Introduction
Acute cholecystitis is an inflammation of the
gallbladder caused by gallstones packed in its neck or the cystic
duct (1). Perforations of the
gallbladder into the liver or the pericholecystic space may lead to
the development of liver abscesses or peritonitis, respectively
(2–4). In order to effectively manage acute
cholecystitis, an accurate diagnosis must be made before the
disease worsens (5). Acute
cholecystitis is diagnosed based on a combination of signs
indicating local and systemic inflammation (6). Local inflammation presents as right
upper quadrant pain. Murphy's sign, which is a pain on taking a
deep breath in the right upper quadrant when the examiner's finger
is on the location of the gallbladder, is considered to be the most
useful indicator for the diagnosis of local inflammation in
patients with acute cholecystitis (7). Systemic inflammation is confirmed based
on the findings of blood tests, with leukocytosis and elevated
levels of C-reactive protein (CRP) indicating systemic inflammation
(8). To confirm the diagnosis of
cholecystitis, diagnostic imaging is useful; computed tomography
(CT) scanning typically reveals thickened walls of the gallbladder,
pericholecystic inflammation, and the presence of liver abscesses
(9).
Diffusion-weighted imaging (DWI) utilizes the random
movement of water molecules to construct images (10). Based on DWI, diffusion-weighted whole
body imaging with background body signal suppression (DWIBS) has
been developed (11,12). DWIBS images are formed with the
suppression of fat signals and the application of heavy diffusion
weighting during free breathing (13). DWIBS shows a strong contrast between
r tissue with high signal intensity and the surrounding tissues
(14). One limitation of DWIBS in
some cases is the difficulty of evaluating high signal intensities
in anatomical settings (15,16). To overcome this disadvantage, DWIBS
images are overlapped with T2-weighted images to create DWIBS
T2-weighted image fusion (DWIBS/T2) (14,17,18).
DWIBS/T2 enables the evaluation of tissues with high signal and
strong contrast in fusion with T2-weighted images (19).
In the present study, the use of DWIBS/T2 and CT
scanning for patients with acute cholecystitis was evaluated.
Materials and methods
Ethical statement
The Ethics Committee of the National Hospital
Organization, Shimoshizu Hospital (Yotsukaidu, Japan) approved the
present study. Patient records were anonymous and retrospectively
analyzed. Written informed consent was obtained from all the
patients prior to DWIBS/T2 and CT, with or without contrast
enhancement, being performed.
Patients
Patients who were treated for acute cholecystitis
and underwent DWIBS/T2 in the National Hospital Organization,
Shimoshizu Hospital between December 2012 and August 2015, and for
whom DWIBS/T2 and CT results were available, were enrolled in the
present study. Ten men (aged 67.7±7.6 years) and 4 women (aged
70.8±13.2 years) were enrolled in the present study. No patients
were treated for acute cholecystitis prior to DWIBS/T2 and CT.
Diagnosis of acute cholecystitis
Diagnosis of acute cholecystitis was based on Tokyo
Guideline 13 (6). Patients were
suspected of acute cholecystitis when they presented with upper
abdominal pain or right upper quadrant abdominal pain accompanied
by leukocytosis or elevated CRP levels. Patients were subjected to
abdominal ultrasonography or CT scanning, with or without contrast
enhancement. Abdominal ultrasonography and CT have previously been
demonstrated to detect an enlarged gallbladder, thickened walls,
and fluid collection between the liver and gallbladder or
pericholecystic space (20). A
positive sonographic for Murphy's sign was a reliable test for the
diagnosis of acute cholecystitis (21).
Magnetic resonance imaging (MRI)
techniques
The MRI studies were performed using a 1.5 Tesla
scanner with Achieva software version 3.2.2 (Philips Healthcare, DA
Best, The Netherlands). Patients were placed in a supine headfirst
position on an extended table platform that allowed for coverage of
the body from the head to the lower legs. The DWIBS/T2 imaging
protocol consisted of unenhanced T1-weighted, T2-weighted, DWI, and
DWIBS imaging. The MRI pulse sequences are presented in Table I. DWIBS images were acquired axially
by a Q-body coil, under free breathing conditions, using an
echo-planar imaging single-shot pulse sequence. DWI gradients were
applied along the X, Y, and Z axes prior to and following a 180°
inversion pre-pulse to obtain fat-saturated isotropic images with
DWI sensitivity. The following parameters were used for a single
stack: B-value, 0 and 800 mm2/sec; repetition time,
6,960 msec; echo time, 7 msec; inversion recovery, 150 msec;
acquisition matrix, 176×115; reconstruction matrix, 256; right/left
field of view, 530 mm; anterior/posterior field of view, 349 mm;
feet/head field of view, 226 mm; slice thickness, 6 mm; and size of
reconstructed voxels, 2.07×2.08×6 mm3. Fused DWIBS/T2
images were constructed using an Extended MR WorkSpace workstation
(Phillips Healthcare). For all patients, five stacks were acquired
consecutively to obtain images from the head to the middle of the
tibia, with each stack consisting of 45slices. Overall, the average
required imaging time was 13.31 min. To rule out T2 shine-through
effects and to differentiate malignant lesions from non-malignant
causes of restricted diffusion, a decreased signal on the apparent
diffusion coefficient (ADC) was used with ADC reduction, to
determine a ‘positive ADC map’ (22).
 | Table I.Pulse sequences used in the present
study. |
Table I.
Pulse sequences used in the present
study.
| Variable | T1-weighted
image | T2-weighted
image | DWI (DWIBS) |
|---|
|
| GRE | Single-shot SE | EPI SE |
| TR, msec | Shortest | 1,000 | 11,250 |
| TE, msec | First: 2.3 (out of
phase), second: 4.6 (in phase) | 90 | 83 |
| Flip angle, ° | 75 | 90 | 90 |
| NSA | 1 | 1 | 4 |
| Slice thickness,
mm | 8 | 8 | 5 |
| Slice gap | 1 | 1 | 0 |
| Fat saturation | No | No | SPAIR |
| Phase encoding
direction |
Posterior-anterior |
Posterior-anterior |
Posterior-anterior |
CT scanning
CT scanning was performed using a 16-detector row CT
scanner (SOMATOM Emotion 16; Siemens AG, Munich, Germany). Imaging
parameters for three-phase contrast-enhanced images were as
follows: Tube voltage, 130 kVp; gantry rotation speed, 0.6
rotations/sec; maximum allowable tube current, 120 mA. For some
patients, contrast medium (100 ml of iopamidol; 3 ml/sec; Konica
Minolta, Inc., Tokyo, Japan) was administered intravenously. CT
images were acquired prior to and at 30, 70, and 180 sec following
injection of contrast medium.
Results
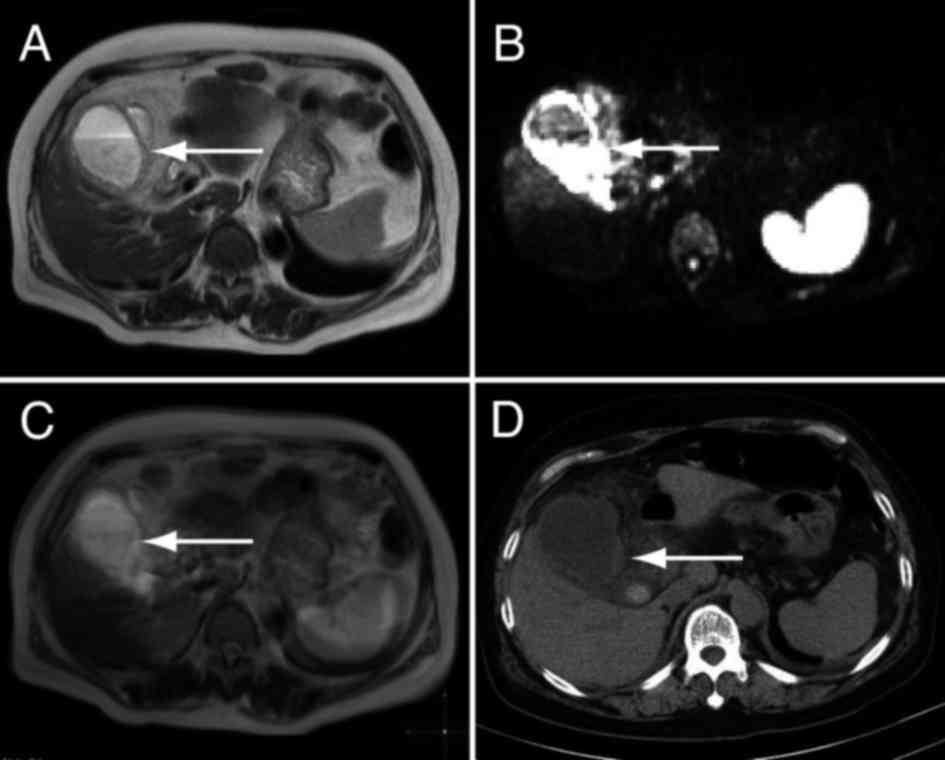
The thickness of the gallbladder wall in DWIBS/T2
and CT images was compared. T2-weighted imaging revealed a
thickened wall (Fig. 1A), and the
signal intensity of the thickened wall was high with DWIBS
(Fig. 1B). Fusion images of DWIBS
and T2-weighted images showed high signal intensity of the
gallbladder wall and allowed for anatomical analysis (Fig. 1C), which was not possible from the
DWIBS image alone. The high signal intensity with DWIBS indicated
inflammation. A thickened gallbladder wall was also observed in the
CT images (Fig. 1D). These results
suggest that gallbladder wall thickening is easily detectable using
both DWIBS/T2 and CT imaging.
Pericholecystic inflammation is one of the
complications of acute cholecystitis (9). T2-weighted imaging revealed a space
around the gallbladder (Fig. 2A),
which gave a high signal intensity with DWIBS (Fig. 2B). DWIBS/T2 showed high signal
intensity for the gallbladder wall (Fig.
2C). The pericholecystic inflammation appeared as a low-density
area in the CT images (Fig. 2D). The
high signal intensity with DWIBS/T2 enabled easier detection than
with CT, indicating that DWIBS/T2 shows pericholecystic
inflammation more clearly than CT (Fig.
2E).
Liver abscess and liver inflammation are additional
complications of acute cholecystitis (9). A weak high intensity signal was
observed in the T2-weighted images for the liver (Fig. 3A). An area with high signal intensity
was observed for the liver with DWIBS (Fig. 3B). DWIBS/T2 images, however, clearly
showed high signal intensity in the liver (Fig. 3C). The high intensity with DWIBS/T2
shown in Fig. 3C was less obvious
than Figs. 1C and 2C. The possible reason was that the
intensity of the inflammation was less severe in that shown in
Fig. 3C compared with Figs. 1C and 2C. A low-density area was observed in liver
when plain CT was used (Fig. 3D),
and contrast-enhanced CT made this low-density area more obvious
(Fig. 3E).
Detectability of inflammation of the pericholecystic
space and the liver were compared between DWIBS/T2 and CT images
(Table II). There was no obvious
difference in detectability for either condition between DWIBS/T2
and CT.
 | Table II.Patient characteristics. |
Table II.
Patient characteristics.
|
|
|
| Liver
inflammation | Pericholecystic
changes | Wall thickening |
|---|
|
|
|
|
|
|
|
|---|
| Patient no. | Age, years | Sex | DWIBS/T2 | CT | DWIBS/T2 | CT | DWIBS/T2 | CT |
|---|
| 1 | 69 | M | (−) | (−) | (−) | (−) | (+) | (+) |
| 2 | 70 | M | (−) | (−) | (−) | (−) | (+) | (+) |
| 3 | 68 | M | (−) | (−) | (−) | (−) | (+) | (+) |
| 4 | 69 | M | (−) | (−) | (−) | (−) | (+) | (+) |
| 5 | 79 | M | (+) | (−) | (+) | (+) | (+) | (+) |
| 6 | 65 | M | (−) | (−) | (+) | (+) | (+) | (+) |
| 7 | 69 | M | (−) | (−) | (+) | (+) | (+) | (+) |
| 8 | 70 | F | (−) | (−) | (+) | (+) | (+) | (+) |
| 9 | 76 | F | (+)a | (+)a | (−) | (−) | (+) | (+) |
| 10 | 70 | M | (+) | (−) | (−) | (−) | (+) | (+) |
| 11 | 72 | F | (−) | (−) | (−) | (−) | (−) | (−)b |
| 12 | 88 | M | (−) | (−) | (+) | (+) | (+) | (+) |
| 13 | 50 | F | (−) | (−) | (+) | (+) | (+) | (+) |
| 14 | 50 | F | (−) | (−) | (−) | (−) | (+) | (+) |
The correlation between positive DWIBS/T2 results
and laboratory test variables was also investigated (Table III). White blood count and CRP
levels tended to be higher in patients whose DWIBS/T2 images
indicated inflammation or liver abscesses; however, this difference
was not statistically significant. These results suggested that
acute cholecystitis is more severe in patients for whom there is a
high signal density on the DWIBS/T2 image. DWIBS/T2 may therefore
be more useful for the detection of severe acute cholecystitis.
 | Table III.Laboratory test variables. |
Table III.
Laboratory test variables.
|
| Liver
inflammation | Pericholecystic
changes |
|---|
|
|
|
|
|---|
| Parameter | (+), n=3 | (−), n=11 | P-value | (+), n=6 | (−), n=8 | P-value |
|---|
| WBC,
×103/ml | 17.4±11.2 | 9.8±4.3 | 0.08 | 11.0±4.3 | 12.0±8.3 | 0.76 |
| CRP, mg/dl | 15.5±5.6 | 6.2±2.4 | 0.09 | 8.4±10.6 | 8.1±7.2 | 0.95 |
| ALP, IU/l | 645±204 | 462±106 | 0.44 | 367±257 | 602±389 | 0.25 |
| AST, IU/l | 63±20 | 123±75 | 0.69 | 36±25 | 168±284 | 0.28 |
| ALT, IU/l | 77±20 | 85±183 | 0.94 | 37±30 | 119±210 | 0.37 |
| γ-GTP, IU/l | 226±70 | 232±273 | 0.97 | 126±83 | 320±313 | 0.17 |
| LDH, IU/l | 214±40 | 300±154 | 0.37 | 228±62 | 305±162 | 0.40 |
| BUN, mg/dl | 16±6 | 17±2 | 0.78 | 14±5 | 17±6 | 0.35 |
| Cre, mg/dl | 0.75±0.25 | 0.82±0.16 | 0.59 | 0.82±0.14 | 0.79±0.20 | 0.78 |
Discussion
MRI imaging typically reveals gallbladder wall
thickening and pericholecystic fluid in patients with acute
cholecystitis (23), and is reported
to be superior to CT for the diagnosis of acute cholecystitis
(24). In the present study,
DWIBS/T2 was as successful as CT imaging in identifying wall
thickening and pericholecystic inflammation. Furthermore, compared
with CT, DWIBS/T2 showed positive results more clearly due to the
strong contrast between the target and surrounding tissues.
Liver abscesses typically appear as an irregularly
shaped, low-density area, with slight signal enhancement in
surrounding tissues (25). One
patient in the present study had a liver abscess, and this was
easily diagnosed via DWIBS/T2 or CT scanning. For this patient, a
small liver abscess was observed in a contrast-enhanced CT image.
DWIBS/T2 revealed an area of high signal intensity around the
abscess. These results indicate that, in this case, DWIBS/T2 was
able to reveal an area of inflammation in the liver that was
developing into a liver abscess. The high signal intensity area
observed with DWIBS/T2 appeared as a slight enhancement in the CT
image, and was easy to detect. However, DWIBS/T2 indicated the area
more clearly as the high signal intensity was strongly contrasted
against the surrounding tissues. These results suggested that
DWIBS/T2 enables easier evaluation of liver abscesses and the
surrounding inflammation compared with CT.
One limitation of the present study was the small
number of patients. Furthermore, the other complications that
typically occur with cholecystitis, such as gallbladder
perforation, emphysema, and gangrene, were not investigated. In
future studies, a greater number of patients should be included to
evaluate a broader set of complications.
In conclusion, the results of the present study
suggest that DWIBS/T2 has the same sensitivity as CT for the
diagnosis of complicated acute cholecystitis. However, the strong
contrast shown by DWIBS/T2 allows for easier evaluation of acute
cholecystitis than CT scanning. These findings may be beneficial in
a clinical setting as they allow doctors to select the most
effective diagnostic imaging technique for patients with suspected
cholecystitis.
References
|
1
|
Knab LM, Boller AM and Mahvi DM:
Cholecystitis. Surg Clin North Am. 94:455–470. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pinto A, Reginelli A, Cagini L, Coppolino
F, Ianora AA Stabile, Bracale R, Giganti M and Romano L: Accuracy
of ultrasonography in the diagnosis of acute calculous
cholecystitis: Review of the literature. Crit Ultrasound J. 5 Suppl
1:S112013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singal R, Mittal A, Gupta S, Singh B and
Jain P: Management of gall bladder perforation evaluation on
ultrasonography: Report of six rare cases with review of
literature. J Med Life. 4:364–371. 2011.PubMed/NCBI
|
|
4
|
Tang S and Wang Y and Wang Y:
Contrast-enhanced ultrasonography to diagnose gallbladder
perforation. Am J Emerg Med. 31:1240–1243. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koti RS, Davidson CJ and Davidson BR:
Surgical management of acute cholecystitis. Langenbecks Arch Surg.
400:403–419. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yokoe M, Takada T, Strasberg SM, Solomkin
JS, Mayumi T, Gomi H, Pitt HA, Gouma DJ, Garden OJ, Büchler MW, et
al: New diagnostic criteria and severity assessment of acute
cholecystitis in revised Tokyo Guidelines. J Hepatobiliary Pancreat
Sci. 19:578–585. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Myrianthefs P, Evodia E, Vlachou I,
Petrocheilou G, Gavala A, Pappa M, Baltopoulos G and Karakitsos D:
Is routine ultrasound examination of the gallbladder justified in
critical care patients? Crit Care Res Pract. 2012.565–617.
2012.
|
|
8
|
Teefey SA, Dahiya N, Middleton WD, Bajaj
S, Dahiya N, Ylagan L and Hildebolt CF: Acute cholecystitis: Do
sonographic findings and WBC count predict gangrenous changes? AJR
Am J Roentgenol. 200:363–369. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shakespear JS, Shaaban AM and Rezvani M:
CT findings of acute cholecystitis and its complications. AJR Am J
Roentgenol. 194:1523–1529. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bonekamp S, Corona-Villalobos CP and Kamel
IR: Oncologic applications of diffusion-weighted MRI in the body. J
Magn Reson Imaging. 35:257–279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sehy JV, Ackerman JJ and Neil JJ: Apparent
diffusion of water, ions, and small molecules in the Xenopus oocyte
is consistent with Brownian displacement. Magn Reson Med. 8:42–51.
2002. View Article : Google Scholar
|
|
12
|
Koike N, Cho A, Nasu K, Seto K, Nagaya S,
Ohshima Y and Ohkohchi N: Role of diffusion-weighted magnetic
resonance imaging in the differential diagnosis of focal hepatic
lesions. World J Gastroenterol. 15:5805–5812. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takahara T, Imai Y, Yamashita T, Yasuda S,
Nasu S and Van Cauteren M: Diffusion weighted whole body imaging
with background body signal suppression (DWIBS): Technical
improvement using free breathing, STIR and high resolution 3D
display. Radiat Med. 22:275–282. 2004.PubMed/NCBI
|
|
14
|
Kwee TC, Takahara T, Ochiai R, Nievelstein
RA and Luijten PR: Diffusion-weighted whole-body imaging with
background body signal suppression (DWIBS): Features and potential
applications in oncology. Eur Radiol. 18:1937–1952. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ohno Y, Koyama H, Onishi Y, Takenaka D,
Nogami M, Yoshikawa T, Matsumoto S, Kotani Y and Sugimura K:
Non-small cell lung cancer: Whole-body MR examination for M-stage
assessment-utility for whole-body diffusion-weighted imaging
compared with integrated FDG PET/CT. Radiology. 248:643–654. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fischer MA, Nanz D, Hany T, Reiner CS,
Stolzmann P, Donati OF, Breitenstein S, Schneider P, Weishaupt D,
von Schulthess GK and Scheffel H: Diagnostic accuracy of whole-body
MRI/DWI image fusion for detection of malignant tumours: A
comparison with PET/CT. Eur Radiol. 21:246–255. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sommer G, Wiese M, Winter L, Lenz C,
Klarhöfer M, Forrer F, Lardinois D and Bremerich J: Preoperative
staging of non-small-cell lung cancer: Comparison of whole-body
diffusion-weighted magnetic resonance imaging and
18F-fluorodeoxyglucose-positron emission tomography/computed
tomography. Eur Radiol. 22:2859–2867. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nechifor-Boilă IA, Bancu S, Buruian M,
Charlot M, Decaussin-Petrucci M, Krauth JS, Nechifor-Boilă AC and
Borda A: Diffusion weighted imaging with background body signal
suppression/T2 image fusion in magnetic resonance mammography for
breast cancer diagnosis. Chirurgia (Bucur). 108:199–205.
2013.PubMed/NCBI
|
|
19
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S and Ishige N: Diffusion-weighted whole body
imaging with background body signal suppression/T2 image fusion is
negative for patients with intraductal papillary mucinous neoplasm.
Hepatogastroenterology. 62:463–465. 2015.PubMed/NCBI
|
|
20
|
Hirota M, Takada T, Kawarada Y, Nimura Y,
Miura F, Hirata K, Mayumi T, Yoshida M, Strasberg S, Pitt H, et al:
Diagnostic criteria and severity assessment of acute cholecystitis:
Tokyo guidelines. J Hepatobiliary Pancreat Surg. 14:78–82. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ralls PW, Halls J, Lapin SA, Quinn MF,
Morris UL and Boswell W: Prospective evaluation of the sonographic
Murphy sign in suspected acute cholecystitis. J Clin Ultrasound.
10:113–115. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Miller FH, Chen ZE, Merrick L,
Mortele KJ, Hoff FL, Hammond NA, Yaghmai V and Nikolaidis P:
Diffusion-weighted MR imaging of solid and cystic lesions of the
pancreas. Radiographics. 31:E47–E64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tonolini M, Ravelli A, Villa C and Bianco
R: Urgent MRI with MR cholangiopancreatography (MRCP) of acute
cholecystitis and related complications: Diagnostic role and
spectrum of imaging findings. Emerg Radiol. 19:341–348. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaura SH, Haghighi M, Matza BW, Hajdu CH
and Rosenkrantz AB: Comparison of CT and MRI findings in the
differentiation of acute from chronic cholecystitis. Clin Imaging.
37:687–691. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feier D, Socaciu M, Anton O, Al Hajjar N
and Badea R: The combined role of intravenous contrast enhanced
ultrasound (CEUS) and computed tomography (CT) in liver abscess
diagnosis. Chirurgia (Bucur). 107:343–351. 2012.PubMed/NCBI
|