Introduction
Regulatory T cells (Tregs) have a critical function
in controlling adaptive immune responses and maintaining
self-tolerance. Tregs interact with various immune cell types, some
of which may be found in the tumor environment (1,2). Thus,
Tregs may have an important impact on cancer immune escape.
Recruitment of Tregs by tumors has been reported to be one aspect
of immune escape (2), so targeting
these cells may provide a mechanism by which antitumor immune
function can be restored. A variety of agents targeting Tregs have
been developed for this reason. These include conventional
chemotherapy, which affects Tregs together with other cell types,
as well as other strategies aimed at Tregs directly, including the
use of specific monoclonal antibodies for CD25 (a depleting
antibody), CTLA4 (a blocking antibody), GITR (an agonistic
antibody) and OX40 (an agonistic antibody) (1,3,4). However, many current immunotherapeutic
strategies that target Tregs may have a negative effect on effector
immune cells (3,5), which may result in the therapy being
unsuccessful. If immunotherapy is to become a viable option in the
treatment of cancer, immunotherapeutic strategies that target Tregs
must also have a positive effect on effector cells, shifting the
balance in favor of immunity.
Cytokine-induced killer (CIK) cells are
heterogeneous in vitro-expanded T lymphocytes, with a
natural killer (NK)/T phenotype and major histocompatibility
complex-unrestricted antitumor ability. These biological features
of CIK cells make them appealing for adoptive immunotherapy and
they have previously displayed encouraging results, which was
indicated by the prolonged survival time following their use in
tumor therapy (6–8).
Lung cancer is one of the most common malignancies
and the leading cause of cancer-related mortality worldwide
(9). Approximately 80% of all lung
cancer cases are non-small cell lung cancer (NSCLC) (10,11) and
the majority of patients have been diagnosed in the advanced stage
(12). At present the primary method
for clinical treatment of advanced NSCLC is drug treatment,
including chemotherapy and biological therapy (which may involve
tumor specific monoclonal antibodies, molecular targeted drugs or
antiangiogenic drugs). However, great toxicity is exhibited during
chemotherapy and great individual differences of clinical antitumor
therapeutic effect with drug treatment. Highly heterogeneous tumor
cells could occur in a series of evolutionary changes in the
molecular level, subcellular, cell, tissue and organ levels in
order to adapt (resistance) or find (transfer) a new living
environment under the external pressure (such as chemotherapy,
targeted therapy and radiotherapy). Despite developments in cancer
treatment and the introduction of novel drugs, advanced lung cancer
remains associated with poor prognosis. Adoptive immunotherapy, as
a new approach to treat solid tumors, has been reported to hold
great potential compared with other traditional treatments
(13–20). Adoptive CIK cell transfer, as one
type of adoptive immunotherapy, has displayed antitumor effects in
various malignant tumor types, including NSCLC (17–21).
Considering the importance of targeting Tregs in
cancer immunotherapy, the current study aimed to determine whether
CIK cell therapy was able to affect Tregs and effector cells in
NSCLC patients. A possible mechanism of adoptive CIK cell therapy
was also explored, which has not yet been elucidated.
Materials and methods
Patient characteristics
A total of 30 patients with stage III–IV NSCLC and
similar clinical characteristics, who were hospitalized in the
Guangzhou Institute of Respiratory Diseases (Guangzhou, China)
between July 2009 and December 2013, with or without CIK cell
therapy, were enrolled in this study and randomly assigned to the
CIK cell treatment group or non-CIK cell treatment group (n=15 per
group). Clinical information, including sex, age, tumor histology
and clinical stage are presented in Table I. Patients received chemotherapy
before CIK cell immunotherapy.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Chemotherapy and
CIK cell therapy (n=15) | Chemotherapy
(n=15) |
|---|
| Mean age
(range) | 59.3 (52–68) | 59.5 (51–73) |
| Sex |
|
|
|
Male | 8 | 8 |
|
Female | 7 | 7 |
| Karnofsky
performance status | 65±10.2 | 62±9.6 |
| Stage |
|
|
|
III | 6 | 5 |
| IV | 9 | 10 |
| Pathology type |
|
|
|
Adenocarcinoma | 10 | 10 |
|
Squamous cell carcinoma | 5 | 5 |
Inclusion criteria were as follows: i) Pathological
or radiographic confirmation of stage III–IV NSCLC tumors; ii)
Karnofsky performance status (22)
≥50; iii) life expectancy ≥3 months; and iv) hemogram (hemachrome
>80 g/l; white blood cell count, >3×107/l), blood
urea nitrogen, serum creatinine, alanine aminotransferase,
aspartate aminotransferase and alkaline phosphatase were close to
normal levels. Rejection criteria were as follows: i) Patients
without consent for treatment; ii) patients who had other immune
system disorders; and iii) patients who had been treated with
steroids within the past 6 weeks.
All patients gave their informed consent prior to
inclusion in the study. The study was approved by the Ethics
Committee of the First Affiliated Hospital of Guangzhou Medical
University (Guangzhou, China).
Preparation of CIK cells and
treatment
Peripheral blood mononuclear cells (PBMCs) were
collected with a COBE spectra blood cell separator (Terumo BCT,
Inc., Lakewood, CO, USA). PBMCs (5.0×106 cells/ml) were
cultured with TexMACS GMP medium (Miltenyi Biotec GmbH, Bergisch
Gladbach, Germany) in the presence of 1.0×106 U/l human
interferon (IFN)-γ (Shanghai Fosun Pharmaceutical Group Co., Ltd.,
Shanghai, China). The cells were incubated for 24 h in a humidified
atmosphere containing 5% CO2 at 37°C. Monoclonal
antibody against CD3 (MAB100; 50 µg/l; R&D Systems, Inc.,
Minneapolis, MN, USA) and 5.0×105 U/l recombinant human
interleukin (IL)-2 (Shandong Quangang Pharmaceutical Co., Ltd.,
Jinan, China) were added after 24 h culture at 37°C. The medium was
changed every 3 days with fresh IL-2 supplement. Cells were
collected after 2 to 3 weeks of culture. Cell phenotypes of CIK
cells were assessed by flow cytometry.
CIK cells were delivered once a day for 3
consecutive days as a course of treatment. The number of cells was
between 2×109 and 6×109 for each
infusion.
Trypan blue assay
The cell suspension and the 0.4% trypan blue
solution (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) were added
in equal parts (100 µl) and 10 µl of this content was transferred
to a Neubauer chamber for counting the four lateral quadrants under
an inverted light microscope. The viability calculation was made
according to the following formula: percentage of viable cells (%)
= (live cells/total cells) × 100. The cells were assessed for
viability using the trypan dye-exclusion test and the viability of
CIK cells detected by trypan blue was 94.0±3.6%.
Bacteria, fungi and endotoxin
assays
CIK cells were checked twice (the first day of
culture and after 2 weeks of culture) for bacteria, fungi or
endotoxins. Detection was performed in the Clinical Laboratory of
the First Affiliated Hospital of Guangzhou Medical University.
Bacteria and fungi detection
A BacT/ALERT 3D 240 Microbial Detection System
(bioMerieux, Inc., Durham, CA, USA) for detection of bacteria and
fungi was used. CIK cell culture medium was collected aseptically
and injected into BacT/ALERT SA (258789) and BacT/ALERT SN (258790;
both from bioMerieux, Inc.) culture bottles. Bottles were detected
by the microbial detection system for bacteria and fungi. The
microbial detection system utilized a colorimetric sensor and
reflected light to monitor the presence and production of
CO2 dissolved in the culture medium. If microorganisms
were present in the test sample, CO2 was produced as the
organisms metabolize the substrates in the culture medium. When the
microorganisms produced CO2, the color of the
gas-permeable sensor installed in the bottom of each culture bottle
changed from blue-green to yellow. The lighter color resulted in an
increase of reflectance units monitored by the system. Bottle
reflectance were monitored and recorded by the instrument every 10
min. Over the course of 7 consecutive days, lack of bacteria and
fungi growth was regarded as a negative result. All CIK cells
infused were negative for bacteria, fungi.
Endotoxin detection
The Limulus Amoebocyte Lysate (LAL) assay was used.
CIK cell culture medium was collected aseptically and assayed using
Endochrome-K lysate (Charles River Laboratories,
Saint-Germain-Nuelles, France) with depyrogenated glass tubes,
pipettes and pipette tips. Microplates were analysed using a
FLUOstar Omega microplate reader with MARS data analysis software
(BMG Labtech, Ortenberg, Germany) and observed at 405 nm with an
optical density value of 0.1 as per manufacturer's recommendations.
The control standard endotoxin was used to construct standard
curves as described for the kinetic turbidimetric LAL assay. The
endotoxin level of CIK cells suspension <0.5 EU/ml indicated a
negative result. All CIK cells infused were negative for
endotoxins.
Isolation of NK cells
Peripheral blood samples (2–3 ml) were used to
purify NK cells using a negative selection NK cell isolation kit
(Miltenyi Biotec GmbH) according to the manufacturer's protocol.
Purified NK cells were subsequently cultured in TexMACS GMP medium
and used in a cytotoxicity assay.
Cytotoxicity assay
Human lung cancer cell line A549 were purchased from
the American Type Culture Collection (Manassas, VA, USA). Effector
cells (CIK cells, PBMC or NK cells) were co-cultured with target
cells (A549 cells) at effector-to-target (E:T) ratios of 10:1 or
20:1 in TexMACS GMP medium. Target cells without effector cells
were used as a negative control. Furthermore, TexMACS GMP medium
without any cells was used as a blank control. Cell Counting Kit-8
(Beyotime Institute of Biotechnology, Jiangsu, China) analysis was
used to measure the cytotoxicity of effector cells according to the
manufacturer's instructions.
Flow cytometry
CD3 FITC (IM1650, 20 µl/test), CD4 FITC/CD8PE/CD3PC5
(6604727, 20 µl/test), CD3 FITC/CD16+56 PE (07735, 20 µl/test),
CD16 FITC/CD56 PE/CD3 ECD (A07728, 20 µl/test), NKG2A PE (IM3291U,
20 µl/test), CD3 ECD (A07748, 10 µl/test), CD56 PC5 (A07789, 10
µl/test), NKG2D PE (A08934, 20 µl/test), CD4 FITC (A07750, 20
µl/test), CD127 PE (IM1980, 20 µl/test), CD25PC5 (IM1646, 10
µl/test), IgG1 FITC (A07795, 20 µl/test), IgG1 PE (A07796, 20
µl/test), IgG1 ECD (A07797, 10 µl/test), and IgG1 PC5 (A07798, 10
µl/test), were purchased from Immunotech (Beckman Coulter, Inc.,
Brea, CA, USA) and used for flow cytometry according to the
manufacturer's protocol using an EPICS-XL flow cytometer (Beckman
Coulter, Inc.). For antibody staining, antibodies were combined
with 5×105 leucocytes according to the manufacturer's
instructions. A total of 100 µl of the test sample was mixed with
antibodies and vortexed gently prior to incubation for 15 min at
room temperature (18–25°C) away from light. After incubation, a
total of 0.5 ml of OptiLyse C (Ref. A11895) was added and vortex
immediately for 1 sec. Samples were incubated for 10 min at room
temperature (18–25°C) protected from light. PBS (0.5 ml) was added
and the samples were left to incubate for at least 5 min at room
temperature away from light. Subsequently, samples were centrifuges
for 5 min at 300 × g at room temperature, the supernatant was
removed by aspiration and the cell pellet was resuspended in 4 ml
PBS. The samples were then centrifuged for 5 min at 300 × g at room
temperature prior to removal of the supernatant by aspiration. The
cell pellet was resuspended in 0.5 or 1 ml of PBS made up with 0.1%
formaldehyde. Samples were analyzed in the flow cytometer
(EPICS-XL; Beckman Coulter, Inc.). For evaluation of the immune
status of CIK cells, cluster of differentiation CD3+,
CD4+, CD8+, CD3+CD56+
and CD3−CD(16+56)+ cells were respectively
determined. For evaluation of the immune status of NSCLC patients,
CD3+, CD4+, CD8+,
CD4+/CD8+, CD3+CD56+,
CD3−CD(16+56)+,
CD3−CD(16+56)+NKG2A+,
CD3−CD(16+56)+NKG2D+,
CD3−CD16dimCD56high,
CD3−CD56dimCD16high cells ratio
and CD4+CD25+CD127+ Treg cells
were determined from peripheral blood 1 day before immunotherapy,
and at weeks 2 and 4 after immunotherapy. Cells were stained
according to the manufacturer's directions. Data were analyzed
using Expo32 analysis software (V1.2; Beckman Coulter, Inc.).
Evaluation of plasma cytokines
Plasma levels of cytokines, including IFN-γ, IFN-α,
IFN-γ-inducible protein 10 (IP-10), monocyte chemoattractant
protein (MCP)-1, macrophage inflammatory protein-1α, tumor necrosis
factor (TNF)-α, transforming growth factor (TGF)-β, MCP-3,
granulocyte-macrophage colony-stimulating factor, IL-4, IL-6, IL-8,
IL-10, IL-12, IL-15, IL-17, IL-18 and IL-21, were measured in all
patients in the CIK cell group before CIK cell therapy, and at
weeks 2 and 4 after cell therapy. Bio-Plex Pro magnetic bead-based
assays were utilized on the Bio-Plex platform (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), according to the
manufacturer's instructions. Bio-Plex Manager software, version 6.0
(Bio-Rad Laboratories, Inc.) was used for bead acquisition and
analysis.
Therapeutic effect evaluation
Overall survival (OS) was defined as the time from
the diagnosis of metastatic NSCLC to patient death.
Progression-free survival (PFS) was defined as the time that
elapsed from the date of diagnosis to the date of the first
event.
Adverse effects
Side-effects of fever, insomnia, anorexia, joint
soreness and skin rash were recorded during the experimental
period.
Statistical analysis
All data are presented as the mean ± standard
deviation. All statistical analyses were performed using SPSS v21.0
(IBM SPSS, Armonk, NY, USA). T and NK cell subsets, cytokines and
cytotoxicity were analyzed with either paired t-tests or
non-parametric Wilcoxon signed-rank tests based upon the normality
of data. Kaplan-Meier analysis with the log-rank test was used to
compare PFS and OS between patient groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
Induction of CIK cells
In order to generate CIK cells in vitro,
non-adherent PBMCs were separated and cultured in the presence of
IFN-γ, CD3 monoclonal antibody and IL-2. At day 7 of cell culture,
the cell area was ~3-fold larger than on day 1. Multiple cells
formed clusters and gathered into cell aggregates.
Proliferation and phenotype of PBMCs after CIK cell
induction varied between individuals. The total cell number had
increased significantly (mean, 8.18-fold) by day 21 after CIK cell
induction compared with before treatment (P<0.001; Fig. 1A). The number of
CD3+CD56+ cells had increased significantly
(range, 23.28 to 138.55-fold; mean, 51.56-fold) by day 21 after CIK
cell induction compared with before treatment (P<0.01; Fig. 1B). The proportion of CD3+,
CD3+/CD8+ and
CD3+/CD56+ cells had significantly increased
by day 21 compared with before treatment (all P<0.01; Fig. 1C), whereas the proportion of
CD3+CD4+ T cells and
CD3−CD16/56+ NK cells significantly decreased
during CIK cell culture (P<0.001 and P<0.05, respectively;
Fig. 1C). Viability of CIK cells
detected by trypan blue was >90% (data not shown). A
cytotoxicity assay indicated that the cytotoxicity of PBMCs against
A549 cell lines by day 21 after CIK cell induction (E:T ratio, 10:1
or 20:1) was significantly increased compared with that of PBMCs
before treatment (P<0.001; Fig.
1D). Cytotoxicity was also significantly increased at an E:T
ratio of 20:1 compared with a ratio of 10:1 (P<0.001; Fig. 1D).
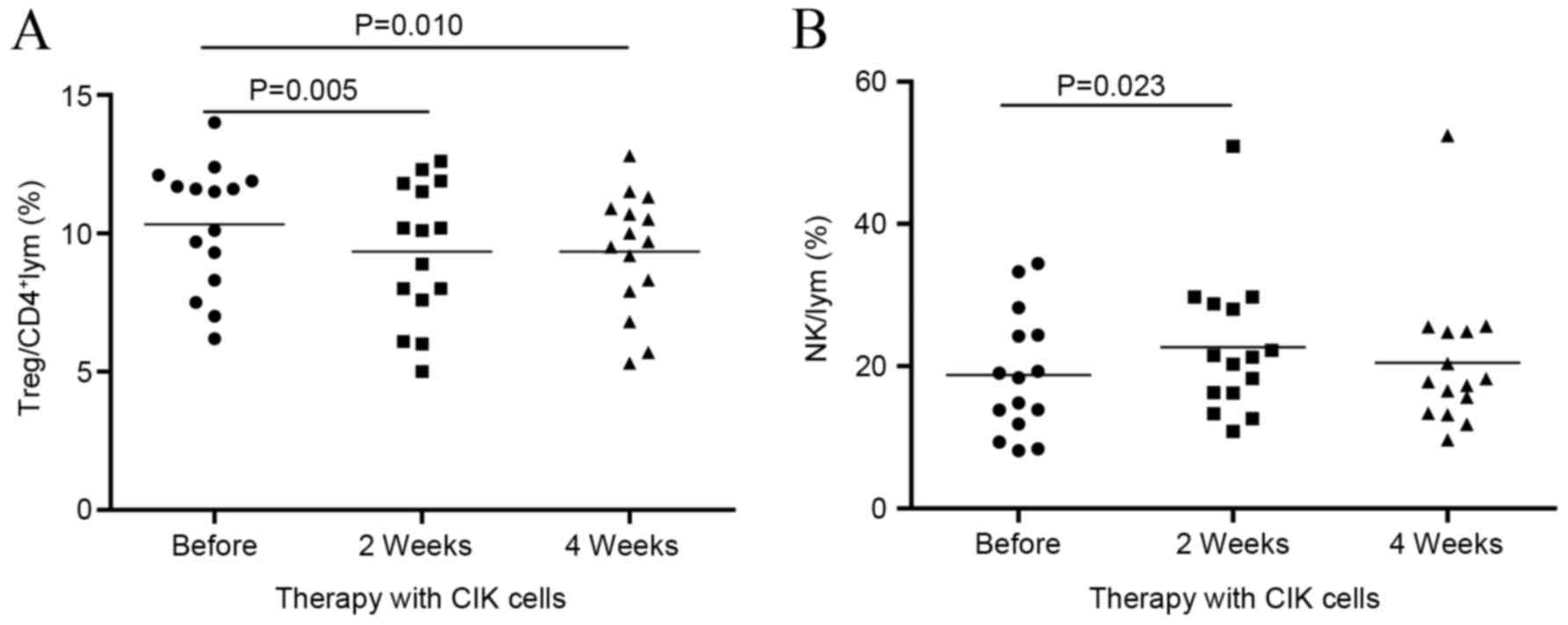
Dynamic changes in Treg and NK cell
subsets
In the current study, to investigate changes within
Tregs and NK cell subsets, blood samples were collected before CIK
cell therapy (baseline) and at 2 and 4 weeks after CIK cell
therapy. It was found that the proportion of Tregs was
significantly reduced at week 2 compared with the baseline (9.99
vs. 9.26%; P<0.01), and remained low at week 4 (Fig. 2A). The percentage of NK cells
significantly increased by week 2 compared with the baseline
(P<0.05; Fig. 2B). This was
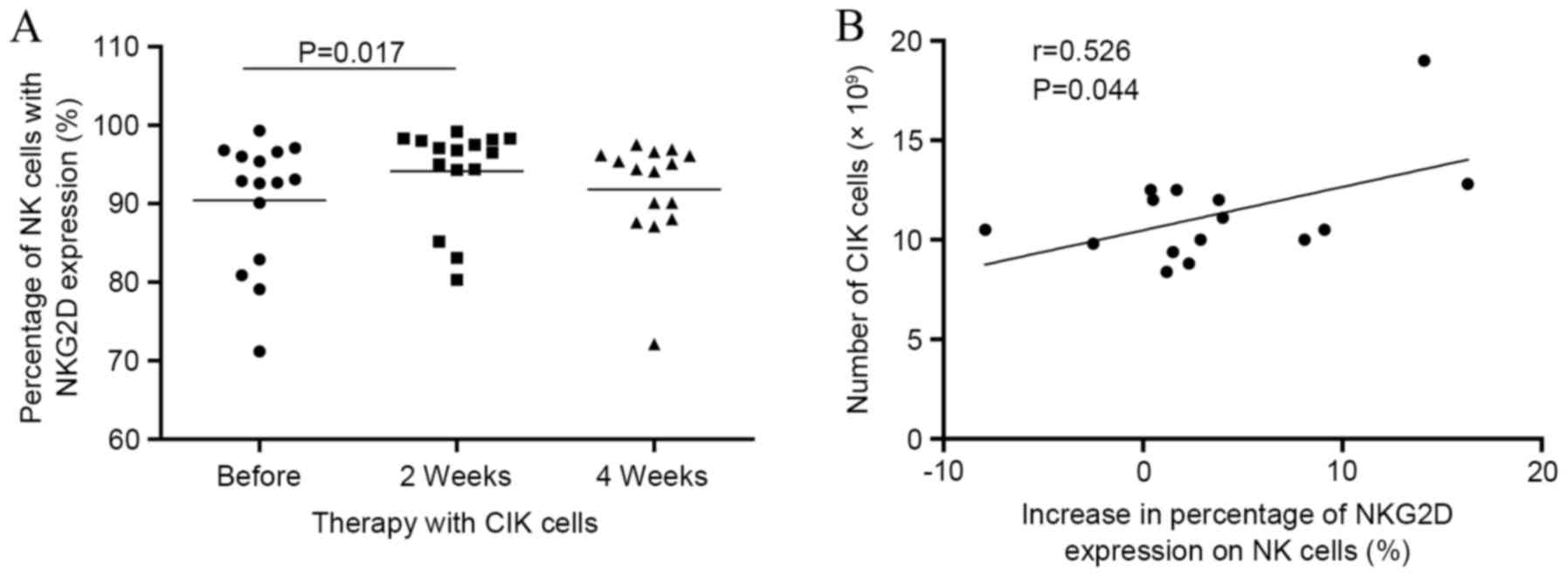
accompanied by a significant increase in NKG2D expression in NK
cells at week 2 compared with the baseline (Fig. 3A). The increase in NKG2D expression
was positively correlated with the number of CIK cells infused
(P<0.05; Fig. 3B).
CD3+, CD4+, CD8+,
CD4+/CD8+, CD3+CD56+,
CD3−CD(16+56)+NKG2A+,
CD3−CD16dimCD56high and
CD3−CD56dimCD16high cell ratios
were also measured but no significant differences were observed
during the therapy (data not shown).
Cytotoxicity of PBMC or isolated NK
cells
Cytotoxicity results with PBMC (n=15) and isolated
NK cells (n=6) from NSCLC patients before or after CIK cell therapy
are shown in Fig. 4. Cytotoxicity of
PBMC and isolated NK cells at 2 weeks after CIK cell thwerapy was
significantly increased compared with the baseline (P<0.05).
However, there were no significant differences in the cytotoxicity
of PBMC or isolated NK cells at 4 weeks compared with the baseline
(Fig. 4).
Evaluation of cytokines in the plasma
of CIK cell therapy patients
Cytokine levels were evaluated in order to identify
those that may be involved in the changes of Tregs and NK cells
following CIK cell therapy. There were significant increases in
IFN-γ (P<0.05), IP-10 (P<0.01), TNF-α (P<0.001), GM-CSF
(P<0.01), MCP-3 (P<0.01) and IL-21 (P<0.05) at 2 weeks
after CIK cell therapy compared with the baseline (Fig. 5A-F). For all these cytokines, the
levels decreased towards baseline levels by week 4. The opposite
trend was observed for plasma TGF-β levels, which significantly
decreased at week 2 compared with the baseline (P<0.05) and
increased towards baseline levels by week 4 (Fig. 5G). IL-4 and IL-10 were only detected
in a small number of samples. For all other cytokines evaluated, no
significant differences were found between the baseline and 2 or 4
weeks after therapy (data not shown).
 | Figure 5.Plasma cytokine profiles of non-small
cell lung cancer patients before and at 2 or 4 weeks after
cytokine-induced killer cell treatment. Antitumor cytokines (A)
IFN-γ, (B) IP-10, (C) TNF-α, (D) GM-CSF, (E) MCP-3, (F) IL-21 and
(G) TGF-β1 were evaluated (n=15). IFN-γ, interferon-γ; IP-10,
IFN-γ-inducible protein 10; TNF-α, tumor necrosis factor-α; GM-CSF,
granulocyte-macrophage colony-stimulating factor; MCP-3, monocyte
chemotactic protein-3; IL-21, interleukin-21; TGF-β1, transforming
growth factor-β1; CIK, cytokine-induced killer. |
Evaluation of survival
Median PFS for patients in the CIK cell therapy
group and control group were 9.98 months (range, 4.2–43.7 months)
and 5.44 months (range, 1.9–27.7 months), respectively. Median OS
rates for patients in the CIK cell group and control group were
24.17 months (range, 11.3–51.5 months) and 20.19 months (range,
12.5–44.0 months) respectively. Median PFS and OS were
significantly increased in patients who were given CIK cell therapy
plus chemotherapy compared with chemotherapy alone (control group;
P=0.038 and P=0.048, respectively; Fig.
6).
Adverse effects
No adverse effects were observed in the CIK cell
therapy group, with the exception of 5 patients who experienced
transient fever; one case had a temperature of 38.5°C, whereas the
other four cases had temperatures of <38°C. All patients with
fever recovered within 24 h without additional treatment.
Discussion
Previous results indicate that an imbalance between
immune suppression and antitumor immunity can promote tumor growth.
If immunotherapy is to become a viable option in the treatment of
cancer, this imbalance in favor of tumor growth needs to be
addressed.
CD4+CD25+FOXP3+
Tregs account for 5–10% of all T lymphocytes in healthy individuals
(23). Evidence suggests that Tregs
contribute to immune suppression by dampening the antitumor
immunity elicited by CD4+ T cells, CD8+ T
cells, dendritic and NK cells (24–27).
Treg accumulations in tumors and peripheral blood have been linked
to unfavorable disease outcomes of tumor invasion, recurrence and
shortened survival for many human solid tumors, including
hepatocellular carcinoma, ovarian carcinoma, pancreatic ductal
carcinoma, cervical cancer, NSCLC and breast cancer (28–33). In
these tumors, Tregs suppress antitumor immunity and mediate immune
tolerance that favors tumor growth. In this context, Treg could be
viewed as a major component of immune evasion from the host immune
system and may be used as a marker for poor prognosis. Indeed,
reducing Treg function and/or numbers in patients with cancer may
result in more effective immune-based therapies, alone or in
combination with traditional chemotherapeutics. Numerous
preclinical and clinical reports support the notion that
elimination of Tregs is crucial to various cancer therapies
(34–37).
Previous studies have demonstrated that human
peripheral CD4+CD25+ Tregs can be accurately
identified and purified using surface expression of CD127, as an
alternative to the transcription factor FOXP3 (38–43).
Therefore, CD4+CD25+CD127dim Tregs
were used to represent
CD4+CD25+FOXP3+ Tregs in the
current study.
A number of therapeutic approaches are aimed at
inhibiting immune suppression within the tumor microenvironment.
Specifically, therapies have been developed to deplete major
immunosuppressive cell types and a number of direct and indirect
methods aimed at depleting Tregs within the tumor microenvironment
already exist (44–50). These include specific depletion with
monoclonal antibodies (anti-CD25) (49) and depletion with the use of
chemotherapeutics, which includes cyclophosphamide (51–54).
This remains an area of active research. A major therapeutic
challenge remains, however, which is the small number of methods
that are able to target Tregs effectively in the clinic.
It is well-established that Tregs are able to affect
CIK cells, cytotoxic T cells and NK cells, but data on CIK cell
potential activity against Tregs are generally absent, let alone
data on the specific effects of CIK cells on Tregs in NSCLC
patients. In the current study, it was found that CIK cell infusion
was able to downregulate Tregs in the circulation of 15 patients
with NSCLC at week 2 post-infusion, and maintained a lower level at
4 weeks post-infusion. Therefore, the present findings suggested
that CIK cell infusion is an effective method for targeting Tregs
in NSCLC patients, which may have a potential application in
therapeutic settings.
The ideal therapeutic strategies that target
suppression factors should also have a positive effect on the
effector cell compartments, in order to shift the balance in favor
of immunity. NK cells are critical for tumor immunity and provide
‘spontaneous cytotoxicity’ against tumor cells as part of innate
immunity. NK cells have the capacity to detect changes in
transformed cells even in the absence of inflammatory signals.
These changes are recognized by NK cells through their inhibitory
and activating receptors (55). NK
cells mediate innate immunity and have an important role in cancer
immunosurveillance. NK cells are able to recognize tumor cells, due
to their decreased HLA expression, and induce lysis before they
grow into larger tumor aggregates (14,56,57).
According to the current data, NK cells
(CD3−CD56+CD16+) increased at the
same time that Tregs decreased, at 2 weeks after CIK cell
therapy.
Expression of activating or inhibitory receptors on
NK cells enables self- and non-self-recognition. Depending on the
balance between inhibitory and activating signals engaged by
ligands expressed on tumor cells, NK cells are triggered to kill or
to ignore target cells. NKG2D is an activating receptor expressed
on the surface of NK cells, which has an important role in immune
responses, including those against tumors (57,58).
Data from the current study indicated that expression of activating
NK cell receptors, such as NKG2D, increased, whereas expression of
inhibitory receptors, such as NKG2A (data not shown), did not vary
with CIK cells therapy, suggesting that CIK cell therapy is able to
activate NK cells in NSCLC patients. By inducing the upregulation
of activating receptors on NK cells, CIK cell therapy may enhance
antitumor immunity. A positive correlation was also observed
between NKG2D expression and the number of CIK cells infused. This
implied that increased CIK cell infusion may contribute to stronger
NK activity on tumor cells. Based on previously published data, the
alteration of NK cell receptors may be induced by cytokines,
including IL-2 (59,60) and IFN (61). Therefore, cytokines of CIK cells may
contribute to the enhancement of NKG2D expression in NK cells.
In vitro cytotoxicity assays demonstrated
that the antitumor activity of PBMC from 15 NSCLC patients was
significantly increased at 2 weeks after CIK cell therapy.
Cytotoxicity assays of isolated NK cells were similar to those of
PBMC assays, although it was only possible to obtain enough p-NK
cells to perform this test in 6/15 NSCLC patients. It was indicated
that the antitumor efficacy of immune cells, including but not
limited to NK cells, from NSCLC was enhanced by CIK cell
therapy.
The results of the current study indicated
significant increases in plasma IFN-γ, IP-10, TNF-α, GM-CSF, MCP-3
and IL-21 levels in patients at 2 weeks after CIK cell therapy. The
general trends were initial increases after treatment was initiated
and a subsequently decrease to approximate pre-therapy levels after
4 weeks. Increased levels of cytokines in the serum of patients who
received CIK cell infusion suggests the presence of CIK
cell-induced T helper 1 (Th1) cell responses. Since Th1 responses
seem to be essential in cancer immunotherapy (62,63),
this may indicate a therapeutic potential of CIK cell therapy. It
was not possible to determine how Tregs and TGF-β interact with
each other in the blood from the current results. However, previous
results suggest that reduction of either Tregs or TGF-β may
contribute to downregulating harmful tumor suppression, which may
favor tumor progression (64–68). In
conjunction, serum cytokine profiles suggested that antitumor
immunity is enhanced at 2 weeks after CIK cell treatment.
In the current study, infusion of CIK cells was
associated with minimal toxicity, and evidence of a disease
response was observed. PFS and OS were prolonged in NSCLC patients
treated with CIK cells compared with the control group. These
results suggest that CIK cell immunotherapy plus chemotherapy for
NSCLC has more potential benefits than chemotherapy alone.
To the best of our knowledge, this is the first
report to evaluate the role of CIK cell therapy in modulating Tregs
in patients with NSCLC. The interaction of CIK cells and regulatory
T cells in the tumor microenvironment requires further research.
Improved understanding of the cellular cross-talk between Tregs and
CIK cells will aid future therapeutic manipulation of Tregs in
cancer treatment.
In conclusion, the current study suggests that CIK
cell therapy can reduce Tregs, increase activating NK cells, create
an antitumor cytokine environment and contribute to improved PFS
and OS in NSCLC patients. These results highlight the potential
benefits of combining conventional chemotherapy and CIK cell
treatment as an approach for reducing Tregs and improving clinical
outcomes for NSCLC patients. Further understanding of the
underlying mechanism of CIK cell therapy and its effects on Tregs
could enhance future therapeutic approaches.
References
|
1
|
Finotello F and Trajanoski Z: New
strategies for cancer immunotherapy: Targeting regulatory T cells.
Genome Med. 9:102017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Farashi-Bonab S and Khansari N: Regulatory
T cells in cancer patients and their roles in cancer
development/progression. MOJ Immunol. 1:00024. 2014.
|
|
3
|
Chen C, Chen Z, Chen D, Zhang B, Wang Z
and Le H: Suppressive effects of gemcitabine plus cisplatin
chemotherapy on regulatory T cells in nonsmall-cell lung cancer. J
Int Med Res. 43:180–187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sakaguchi S, Miyara M, Costantino CM and
Hafler DA: FOXP3+ regulatory T cells in the human immune system.
Nat Rev Immunol. 10:490–500. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Q, Yang L, Xu F, Wang J, An G and Ma
Y: Changes of lymphocyte subgroups in non-small cell lung cancer
patients before and during chemotherapy. Clin Lab. 61:1343–1351.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pan K, Guan XX, Li YQ, Zhao JJ, Li JJ, Qiu
HJ, Weng DS, Wang QJ, Liu Q, Huang LX, et al: Clinical activity of
adjuvant cytokine-induced killer cell immunotherapy in patients
with post-mastectomy triple-negative breast cancer. Clin Cancer
Res. 20:3003–3011. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang M, Shi SB, Qi JL, Tang XY and Tian J:
S-1 plus CIK as second-line treatment for advanced pancreatic
cancer. Med Oncol. 30:7472013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen JL, Lao XM, Lin XJ, Xu L, Cui BK,
Wang J, Lin GH, Shuang ZY, Mao YZ, Huang X, et al: Adjuvant
cytokine-induced killer cell therapy improves disease-free and
overall survival in solitary and nonmicrovascular invasive
hepatocellular carcinoma after curative resection. Medicine
(Baltimore). 95:e26652016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Razzaghi H, Quesnel-Crooks S, Sherman R,
Joseph R, Kohler B, Andall-Brereton G, Ivey MA, Edwards BK, Mery L,
Gawryszewski V and Saraiya M: Leading causes of cancer
mortality-Caribbean region, 2003–2013. MMWR Morb Mortal Wkly Rep.
65:1395–1400. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tian J and Han S: Role of RRM1 in the
treatment and prognosis of advanced non-small cell lung cancer.
Zhongguo Fei Ai Za Zhi. 18:381–386. 2015.(In Chinese). PubMed/NCBI
|
|
11
|
Daga A, Ansari A, Patel S, Mirza S, Rawal
R and Umrania V: Current drugs and drug targets in non-small cell
lung cancer: Limitations and opportunities. Asian Pac J Cancer
Prev. 16:4147–4156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vigneron N: Human tumor antigens and
cancer immunotherapy. Biomed Res Int. 2015:9485012015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu K, Liu X, Peng Z, Sun H, Zhang M,
Zhang J, Liu S, Hao L, Lu G, Zheng K, et al: Retargeted human
avidin-CAR T cells for adoptive immunotherapy of EGFRvIII
expressing gliomas and their evaluation via optical imaging.
Oncotarget. 6:23735–23747. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bigley AB and Simpson RJ: NK cells and
exercise: Implications for cancer immunotherapy and survivorship.
Discov Med. 19:433–445. 2015.PubMed/NCBI
|
|
16
|
Ascierto ML, Melero I and Ascierto PA:
Melanoma: From incurable beast to a curable bet. The success of
immunotherapy. Front Oncol. 5:1522015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang M, Cao JX, Pan JH, Liu YS, Xu BL, Li
D, Zhang XY, Li JL, Liu JL, Wang HB and Wang ZX: Adoptive
immunotherapy of cytokine-induced killer cell therapy in the
treatment of non-small cell lung cancer. PLoS One. 9:e1126622014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang J, Zhu L, Du H, He X, Yin Y, Gu Y,
Liu L, Lu K, Guo R, Liu P and Shu Y: Autologous cytokine-induced
killer cell therapy in lung cancer patients: A retrospective study.
Biomed Pharmacother. 70:248–252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang B, Lu XC, Zhu HL, Han WD, Wang Y, Fan
H, Li SX, Liu Y, Dai HR and Yao SQ: Clinical study of autologous
cytokine induced killer cells combined with IL-2 for therapy of
elderly patients with B-cell malignant lymphoma. Zhongguo Shi Yan
Xue Ye Xue Za Zhi. 18:1244–1249. 2010.(In Chinese). PubMed/NCBI
|
|
20
|
Zhang S, He X, Li X and Ren Y: Clinical
study on cytokine induced killer cells therapy to laryngeal cancer
after radiotherapy. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
25:61–63. 2011.(In Chinese). PubMed/NCBI
|
|
21
|
Li XD, Ji M, Zheng X, Ning ZH, Wu J, Lu B,
Wu CP and Jiang JT: Evaluation of tumor response to
cytokine-induced killer cells therapy in malignant solid tumors. J
Transl Med. 12:2152014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Walasek T, Sas-Korczyńska B, Dąbrowski T,
Reinfuss M, Jakubowicz J, Blecharz P, Łuczyńska E, Darasz Z and
Skotnicki P: Palliative thoracic radiotherapy for patients with
advanced non-small cell lung cancer and poor performance status.
Lung Cancer. 87:130–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kverneland AH, Streitz M, Geissler E,
Hutchinson J, Vogt K, Boës D, Niemann N, Pedersen AE, Schlickeiser
S and Sawitzki B: Age and gender leucocytes variances and
references values generated using the standardized ONE-Study
protocol. Cytometry A. 89:543–564. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arias DA Alvarez, Kim HJ, Zhou P,
Holderried TA, Wang X, Dranoff G and Cantor H: Disruption of CD8+
Treg activity results in expansion of T follicular helper cells and
enhanced antitumor immunity. Cancer Immunol Res. 2:207–216. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bauer CA, Kim EY, Marangoni F, Carrizosa
E, Claudio NM and Mempel TR: Dynamic Treg interactions with
intratumoral APCs promote local CTL dysfunction. J Clin Invest.
124:2425–2440. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guzmán-Flores JM and Portales-Pérez DP:
Mechanisms of suppression of regulatory T-cells (Treg). Gac Med
Mex. 149:630–638. 2013.(In Spanish). PubMed/NCBI
|
|
27
|
Duan MC, Zhong XN, Liu GN and Wei JR: The
Treg/Th17 paradigm in lung cancer. J Immunol Res. 2014:7303802014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Perrone G, Ruffini PA, Catalano V, Spino
C, Santini D, Muretto P, Spoto C, Zingaretti C, Sisti V,
Alessandroni P, et al: Intratumoural FOXP3-positive regulatory T
cells are associated with adverse prognosis in radically resected
gastric cancer. Eur J Cancer. 44:1875–1882. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bates GJ, Fox SB, Han C, Leek RD, Garcia
JF, Harris AL and Banham AH: Quantification of regulatory T cells
enables the identification of high-risk breast cancer patients and
those at risk of late relapse. J Clin Oncol. 24:5373–5380. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mougiakakos D, Choudhury A, Lladser A,
Kiessling R and Johansson CC: Regulatory T cells in cancer. Adv
Cancer Res. 107:57–117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nishikawa H and Sakaguchi S: Regulatory T
cells in tumor immunity. Int J Cancer. 127:759–767. 2010.PubMed/NCBI
|
|
32
|
Curiel TJ, Coukos G, Zou L, Alvarez X,
Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L,
Burow M, et al: Specific recruitment of regulatory T cells in
ovarian carcinoma fosters immune privilege and predicts reduced
survival. Nat Med. 10:942–949. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bergmann C, Strauss L, Wang Y, Szczepanski
MJ, Lang S, Johnson JT and Whiteside TL: T regulatory type 1 cells
in squamous cell carcinoma of the head and neck: Mechanisms of
suppression and expansion in advanced disease. Clin Cancer Res.
14:3706–3715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Alizadeh D and Larmonier N:
Chemotherapeutic targeting of cancer-induced immunosuppressive
cells. Cancer Res. 74:2663–2668. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou S, Chen L, Qin J, Li R, Tao H, Zhen
Z, Chen H, Chen G, Yang Y, Liu B, et al: Depletion of CD4+ CD25+
regulatory T cells promotes CCL21-mediated antitumor immunity. PLoS
One. 8:e739522013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bulliard Y, Jolicoeur R, Zhang J, Dranoff
G, Wilson NS and Brogdon JL: OX40 engagement depletes intratumoral
Tregs via activating FcgRs, leading to antitumor efficacy. Immunol
Cell Biol. 92:475–480. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mattarollo SR, Steegh K, Li M, Duret H,
Ngiow S Foong and Smyth MJ: Transient Foxp3(+) regulatory T-cell
depletion enhances therapeutic anticancer vaccination targeting the
immune-stimulatory properties of NKT cells. Immunol Cell Biol.
91:105–114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yu N and Li X, Song W, Li D, Yu D, Zeng X,
Li M, Leng X and Li X: CD4(+)CD25 (+)CD127 (low/−) T cells: A more
specific Treg population in human peripheral blood. Inflammation.
35:1773–1780. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dasgupta A, Mahapatra M and Saxena R: Flow
cytometric immunophenotyping of regulatory T cells in chronic
lymphocytic leukemia: Comparative assessment of various markers and
use of novel antibody panel with CD127 as alternative to
transcription factor FoxP3. Leuk Lymphoma. 54:778–789. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Su H, Longhi MS, Wang P, Vergani D and Ma
Y: Human CD4+CD25(high)CD127 (low/neg) regulatory T cells. Methods
Mol Biol. 806:287–299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Drennan S, Stafford ND, Greenman J and
Green VL: Increased frequency and suppressive activity of
CD127(low/−) regulatory T cells in the peripheral circulation of
patients with head and neck squamous cell carcinoma are associated
with advanced stage and nodal involvement. Immunology. 140:335–343.
2013.PubMed/NCBI
|
|
42
|
Jun C, Ke W, Qingshu L, Ping L, Jun D, Jie
L, Bo C and Su M: Protective effect of CD4(+)CD25(high)CD127(low)
regulatory T cells in renal ischemia-reperfusion injury. Cell
Immunol. 289:106–111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Marek-Trzonkowska N, Myśliwiec M, Dobyszuk
A, Grabowska M, Derkowska I, Juścińska J, Owczuk R, Szadkowska A,
Witkowski P, Młynarski W, et al: Therapy of type 1 diabetes with
CD4(+)CD25(high)CD127-regulatory T cells prolongs survival of
pancreatic islets - results of one year follow-up. Clin Immunol.
153:23–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sugiyama D, Nishikawa H, Maeda Y, Nishioka
M, Tanemura A, Katayama I, Ezoe S, Kanakura Y, Sato E, Fukumori Y,
et al: Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+
regulatory T cells, evoking antitumor immune responses in humans.
Proc Natl Acad Sci USA. 110:pp. 17945–17950. 2013; View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liakou CI, Kamat A, Tang DN, Chen H, Sun
J, Troncoso P, Logothetis C and Sharma P: CTLA-4 blockade increases
IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector
to regulatory T cells in cancer patients. Proc Natl Acad Sci USA.
105:pp. 14987–14992. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hodi FS, Butler M, Oble DA, Seiden MV,
Haluska FG, Kruse A, Macrae S, Nelson M, Canning C, Lowy I, et al:
Immunologic and clinical effects of antibody blockade of cytotoxic
T lymphocyte-associated antigen 4 in previously vaccinated cancer
patients. Proc Natl Acad Sci USA. 105:pp. 3005–3010. 2008;
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shimizu J, Yamazaki S and Sakaguchi S:
Induction of tumor immunity by removing CD25+CD4+ T cells: A common
basis between tumor immunity and autoimmunity. J Immunol.
163:5211–5218. 1999.PubMed/NCBI
|
|
48
|
Ko K, Yamazaki S, Nakamura K, Nishioka T,
Hirota K, Yamaguchi T, Shimizu J, Nomura T, Chiba T and Sakaguchi
S: Treatment of advanced tumors with agonistic anti-GITR mAb and
its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T
cells. J Exp Med. 202:885–891. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Onizuka S, Tawara I, Shimizu J, Sakaguchi
S, Fujita T and Nakayama E: Tumor rejection by in vivo
administration of anti-CD25 (interleukin-2 receptor alpha)
monoclonal antibody. Cancer Res. 59:3128–3133. 1999.PubMed/NCBI
|
|
50
|
Mitsui J, Nishikawa H, Muraoka D, Wang L,
Noguchi T, Sato E, Kondo S, Allison JP, Sakaguchi S, Old LJ, et al:
Two distinct mechanisms of augmented antitumor activity by
modulation of immunostimulatory/inhibitory signals. Clin Cancer
Res. 16:2781–2791. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dannull J, Su Z, Rizzieri D, Yang BK,
Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E and Vieweg
J: Enhancement of vaccine-mediated antitumor immunity in cancer
patients after depletion of regulatory T cells. J Clin Invest.
115:3623–3633. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tao Q, Chen T, Tao L, Wang H, Pan Y, Xiong
S and Zhai Z: IL-15 improves the cytotoxicity of cytokine-induced
killer cells against leukemia cells by upregulating CD3+CD56+ cells
and downregulating regulatory T cells as well as IL-35. J
Immunother. 36:462–467. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ganesan AP, Johansson M, Ruffell B,
Yagui-Beltrán A, Lau J, Jablons DM and Coussens LM:
Tumor-infiltrating regulatory T cells inhibit endogenous cytotoxic
T cell responses to lung adenocarcinoma. J Immunol. 191:2009–2017.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Walter S, Weinschenk T, Stenzl A, Zdrojowy
R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY,
Mendrzyk R, et al: Multipeptide immune response to cancer vaccine
IMA901 after single-dose cyclophosphamide associates with longer
patient survival. Nat Med. 18:1254–1261. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lanier LL: NK cell recognition. Annu Rev
Immunol. 23:225–274. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
McDowell KA, Hank JA, DeSantes KB,
Capitini CM, Otto M and Sondel PM: NK cell-based immunotherapies in
pediatric oncology. J Pediatr Hematol Oncol. 37:79–93. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Crouse J, Xu HC, Lang PA and Oxenius A: NK
cells regulating T cell responses: Mechanisms and outcome. Trends
Immunol. 36:49–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sentman CL and Meehan KR: NKG2D CARs as
cell therapy for cancer. Cancer J. 20:156–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sarkar S, Germeraad WT, Rouschop KM,
Steeghs EM, van Gelder M, Bos GM and Wieten L: Hypoxia induced
impairment of NK cell cytotoxicity against multiple myeloma can be
overcome by IL-2 activation of the NK cells. PLoS One.
8:e648352013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hromadnikova I, Pirkova P and Sedlackova
L: Influence of in vitro IL-2 or IL-15 alone or in combination with
Hsp-70-derived 14-mer peptide (TKD) on the expression of NK cell
activatory and inhibitory receptors. Mediators Inflamm.
2013:4052952013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Konjevic G, Jurisic V, Jovic V, Vuletic A,
Martinovic K Mirjacic, Radenkovic S and Spuzic I: Investigation of
NK cell function and their modulation in different malignancies.
Immunol Res. 52:139–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Luo Y, Henning J and O'Donnell MA: Th1
cytokine-secreting recombinant Mycobacterium bovis bacillus
Calmette-Guérin and prospective use in immunotherapy of bladder
cancer. Clin Dev Immunol. 2011:7289302011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ito N, Nakamura H, Tanaka Y and Ohgi S:
Lung carcinoma: Analysis of T helper type 1 and 2 cells and T
cytotoxic type 1 and 2 cells by intracellular cytokine detection
with flow cytometry. Cancer. 85:2359–2367. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Romano S, D'Angelillo A, D'Arrigo P,
Staibano S, Greco A, Brunetti A, Scalvenzi M, Bisogni R, Scala I
and Romano MF: FKBP51 increases the tumour-promoter potential of
TGF-beta. Clin Transl Med. 3:12014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhuo C, Xu Y, Ying M, Li Q, Huang L, Li D,
Cai S and Li B: FOXP3+ Tregs: Heterogeneous phenotypes and
conflicting impacts on survival outcomes in patients with
colorectal cancer. Immunol Res. 61:338–347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wilson EB, El-Jawhari JJ, Neilson AL, Hall
GD, Melcher AA, Meade JL and Cook GP: Human tumour immune evasion
via TGF-β blocks NK cell activation but not survival allowing
therapeutic restoration of anti-tumour activity. PLoS One.
6:e228422011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Itoh S and Itoh F: Implication of TGF-β as
a survival factor during tumour development. J Biochem.
151:559–562. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Du Y, Chen X, Lin XQ, Wu W and Huang ZM:
Tumor-derived CD4+CD25+ Tregs inhibit the maturation and
antigen-presenting function of dendritic cells. Asian Pac J Cancer
Prev. 16:2665–2669. 2015. View Article : Google Scholar : PubMed/NCBI
|