephrol Dial Transplant. 21:2399–2405. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iglarz M, Touyz RM, Viel EC, Paradis P,
Amiri F, Diep QN and Schiffrin EL: Peroxisome
proliferator-activated receptor-alpha and receptor-gamma activators
prevent cardiac fibrosis in mineralocorticoid-dependent
hypertension. Hypertension. 42:737–743. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leclercq IA, Sempoux C, Stärkel P and
Horsmans Y: Limited therapeutic efficacy of pioglitazone on
progression of hepatic fibrosis in rats. Gut. 55:1020–1029. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kayalar O and Oztay F: Retinoin induced
repair in the lung of adult hyperoxic mice, reducing transforming
growth factor-β1 (TGF-β1) mediated abnormal alterations. Acta
Histochem. 116:810–819. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Davis BH, Kramer RT and Davidson NO:
Retinoin modulates rat Ito cell proliferation, collagen, and
transforming growth factor beta production. J Clin Invest.
86:2062–2070. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Szapiel SV, Elson NA, Fulmer JD,
Hunninghake GW and Crystal RG: Bleomycin-induced interstitial
pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis.
120:893–899. 1979.PubMed/NCBI
|
|
15
|
Chua F, Gauldie J and Laurent GJ:
Pulmonary fibrosis: Searching for model answers. Am J Respir Cell
Mol Biol. 33:9–13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Limjunyawong N, Mitzner W and Horton MR: A
mouse model of chronic idiopathic pulmonary fibrosis. Physiol Rep.
2:e002492014. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee
PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, et al:
Early growth response gene 1-mediated apoptosis is essential for
transforming growth factor beta1-induced pulmonary fibrosis. J Exp
Med. 200:377–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim KK, Wei Y, Szekeres C, Kugler MC,
Wolters PJ, Hill ML, Frank JA, Brumwell AN, Wheeler SE, Kreidberg
JA and Chapman HA: Epithelial cell alpha3beta1 integrin links
beta-catenin and Smad signaling to promote myofibroblast formation
and pulmonary fibrosis. J Clin Invest. 119:213–224. 2009.PubMed/NCBI
|
|
19
|
Asada K, Sasaki S, Suda T, Chida K and
Nakamura H: Antiinflammatory roles of peroxisome
proliferator-activated receptor gamma in human alveolar
macrophages. Am J Respir Crit Care Med. 169:195–200. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Burgess HA, Daugherty LE, Thatcher TH,
Lakatos HF, Ray DM, Redonnet M, Phipps RP and Sime PJ: PPARgamma
agonists inhibit TGF-beta induced pulmonary myofibroblast
differentiation and collagen production: Implications for therapy
of lung fibrosis. Am J Physiol Lung Cell Mol Physiol.
288:L1146–L1153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Genovese T, Cuzzocrea S, Di Paola R,
Mazzon E, Mastruzzo C, Catalano P, Sortino M, Crimi N, Caputi AP,
Thiemermann C and Vancheri C: Effect of rosiglitazone and
15-deoxy-Delta12,14-prostaglandin J2 on bleomycin-induced lung
injury. Eur Respir J. 25:225–234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Milam JE, Keshamouni VG, Phan SH, Hu B,
Gangireddy SR, Hogaboam CM, Standiford TJ, Thannickal VJ and Reddy
RC: PPAR-gamma agonists inhibit profibrotic phenotypes in human
lung fibroblasts and bleomycin-induced pulmonary fibrosis. Am J
Physiol Lung Cell Mol Physiol. 294:L891–L901. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cifelli CJ and Ross AC: All-trans-retinoin
distribution and metabolism in vitamin A-marginal rats. Am J
Physiol Gastrointest Liver Physiol. 291:G195–G202. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang L, Potter JJ, Rennie-Tankersley L,
Novitskiy G, Sipes J and Mezey E: Effects of retinoic acid on the
development of liver fibrosis produced by carbon tetrachloride in
mice. Biochim Biophys Acta. 1772:66–71. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fleissner D, Frede A, Knott M, Knuschke T,
Geffers R, Hansen W, Dobos G, Langhorst J, Buer J and Westendorf
AM: Generation and function of immunosuppressive human and murine
CD8+ T cells by transforming growth factor-β and retinoic acid.
Immunology. 134:82–92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Redlich CA, Delisser HM and Elias JA:
Retinoin inhibition of transforming growth factor-beta-induced
collagen production by human lung fibroblasts. Am J Respir Cell Mol
Biol. 12:287–295. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Massaro GD, Massaro D and Chambon P:
Retinoin receptor-alpha regulates pulmonary alveolus formation in
mice after, but not during, perinatal period. Am J Physiol Lung
Cell Mol Physiol. 284:L431–L433. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schüle R, Rangarajan P, Yang N, Kliewer S,
Ransone LJ, Bolado J, Verma IM and Evans RM: Retinoin is a negative
regulator of AP-1-responsive genes. Proc Natl Acad Sci USA. 88:pp.
6092–6096. 1991; View Article : Google Scholar : PubMed/NCBI
|

















