Introduction
Complete surgical resection is the most effective
treatment for tumors; however, 60–70% patients are not candidates
for this treatment (1), due to their
tumor size, number and location, organ function and the presence of
comorbidities. Due to the advantages of short treatment time,
little injury and short recovery time, ablation technologies, such
as radiation therapy and croablation, have increasingly attracted
attention in the non-surgical treatment of tumors, including liver,
spleen and prostate tumors (2).
Although these traditional ablation methods can decrease the tumor
burdens for patients, their side effects, such as causing damage to
adjacent tissues (including vessels and nerves), limit their use
and are a risk to health (2).
Therefore, it is important to assess alternative treatment
methods.
Irreversible electroporation (IRE) is an emerging
ablation technology, which consists of the application of a high
voltage field that generates nanopores in the membrane of target
cells (3,4). As a result, cellular homeostasis is
disrupted, leading to cell necrosis and apoptosis (5,6).
Compared with other thermal ablation technologies, IRE does not
rely on thermal energy, maintains connective tissue integrity and
has little effects on vessels, nerves, bile and pancreatic ducts
(1,7,8).
In 2012, the US Food Drug Administration agency
approved the clinical application of IRE. Even though it has been
successfully applied in the treatment of hepatic (9), pancreatic (10) and renal (11) carcinomas, IRE may cause injury to the
stomach wall of certain patients, due to the close proximity of the
organ to the liver and pancreas. The present study evaluated the
effects of IRE on the stomach wall using a pig model.
Materials and methods
Animals
The present study was approved by the Animal
Experimental Center of Southern Medical University (Guangzhou,
China). All animals were under necessary and appropriate human care
from professional staff. A total of 8 female Tibetan mini-pigs
(weight, 25–30 kg; age, 4–6 months) were purchased from Southern
Medical University and studied under the supervision of the
Division of Laboratory Animal Medicine at Southern Medical
University. The housing conditions were: Temperature, 20–25°C;
relative humidity, 40–60%; light/dark cycle, 12 h. All animals had
free access to food and water. As generating six lesions on the
stomach wall may lead to overlapping of the lesions and cause
extended damage to the stomach, the animals were randomly allocated
into two groups, A and B, with four animals in each group. In group
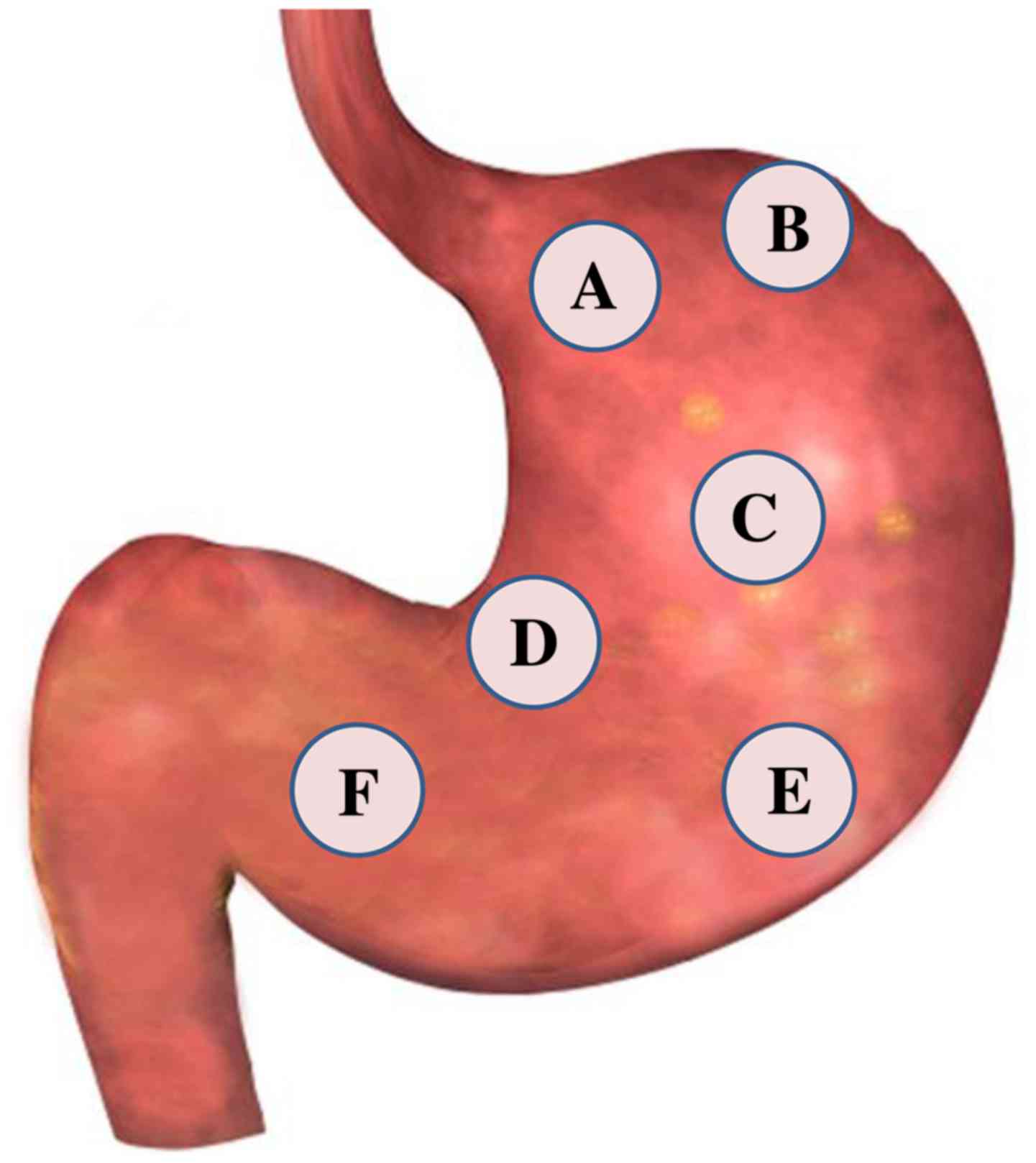
A, three lesions in three separate areas (Fig. 1A-C) of the stomach wall of the
animals were generated, including the gastric cardia, fundus and
body. In group B, three lesions were created in areas (Fig. 1D-F) of the lesser and greater gastric
curvature and in the stomach pylorus of the pigs.
Anesthesia
All pigs were administered 20 mg/kg ketamine (Gutian
Pharmaceutical Co., Ltd., Fujian, China) and 10 mg/kg promethazine
(Suicheng Pharmaceutical Co., Ltd., Xinzheng, China) by
intramuscular injection. General anesthesia was maintained with 2%
isoflurane (Yapei Pharmaceutical Co., Ltd., Shanghai, China) per
continuous inhalation and 0.1 mg/kg atropine (Jinyao Pharmaceutical
Co., Ltd., Tianjin, China) via intramuscular injection. Respiratory
support was administered via mechanical ventilation. Cisatracurium
besylate (Hengrui Medicine Co., Ltd., Jiangsu, China) was
administered intravenously at 60 µg/kg at the start of IRE and
maintained by intravenous perfusion at 10 µg/kg/min during the
procedure to reduce muscle contraction.
IRE procedure
Pigs were fasted for 24 h prior to IRE. Belladonna
and aluminium capsules II (Jiangsu Hengrui Medicine Co., Ltd.,
Jiangsu, China) were administered to reduce gastric motility. Pigs
were placed on a surgical table in a supine position, and the legs
were fastened to ensure that the procedure was performed smoothly.
The skin overlying the center of the abdomen was shaved, cleaned
and sterilized prior to a 15-cm long midline laparotomy being
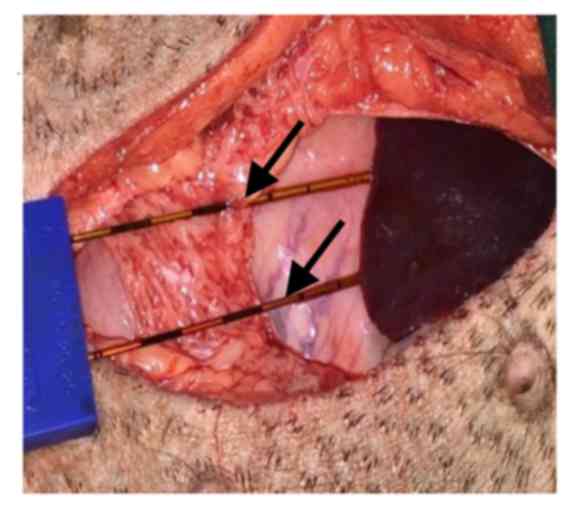
performed. To simulate tumor ablation close to the stomach wall,
electrode needles were placed in the space between the stomach wall
and the liver (Fig. 2). Needles were
not inserted into the stomach tissue, but sutures were used to fix
the needles to the serosal surface of the stomach wall. The 16-g
monopolar IRE probes (AngioDynamics, Queensbury, NY, USA) were
attached to the stomach wall and secured with a spacer device; 10
IRE (NanoKnife; AngioDynamics) pulses at 1,500 V/cm were initially
performed. There was an interruption in the IRE procedure in
certain cases, due to high current, thereby leading to a reduced
and adapted electric field. The final IRE electric field strength
and other parameters were recorded (Table I). As the present study aimed to
evaluate the most severe damage of IRE on the stomach wall, the
above parameters were selected. All IRE pulses were delivered using
an electrocardiographic synchronization instrument (AngioDynamics,
Inc., Latham, NY, USA) to avoid cardiac arrhythmias. The skin
incision was sutured at the end of the procedure. Pigs fully
recovered within 2 h post-IRE and received daily intramuscular
injections of 40 mg/kg body weight cephazolin (Qilu Pharmaceutical
Co., Ltd., Jinan, China) for 1 week to reduce the risk of
infection. To reduce pain, animals were administered intramuscular
buprenorphine (0.01 mg/kg; Jin Lan Pharmaceutical Group Co., Ltd.,
Tianjin, China) and oral meloxicam (0.4 mg/kg; Boehringer Ingelheim
Pharmaceutical Co., Ltd., Shanghai, China) for the first week.
 | Table I.Irreversible electroporation
applications. |
Table I.
Irreversible electroporation
applications.
| Animal no. | Ablated areas | Electric field,
V/cm | Feedback current,
A |
|---|
| 1 | a | 1,500 | 28 |
|
| b | 1,400 | 26 |
|
| c | 1,500 | 29 |
| 2 | a | 1,400 | 25 |
|
| b | 1,300 | 23 |
|
| c | 1,500 | 31 |
| 3 | a | 1,500 | 27 |
|
| b | 1,400 | 26 |
|
| c | 1,400 | 27 |
| 4 | a | 1,300 | 28 |
|
| b | 1,300 | 24 |
|
| c | 1,500 | 29 |
| 5 | d | 1,500 | 28 |
|
| e | 1,500 | 30 |
|
| f | 1,400 | 28 |
| 6 | d | 1,500 | 29 |
|
| e | 1,400 | 25 |
|
| f | 1,500 | 30 |
| 7 | d | 1,300 | 25 |
|
| e | 1,300 | 23 |
|
| f | 1,400 | 26 |
| 8 | d | 1,400 | 25 |
|
| e | 1,400 | 27 |
|
| f | 1,500 | 30 |
Serum aminotransferases and white
blood cells
Blood samples were collected prior to IRE and on
days 1, 3, 5 and 7 post-IRE. To monitor hepatic function and the
presence of inflammation, the levels of alanine aminotransferase
(ALT), aspartate aminotransferase (AST) and white blood cells (WBC)
were assessed using an automatic biochemical analyzer, KHA-220
(Beijing Science and Technology Co., Ltd., Beijing, China).
Gastroscopy and endoscopic
ultrasonography
Gastroscopy and endoscopic ultrasonography images
were obtained on days 7 and 28 post-IRE. Gastroscopy and endoscopic
ultrasonography were performed by two professional endoscopic
doctors, who were blind to the aims of the study.
Gross pathology and
histopathology
The present experiment was performed by a
well-trained and professional pathologist with ~20 years
experience. From each group, 2 pigs were sacrificed on day 7
post-IRE. The remaining 4 pigs were sacrificed on day 28 post-IRE.
The ablated stomach wall tissue was grossly examined for any signs
of perforation, edema, swelling and color changes. The mucosal and
serosal surfaces of the ablated tissue and nearby normal tissue
were imaged. The largest diameter of each lesion in the mucosal and
serosal surface was measured with a ruler. Ablated tissue and
surrounding normal tissue were serially sectioned at 5-mm
intervals, fixed in 10% formalin for 24 h, and stained with
hematoxylin and eosin and with Masson's trichome for
histomorphologic and collagen proliferation analyzes, respectively.
The photos were imaged using a NIKON LV150L microscope (Nikon
Corporation, Tokyo, Japan), with magnification, ×100.
Statistical analysis
Data were analyzed with SPSS version 22.0 (SPSS,
IBM, Armonk, NY, USA). The size of the lesions was analyzed using
Student's t-test. P<0.05 was determined to indicate a
statistically significant difference.
Results
Imaging findings
Gastroscopy, which was performed on days 7 and 28
post-IRE, revealed differing degrees of gastric ulcers in the
stomach wall of the pigs. Additionally, damage in the stomach wall
gradually decreased. On day 7 post-IRE, tissue samples presented
round/oval ulcers with whitish/yellowish fur and slight edema in
the surrounding mucosa (Fig. 3A).
Comparatively, on day 28 post-IRE, tissue samples had less
pronounced edema, shallower and smaller ulcers, and smooth mucosal
surfaces (Fig. 3B). The
yellowish/whitish fur disappeared in the majority of the samples.
In 2 animals of group A, 2 lesions in the gastric body did not
present the visible healing changes (small ulcers and smooth
mucosal surface) observed in the other animals on day 28 post-IRE.
In these animals, the two lesions were shallow but not smaller in
size.
It was challenging to completely eliminate air
interferences during the endoscopic ultrasonography. On day 7
post-IRE, only fusion of stomach layers and edema in the ablated
areas was observed (Fig. 3C). At day
28 post-IRE, there was no visible edema; however, it remained
challenging to differentiate among the stomach wall layers
(Fig. 3D).
Clinical course
In 3 pigs in group A and 2 pigs in group B, the body
temperature increased (>40°C) within 48 h after IRE.
Administration of meloxicam (0.4 mg/kg; Boehringer Ingelheim
Pharmaceutical Co., Ltd.) reduced body temperature to normal levels
(38–39.5°C). Notably, 3 pigs in group A and 2 pigs in group B
presented with distended abdomens for 3 days. All pigs had reduced
appetite and decreased fecal output for 5 days. Animal moaning and
bruxism (teeth grinding), which are classic behavioral indicators
of pain, were noted when the abdomen was pressed down firmly.
Changes in serum aminotransferase
levels and white blood cell count
There were no significant differences in the levels
of serum aminotransferase or WBC between groups A and B (data not
shown). The results from group A and B were then pooled and are
presented in Fig. 4. AST and WBC
levels increased on day 1 post-IRE and gradually decreased
thereafter, reaching normal levels by day 7 post-IRE (Fig. 4). Changes in serum aminotransferase
levels, which are indicative of hepatic damage, occurred for ~7
days post-IRE. The changes in WBC levels suggested that there may
have been mild inflammation during the first week
post-treatment.
Gross pathology
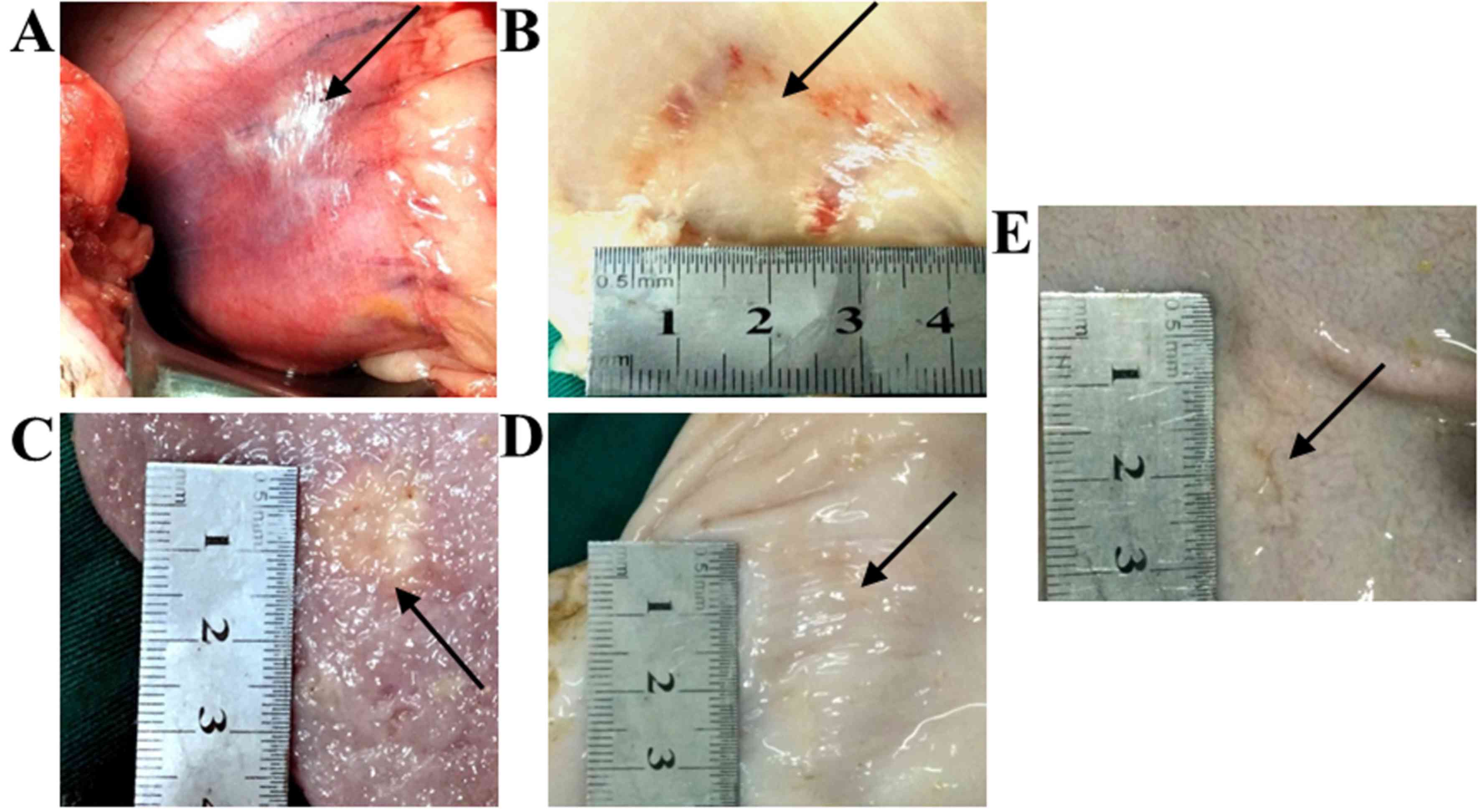
A whitish lesion with surrounding reddish areas was
observed immediately following the ablation procedure (Fig. 5A). Demarcation between the whitish
lesion and surrounding reddish parts was sharp. The appearance was
similar for all ablation areas and there were no signs of
perforation. At day 7 post-IRE, the lesions on the serosal surface
of the stomach wall were observed to be a rectangular shape with
dark red color where the electrodes had been attached (Fig. 5B). On the mucosal surface of the
stomach wall, round/oval ulcers with or without whitish fur were
observed (Fig. 5C). The demarcation
between the lesion and nearby normal stomach tissue was sharp in
all the pigs. However, at 28 days post-IRE, the lesions on the
serosal surface were crumpled, markedly smaller in size and had the
same color as that of nearby normal tissues (white or slightly
reddish) with no signs of edema (Fig.
5D). Ulcers on the mucosal surface were shallower and smaller,
and the color was similar to that of untreated tissue (Fig. 5E), with the exception of 2 lesions in
the gastric body of 2 pigs in group A. The sizes of the lesions at
7 and 28 days post-IRE are presented in Table II. There was a significant
difference in lesion size between the serosal and mucosal surfaces
of the stomach wall.
 | Table II.Size of stomach lesions on days 7 and
28 post-irreversible electroporation. |
Table II.
Size of stomach lesions on days 7 and
28 post-irreversible electroporation.
|
| Diameter of stomach
lesions, cm |
|
|---|
|
|
|
|
|---|
| Days post
procedure | Serosal surface
(n=12) | Mucosal surface
(n=12) | P-value |
|---|
| 7 | 3.13±0.46 | 1.78±0.31 | <0.05 |
| 28 | 3.01±0.42 | 1.61±0.23 | <0.05 |
Histopathology
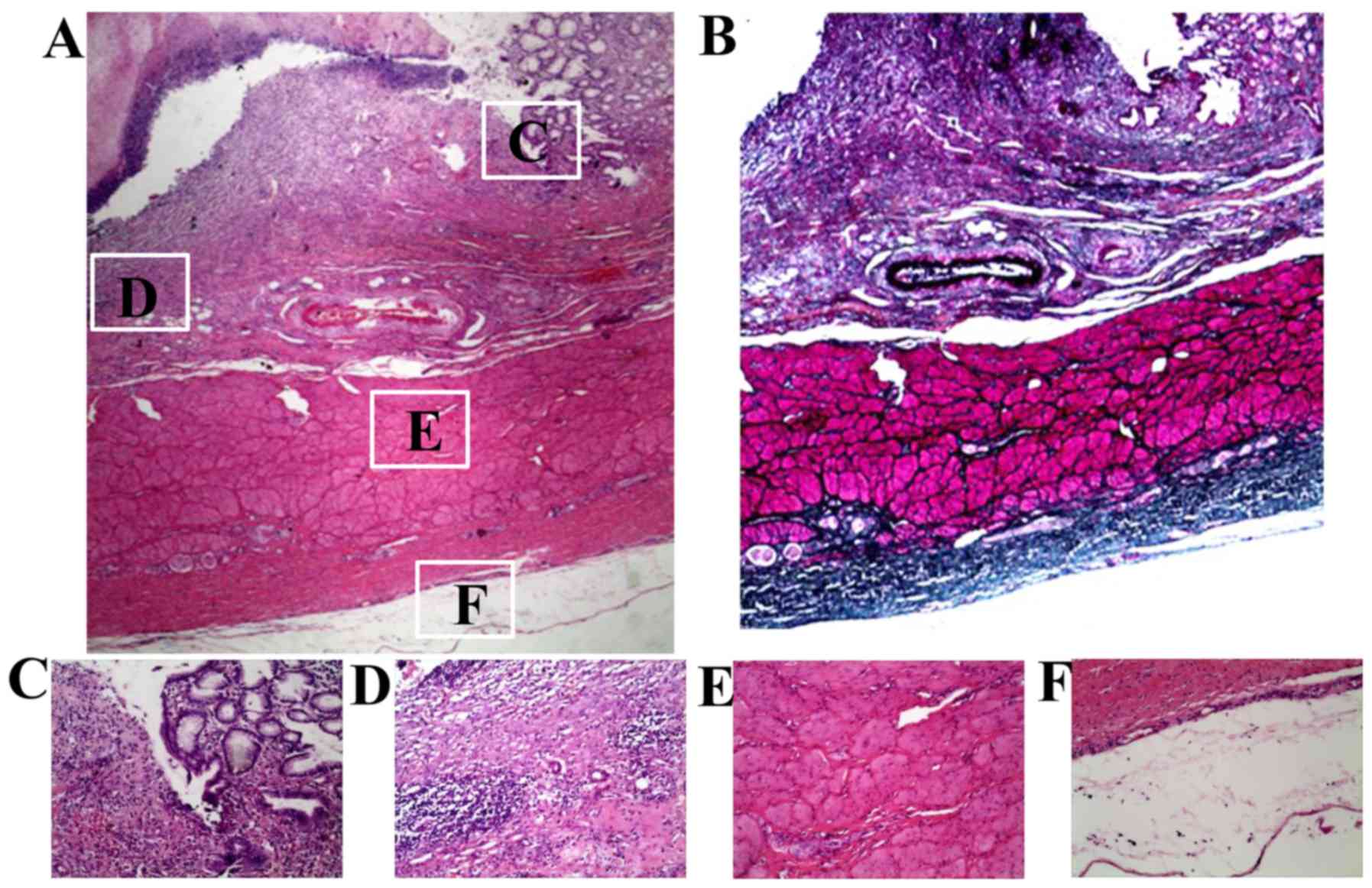
At 7 days post-IRE, all layers of the stomach wall
were damaged by IRE. Stomach wall damage was characterized by the
presence of fibrosis and mild-to-moderate inflammation (plasma
cells, lymphocytes, and eosinophils; Fig. 6A and B). The damage was present
through the entire thickness of the stomach wall and the lesions
were demarcated by sharp borders (Fig.
6C). The mucosa was obliterated by different degrees of
inflammatory liquefactive necrosis, and the submucosa changes were
characterized by hyperemia and edema (Fig. 6D). Muscle fibers in the muscular
layer exhibited differing degrees of swelling and degeneration
(Fig. 6E). The serosa of the stomach
wall was directly exposed to IRE and was obliterated by
inflammation, liquefactive necrosis and fibrous tissues (Fig. 6F). At 28 days post-IRE, the
histopathology results revealed the presence of an almost normal
stomach wall (Fig. 7A) and more
fibrosis in the stomach layers (Fig.
7B). Small glands and short villus in the mucosa were also
observed (Fig. 7C).
Discussion
IRE is an emerging technology that consists of the
application of a high voltage electric field across target cells to
generate nanopores on the cell membranes, thereby contributing to
cell necrosis and apoptosis (12,13).
IRE, which selectively targets cell membranes and preserves
connective tissues, induces rapid tissue regeneration (7,14). IRE
has been widely used in the treatment of hepatic and pancreatic
tumors; however, there is little evidence on the effects of IRE on
the stomach wall.
In a study by Schoellnast et al (15), computed tomography (CT)-guided IRE
adjacent to the rectum wall was performed in 5 pigs without a
water-filled endorectal coil (group A) and in 6 pigs with the coil
(group B) to avoid displacement of the rectum wall. A 16-g bipolar
probe was inserted adjacent and tangential to the rectum wall and
adjacent to the internal obturator muscle. The results revealed
that transmural necrosis was present in all group B animals, while
necrosis was limited to the external layer of the muscularis
propria in the group A animals (15). Schoellnast et al (15) therefore, concluded that IRE ablation
of the rectum may have harmful effects when the rectum wall is
adjacent to the IRE-probe. In the present study, the IRE probes
were placed in the space between the stomach wall and the liver,
fixed in placed by sutures. As a result, no tissues were present
between the IRE probes and the stomach wall; therefore, IRE
ablation directly affected the stomach. As the electrode tips
cannot be exposed to air while ablating, the liver was slightly
pressed down to ensure the successful completion of the IRE
procedure. This step may limit stomach contraction during ablation,
which may contribute to more injury (15). In a study by Srimathveeravalli et
al (16), 2 endorectal IRE
ablations were performed. The first ablation procedure (1,050–1,125
V) was performed on the mucosal and submucosal layers but not on
the muscularis propria. The second procedure (2,100 V) consisted of
a transmural ablation of the rectal wall. In the current study, the
stomach wall was ablated using an electric field >2,100 V, which
likely induced cell death in the stomach layers. Transmural
necrosis was observed in the lesions of the stomach wall, without
any evidence of perforations. The stomach wall was structurally
intact, characterized by the presence of fiber and regenerated
mucosa.
The conditions of the present study, including IRE
probes placement and ablation parameter settings, contributed to
marked damage to the stomach wall. However, when ablating tumors
adjacent to the stomach, the IRE probes cannot affect the stomach
wall directly and the IRE settings may be modified to limit stomach
injury. Therefore, the damage to the stomach wall must be lighter
when ablating tumors adjacent to the stomach.
Compared with other thermal ablation technologies,
IRE, which primarily targets the cellular membrane, preserves the
basic structure of tissue (17,18). In
the present study, the mucosa layer of the stomach presented with
marked inflammatory liquefactive necrosis. The stomach wall
structure was preserved, which allowed collagen, elastic fiber and
regenerated stomach tissue to replace the damaged cells. Edema and
yellowish/whitish furs disappeared by 28 days post-IRE. At day 28
post-IRE, the presence of regenerated mucosa was indicative of
healing. In the present study, there were no evident complications
associated with IRE treatment in the pigs.
In 2 pigs of group A, 2 gastric body lesions did not
heal by day 28 post-IRE. This was attributed to the convenient
ablation area of the gastric body, which is just beneath the
exposed hepatic tissue. IRE probes were fixed tightly to the
stomach by sutures, and movement of the liver or of the IRE probes
was less possible, which may contribute to more severe effects of
IRE on the gastric body. However, in other ablation lesions, due to
the inconvenient ablation area, it was difficult to avoid the
electrode dislocation during the whole procedure and this may
lighten the IRE effects on the stomach wall.
The current study had a number of limitations.
Firstly, the three lesions may increase stomach wall damage and
decrease the healing process, although normal tissues were observed
between the lesions in this study. Secondly, there was an
interruption caused by the high IRE current, and the voltage
required adjusting to complete the ablation procedure, which may
have affected the extent of the stomach wall damage. Thirdly, IRE
electrodes were placed on the stomach wall, not inserted into the
stomach tissue; any probe movement during ablation was able to
affect the procedure. Also, the IRE effects on the anterior stomach
wall were evaluated, but not on the posterior stomach wall.
Finally, while there were no perforations in the stomach wall,
gastrointestinal function was not assessed in these pigs
post-IRE.
In conclusion, the current study demonstrated that
IRE does cause injury to the stomach if it is applied directly on
the organ surface. However, no perforation was observed in the pigs
used in the present study. If translating the results of the
current study into clinical use, IRE of hepatic or pancreatic
tumors, which are adjacent to the stomach wall cannot lead to
stomach perforation. However, further studies that evaluate
different IRE settings and assess gastric function post-IRE are
required.
Acknowledgements
The current study was supported by International
Science Foundation of Guangzhou Fuda Cancer Hospital (grant no.
Y2015-ZD-001). The authors would also like to thank Elixigen Corp.
(Huntington Beach, CA, USA) for helping in proofreading and editing
the English of final manuscript.
References
|
1
|
Cannon R, Ellis S, Hayes D, Narayanan G
and Martin RC II: Safety and early efficacy of irreversible
electroporation for hepatic tumors in proximity to vital
structures. J Surg Oncol. 107:544–549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ahmed M and Goldberg SN: Basic science
research in thermal ablation. Surg Oncol Clin N Am. 20:237–258.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee EW, Wong D, Prikhodko SV, Perez A,
Tran C, Loh CT and Kee ST: Electron microscopic demonstration and
evaluation of irreversible electroporation-induced nanopores on
hepatocyte membranes. J Vasc Interv Radiol. 23:107–113. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Novickij V, Grainys A, Novickij J and
Markovskaja S: Irreversible magnetoporation of micro-organisms in
high pulsed magnetic fields. IET Nanobiotechnol. 8:157–162. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scheffer H, Nielsen K, van Tilborg A,
Vieveen JM, van den Tol MP and Meijerink MR: Irreversible
electroporation: A new form of image-guided tumour ablation. Ned
Tijdschr Geneeskd. 158:A71762014.(In Dutch). PubMed/NCBI
|
|
6
|
Al-Sakere B, André F, Bernat C, Connault
E, Opolon P, Davalos RV, Rubinsky B and Mir LM: Tumor ablation with
irreversible electroporation. PLoS One. 2:e11352007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee EW, Chen C, Prieto VE, Dry SM, Loh CT
and Kee ST: Advanced hepatic ablation technique for creating
complete cell death: Irreversible electroporation. Radiology.
255:426–433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schoellnast H, Monette S, Ezell PC,
Deodhar A, Maybody M, Erinjeri JP, Stubblefield MD, Single GW Jr,
Hamilton WC Jr and Solomon SB: Acute and subacute effects of
irreversible electroporation on nerves: Experimental study in a pig
model. Radiology. 260:421–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Narayanan G, Froud T, Lo K, Barbery KJ,
Perez-Rojas E and Yrizarry J: Pain analysis in patients with
hepatocellular carcinoma: Irreversible electroporation versus
radiofrequency ablation-initial observations. Cardiovasc Intervent
Radiol. 36:176–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martin RC II, McFarland K, Ellis S and
Velanovich V: Irreversible electroporation in locally advanced
pancreatic cancer: Potential improved overall survival. Ann Surg
Oncol. 20 Suppl 3:S443–S449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pech M, Janitzky A, Wendler JJ, Strang C,
Blaschke S, Dudeck O, Ricke J and Liehr UB: Irreversible
electroporation of renal cell carcinoma: A first-in-man phase I
clinical study. Cardiovasc Intervent Radiol. 34:132–138. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maor E, Ivorra A, Leor J and Rubinsky B:
The effect of irreversible electroporation on blood vessels.
Technol Cancer Res Treat. 6:307–312. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Edd JF, Horowitz L, Davalos RV, Mir LM and
Rubinsky B: In vivo results of a new focal tissue ablation
technique: Irreversible electroporation. IEEE Trans Biomed Eng.
53:1409–1415. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Onik G, Mikus P and Rubinsky B:
Irreversible electroporation: Implications for prostate ablation.
Technol Cancer Res Treat. 6:295–300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schoellnast H, Monette S, Ezell PC, Single
G, Maybody M, Weiser MR, Fong Y and Solomon SB: Irreversible
electroporation adjacent to the rectum: Evaluation of pathological
effects in a pig model. Cardiovasc Intervent Radiol. 36:213–220.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Srimathveeravalli G, Wimmer T, Monette S,
Gutta NB, Ezell PC, Maybody M, Weiser MR and Solomon SB: Evaluation
of an endorectal electrode for performing focused irreversible
electroporation ablations in the Swine rectum. J Vasc Interv
Radiol. 24:1249–1256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deipolyi AR, Golberg A, Yarmush ML,
Arellano RS and Oklu R: Irreversible electroporation: Evolution of
a laboratory technique in interventional oncology. Diagn Interv
Radiol. 20:147–154. 2014.PubMed/NCBI
|
|
18
|
Rubinsky B, Onik G and Mikus P:
Irreversible electroporation: A new ablation modality-clinical
implications. Technol Cancer Res Treat. 6:37–48. 2007. View Article : Google Scholar : PubMed/NCBI
|