Introduction
Parkinson's disease (PD) is a neurodegenerative
disorder (1) characterized by the
degradation of dopaminergic cells within the substantia nigra pars
compacta (2) and by the aberrant
aggregation of α-Synuclein (α-Syn) in the dorsal motor nucleus of
the vagus (3–5). α-Syn belongs to a family of
structurally associated proteins in the brain (6,7), which
serve an important role in neurotransmitter release (8) by promoting the assembly of the soluble
N-ethylmaleimide-sensitive fusion attachment protein machinery
(9). Impaired α-Syn degeneration has
been widely accepted to be the key pathological course of
pathogenic protein accumulation (10). However, the mechanism that impairs
the degeneration of α-Syn in PD remains to be determined (11,12).
Besides the increased α-Syn synthesis in PD
(13), aberrant α-Syn aggregation in
PD may predominantly be caused by impaired α-Syn degradation
(14–16). Molecular chaperones, most of which
are heat shock proteins (Hsps), are the primary defense against
protein misfolding and aggregation, as they bind to unfolded
proteins and maintain them in a folding-competent state. In
addition, they may be associated with dissolving aggregates and
targeting misfolded proteins for degradation (17). It has also been demonstrated that Hsp
overexpression may refold and ameliorate aberrantly aggravated
α-Syn (18,19), suggesting that this may be the key
role of Hsp70 in PD (19,20). The negative regulation of α-Syn
aggregation and of α-Syn-induced cellular toxicity by Hsp70 has
been previously demonstrated (20,21).
Therefore, Hsp70 may be a potential therapeutic agent for the
treatment of PD, as it may block and reverse α-Syn-induced
toxicity.
The ubiquitin-proteasome system is the principal
degradation system for short-lived and misfolded proteins in
eukaryotic cells. The ubiquitinated target protein containing the
serially-activated E1ubiquitin-activating enzyme, E2
ubiquitin-conjugating enzyme and the E3 ligase is recognized by and
degraded in the proteasome by the 26S proteasome complex (22,23).
Recently, it has been determined that the dysfunction of the
lysosome and ubiquitin-proteasome system is associated with the
abnormal aggregation of α-Syn in neuroblastoma PC12 cells (22). Furthermore, enhanced
ubiquitin-dependent degradation of α-Syn by neural precursor cell
expressed developmentally down-regulated protein 4 was confirmed in
an animal model of PD (24).
However, the association between the molecular chaperone- and the
ubiquitin/proteasome system-mediated degradation of α-Syn in
neuroblastoma cells remains to be elucidated.
In the present study, the regulatory role of
glutamine on Hsp70 expression in neuroblastoma PC12 cells was
investigated. Subsequently, the regulatory role of glutamine on
α-Syn degradation in α-Syn-overexpressed PC12 cells with or without
treatment with the proteasomal inhibitor lactacystin (Lac) was also
investigated. The present study indicates that glutamine may be
effective at preventing α-Syn aggregation in PD.
Materials and methods
Reagents, cell culture and
treatments
L-glutamine (Gln; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and Lac (Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) were dissolved in Dulbecco's modified Eagle's medium
(DMEM) supplemented with 2% FBS (both from Invitrogen; Thermo
Fisher Scientific, Inc.). Cells from the pheochromocytoma cell line
PC12 were purchased from the American Type Culture Collection
(Manassas, VA, USA) and were cultured for 2 days to ~85% confluencu
in the DMEM supplemented with 10% (for growth) or 2% (for
maintenance) FBS and 100 U/ml penicillin/streptomycin (CSPC
Pharmaceutical Group, Ltd., Shijiazhuang, China) at 37°C in a humid
(100% humidity) incubator. For Gln treatment, PC12 cells at
85%-confluence were incubated at 37°C with DMEM supplemented with
2% FBS and with 0, 5, 10 or 20 mM Gln for 0, 4, 8, 12, 24 or 48 h.
For Lac treatment, PC12 cells at 85% confluence were incubated at
37°C with 0, 2, 5 or 10 µM Lac for 0, 6, 12 or 24 h. To knockdown
expression of heat shock factor (HSF), 20 or 40 nM HSF-1-specific
short interfering (si)RNA (5′-UGAUGUCGGAGAUGAUGGGTT-3′) or control
siRNA (siRNA-con, scramble; Sangon Biotech Co., Ltd., Shanghai,
China) was transfected into PC12 (α-Syn+) cells using Lipofectamine
RNAiMax transfection reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) to abrogate HSF-1 expression.
To induce overexpression of α-Syn in PC12 cells, the
α-Syn coding sequence was amplified with Pfu DNA polymerase
(Promega Corporation, Madison, WI, USA) and was cloned into the
pcDNA3. 1(+) eukaryotic expression vector (Invitrogen; Thermo
Fisher Scientific, Inc.). α-Syn cDNA was synthesized via reverse
transcription (95°C for 2 min followed by 42°C for 60 min) with
poly-dT primer (5′-TTTTTTTTTTTT-3′) (Invitrogen; Thermo Fisher
Scientific, Inc.). The polymerase chain reaction (PCR) was
performed under the conditions of an initial denaturation for 1 min
at 95°C followed by 40 cycles of denaturation for 40 sec at 94°C,
annealing for 1 min at 55°C and extension for 5 min at 72°C, and a
final extension for 10 min at 72°C. The α-Syn-specific primer pairs
were as follows: Forward, 5′-CTCCTCGAGAGGAGAAGGAGAAGG-3′; and
reverse, 5′-CGCAAGCTTTATTTTCATATATGT-3′. The recombinant
α-Syn-pcDNA3. 1(+) plasmid was subsequently transfected using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
into PC12 cells. The coding sequence for chloramphenicol acetyl
transferase (CAT) was also cloned into pcDNA3. 1(+) to construct
CAT-pcDNA3. 1(+) plasmids, which were also transfected into PC12
cells as a control with Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). PC12 cells following transfection were
cultured at 37°C (medium was replaced every 48 h) in the presence
of 200 µg/ml G418 (Thermo Fisher Scientific, Inc.) to select the
positive clone, PC12 (α-Syn+). PC12 (con) cells were maintained in
the presence of 80 µg/ml G418.
mRNA preparation and quantitative
analysis with reverse transcription-quantitative PCR (RT-qPCR)
Cellular mRNA was prepared using an mRNA Isolation
and Purification kit (Clontech Laboratories, Inc., Mountainview,
CA, USA) according to the manufacturer's protocol. The mRNA
expression of Hsp70, HSF-1, or α-Syn was quantified using RT-qPCR.
cDNA for each marker was synthesized using the Quantitect Reverse
Transcription kit (Qiagen, Inc., Valencia, CA, USA) under the
conditions of 95°C for 30 sec followed by 42°C for 60 min with
poly-dT primer (5′-TTTTTTTTTTTT-3′). qPCR was performed using a
SYBR-Green-based Quantitative PCR kit (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) with a Lightcycler 480 II (Roche Diagnostics
GmbH, Mannheim, Germany) under the following conditions: 95°C for 1
min (1 cycle), followed by 40 cycles of 94°C for 15 sec, 55°C for
15 sec and 70°C for 20 sec. The primer sequences were as follows:
α-Syn forward, 5′-AGGACTTTCAAAGGCCAAGG-3′; α-Syn reverse,
5′-TCCTCCAACATTTGTCACTTGC-3′; HSP70 forward,
5′-TGTGTCTGCTTGGTAGGAATGGTGGTA-3′, HSP70 reverse,
5′-TTACCCGTCCCCGATTTGAAGAAC-3′; HSF-1 forward,
5′-CGACAGTGGCTCAGCACATTCC-3′, HSF-1 reverse,
5′-CAGCTCGGTGATGTCGGAGATG-3′; β-actin forward,
5′-TGTCCACCTTCCAGCAGATGT-3′, β-actin reverse,
5′-AGCTCAGTAACAGTCCGCCTAGA-3′. The 2−∆∆Cq method was
used to relatively determine the mRNA level for each marker, with
β-actin as an internal control (25). This experiment was repeated three
times.
Western blotting
Following treatment, PC12 (con) or PC12 (α-Syn+)
cells were washed with cold phosphate-buffered saline, and
cytoplasmic proteins were isolated using the NE-PER Nuclear and
Cytoplasmic Extraction kit (Thermo Fisher Scientific, Inc.) and a
protease inhibitor cocktail (Abcam, Cambridge, UK). Protein
concentration was quantified with bicinchoninic acid protein assay
reagent (Sigma-Aldrich; Merck KGaA). Proteins were then subjected
to sodium dodecyl sulphate-polyacrylamide gel electrophoresis
(SDS-PAGE) electrophoresis with 10% gradient gel. Separated
proteins were subsequently transferred to PVDF membranes (EMD
Millipore, Billerica, MA, USA), which were blocked with 2% bovine
serum albumin (Sigma-Aldrich; Merck KGaA) at 4°C overnight. Primary
rabbit polyclone antibodies against Hsp70 (1:1,000; ab2787; Abcam,
Cambridge, UK), HSF-1 (1:500; sc-17756; Santa Cruz Biotechnology,
Santa Cruz, CA, USA), α-Syn (1:1,000; #2642; Cell Signaling
Technology, Inc., Danvers, MA, USA) or β-actin (1:2,000; A2066;
Sigma-Aldrich; Merck KGaA) were incubated to allow specific binding
for 2 h at 4°C. The horseradish peroxidase-conjugated secondary
anti-rabbit antibody (1:5,000; 111-035-003; Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA) was used to detect the
specific antigen-antibody binding for 1 h at room temperature.
Finally, each target protein was visualized using an enhanced
chemiluminescence detection kit (GE Healthcare Life Sciences,
Chalfont, UK) according to the manufacturer's protocol.
Proteasomal activity assay
Proteasome activity in PC12 (con) or PC12 (α-Syn+)
cells was assayed, following cell lysis, by measuring the release
of 7-amino-4-methylcoumarin (AMC) from the fluorogenic peptides
Suc-Leu-Leu-Val-Tyr-AMC and Z-Leu-Leu-Glu-AMC. Cells were lysed in
20 mM Tris-HCl, 1 mM EDTA buffer (pH 7.5) and were centrifuged at
12,000 × g for 30 min at 4°C to remove cellular debris. The
supernatant was collected and was diluted to a concentration of 100
µg/µl. Protein sample (1–3 µl) was added to the assay mixture in a
total volume of 300 µl containing 50 µM Z-Leu-Leu-Glu-AMC, 50 µM
Suc-Leu-Leu-Val-Tyr-AMC, 5 mM adenosine triphosphate and 5 mM Mg
acetate in Tris-EDTA buffer with or without Lac (0, 2, 5 or 10 µM).
The mixture was incubated at 37°C for 30 min followed by the
fluorescence measurement at λex 360 nm and λem 465 nm with a
fluorescence spectrophotometer (Hitachi Fluorescence
Spectrophotometer F-2000; Hitachi Ltd., Tokyo, Japan). The assay
produced proportional responses up to 300 µg protein. Proteasomal
activity was presented as a relative level to the control group (0
µM Lac or 0 mM Gln). Five replicates were performed for each
experiment.
Statistical analysis
Quantitative results were presented as the mean ±
standard deviation. The difference between the experimental and
control groups was analyzed using Student's two-tailed t-test, with
SPSS 13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Gln upregulates Hsp70 expression in
PC12 neuroblastoma cells HSF-1-dependently
Previous studies have demonstrated that Gln enhances
the expression of Hsp (26–29), which serves a key regulatory role in
α-Syn degradation. Therefore, the regulation by Gln on Hsp70
expression in PC12 neuroblastoma cells was investigated. Hsp70 mRNA
expression was significantly increased in PC12 cells following Gln
treatment for 8 h (P<0.05 for 10 mM; P<0.01 for 20 mM) and
there was a significant increase in Hsp70 mRNA expression in the 20
mM group compared with the 10 mM group (P<0.05; Fig. 1A). Such promotion was also
time-dependent, as significant Hsp70 mRNA upregulation was observed
at 8 h following the treatment with 20 mM Gln (P<0.05) and at 12
h post treatment (P<0.01) compared with cells that were not
treated with Gln; with a significant increase in Hsp70 mRNA levels
at 12 h compared with 8 h (P<0.05; Fig. 1B). Furthermore, promotion of Hsp70
protein expression by Gln was also confirmed in PC12 cells, as
significantly increased levels of Hsp70 were detected in the 24 h
Gln-treated PC12 cells compared with those that were not treated
with Gln (P<0.05 for 10 mM; P<0.01 for 20 mM; Fig. 1C), with a significant increase
observed in the 20 mM group compared with the 10 mM group
(P<0.05).
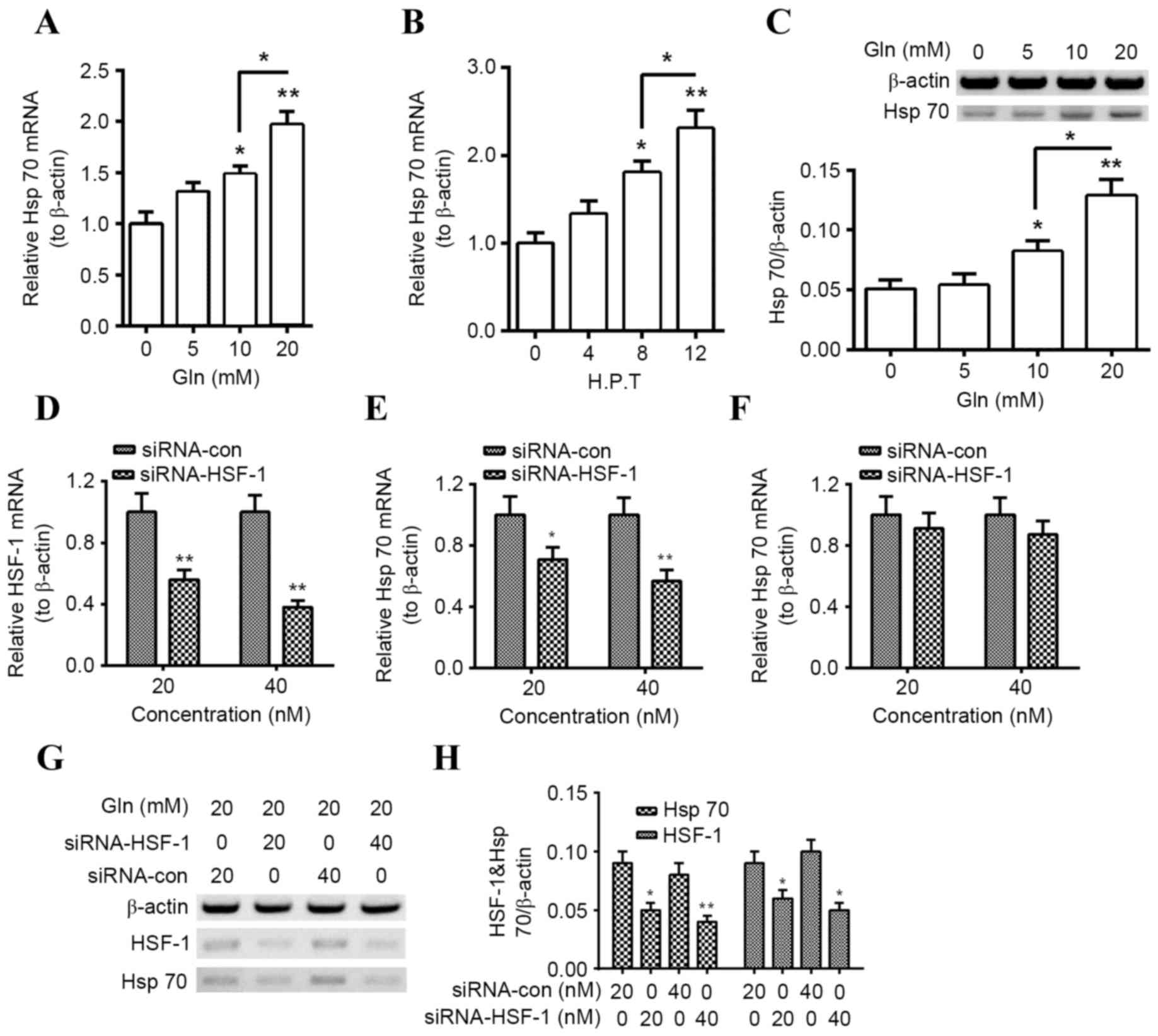 | Figure 1.Gln upregulates Hsp70 expression in
PC12 cells HSF-1-dependently. (A) Levels of Hsp70 mRNA in PC12
cells following treatment with 0, 5, 10, or 20 mM Gln for 8 h. (B)
Levels of Hsp70 mRNA in PC12 cells following 20 mM Gln treatment
for 0, 4, 8 or 12 h. (C) Western blot analysis of Hsp70 protein
levels in PC12 cells following treatment with 0, 5, 10, or 20 mM
Gln for 24 h, with β-actin as an internal control. (D) Levels of
HSF-1 mRNA in PC12 cells 12 h following transfection with 20 or 40
nM siRNA-HSF-1 or siRNA-con. Levels of Hsp70 mRNA in PC12 cells (E)
with or (F) without the treatment with 20 mM Gln, following
transfection with 20 or 40 nM siRNA-HSF-1 or siRNA-Con. (G and H)
Hsp70 and HSF-1 protein levels measured via western blot analysis
in PC12 cells transfected with 20 or 40 nM siRNA-HSF-1, or
siRNA-con, in the presence of 20 mM Gln. All experiments were
performed in triplicate and data are presented as the mean ±
standard deviation. *P<0.05, **P<0.01 vs. control (0 h, 0 mM
Gln or siRNA Con as indicated). Gln, glutamine; Hsp, heat shock
protein; HSF, heat shock factor; siRNA, short interfering RNA;
siRNA-HSF-1, HSF-1-targeted siRNA; con, control; H. P. T., h
post-transfection. |
Given the key role of HSF in the expression of Hsps,
including Hsp70 (30), the
association of HSF-1 knockdown on Gln-promoted Hsp70 expression was
investigated. Transfection with the HSF-1-specific siRNA,
siRNA-HSF-1, significantly downregulated levels of HSF-1 mRNA
(P<0.01 for 20 and 40 nM) in PC12 cells (Fig. 1D) and significantly reduced Hsp70
mRNA levels in PC12 cells treated with 20 nM Gln (P<0.05 for 20
nM and P<0.01 for 40 nM; Fig.
1E), compared with siRNA-con. Notably, downregulation of Hsp70
mRNA was not significant in PC12 cells transfected with siRNA-HSF1
that did not receive Gln treatment (Fig.
1F). In addition, western blotting demonstrated that HSF-1 and
Hsp70 were significantly downregulated following siRNA-HSF-1
transfection (Hsp70, P<0.05 for 20 nM, P<0.01 for 40 nM;
HSF-1, P<0.05 for both; Fig. 1G and
H). These results suggest that Gln promotes Hsp70 expression
HSF-1-dependently in PC12 cells.
Upregulation of Hsp70 by Gln increases
α-Syn degradation in PC12 (α-Syn+) cells
To investigate the regulation of Gln-promoted Hsp70
on α-Syn degradation in PC12 cells, wild-type α-Syn was
overexpressed in PC12. Significantly increased levels of α-Syn mRNA
were observed in PC12 (α-Syn+) cells following incubation with
α-Syn for 6, 12 or 24 h (P<0.001 for all; Fig. 2A). Levels of α-Syn protein were also
significantly higher in PC12 (α-Syn+) cells than in the control
PC12 (con) cells following incubation for 12, 24 or 48 h
(P<0.001 for all; Fig. 2B and C).
Subsequently, it was investigated whether overexpressed α-Syn
regulated Hsp70 expression and it was determined that there was no
significant difference in Hsp70 mRNA levels between PC12 (α-Syn+)
and PC12 (con) cells (Fig. 2D). Gln
was also demonstrated to serve a regulatory role on α-Syn
degradation in PC12 (α-Syn+) cells. Treatment with 10 mM Gln
treatment had no significant influence on the α-Syn mRNA levels in
the PC12 (α-Syn+) cells, at 6, 12 or 24 h following treatment
(Fig. 2E). However, levels of α-Syn
protein were significantly downregulated in PC12 (α-Syn+) cells
treated with 10 or 20 mM Gln (P<0.01 and P<0.001,
respectively; Fig. 2F), with a
significant decrease in α-Syn levels in the 20 nM Gln group
compared with the 10 nM group (P<0.05). These results suggest
that Gln treatment promotes α-Syn degradation in PC12 (α-Syn+)
cells.
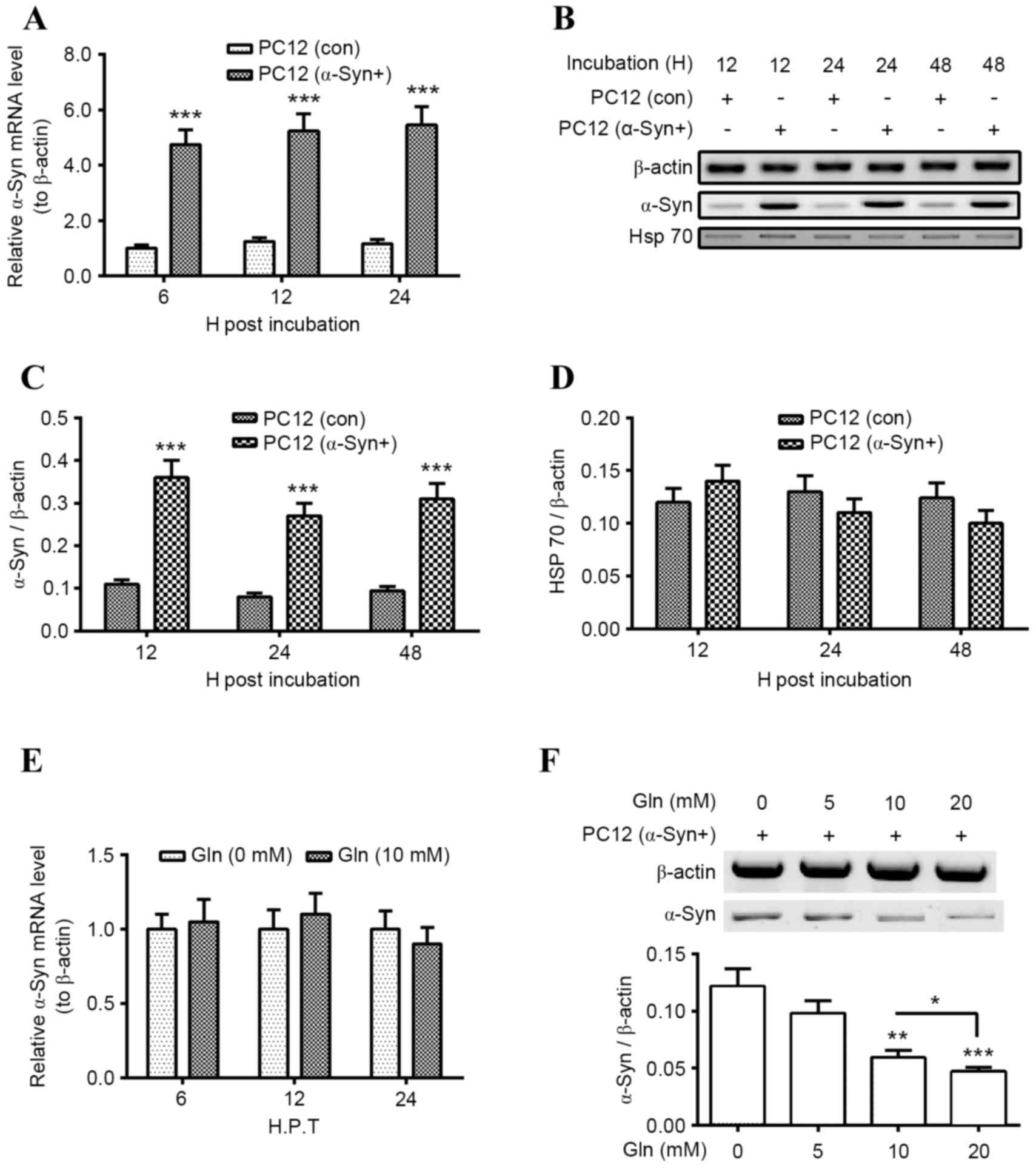 | Figure 2.Gln treatment promotes α-Syn
degradation in PC12 (α-Syn+) cells. (A) Levels of α-Syn mRNA in
PC12 (α-Syn+) cells or in PC12 (con) cells, following an incubation
for 6, 12 or 24 h. (B) Western blot analysis of α-Syn and Hsp70
protein levels in PC12 (α-Syn+) or PC12 (Con) cells, following an
incubation time for 12, 24 or 48 h. Relative levels of (C) α-Syn or
(D) Hsp70 in PC12 (α-Syn+) or PC12 (Con) cells, following an
incubation time for 12, 24 or 48 h. (E) Relative levels of α-Syn
mRNA in the PC12 (α-Syn+) cells with or without 10 mM Gln treatment
for 6, 12 or 24 h. (F) Western blot analysis of α-Syn protein
levels in the PC12 (α-Syn+) cells, following the treatment with 0,
5, 10 or 20 mM Gln for 24 h. All results are presented as mean ±
standard deviation for triple experiments. *P<0.05, **P<0.01,
***P<0.001 vs. control. Gln, glutamine; α-Syn, α-Synuclein; PC12
(α-Syn+), α-Syn-overexpressed PC12; con, control; Hsp, heat shock
protein; H. P. T., h post transfection; H, h. |
Upregulation of Hsp70 by Gln inhibits
proteasomal inhibitor-induced α-Syn accumulation in PC12 (α-Syn+)
cells
Proteasomal impairment has been suggested as another
mechanism associated with abnormal α-Syn accumulation in PD
(31,32). To investigate the regulation of
proteasomal activity on α-Syn degradation, the proteasomal activity
and α-Syn protein levels in PC12 (α-Syn+) cells and normal PC12
cells, which were treated with Lac, were investigated. It was
determined that treatment with 5 or 10 µM Lac significantly reduced
proteasomal activity in normal PC12 cells (P<0.01 and
P<0.001, respectively; Fig. 3A),
and in PC12 (α-Syn+) cells (P<0.001 and P<0.0001,
respectively; Fig. 3B). This
reduction was greater in cells treated with 10 µM Lac than in those
treated with 5 µM Lac in both groups [P<0.05 in PC12 cells;
P<0.01 in PC12 (α-Syn+) cells]. In addition, proteasomal
activity in both types of PC12 cells was investigated following
treatment with Gln. It was demonstrated that treatment with 5, 10
or 20 mM Gln did not result in significant upregulation or
downregulation of the proteasomal activity in PC12 (con) cells or
in PC12 (α-Syn+) cells (Fig. 3C and
D).
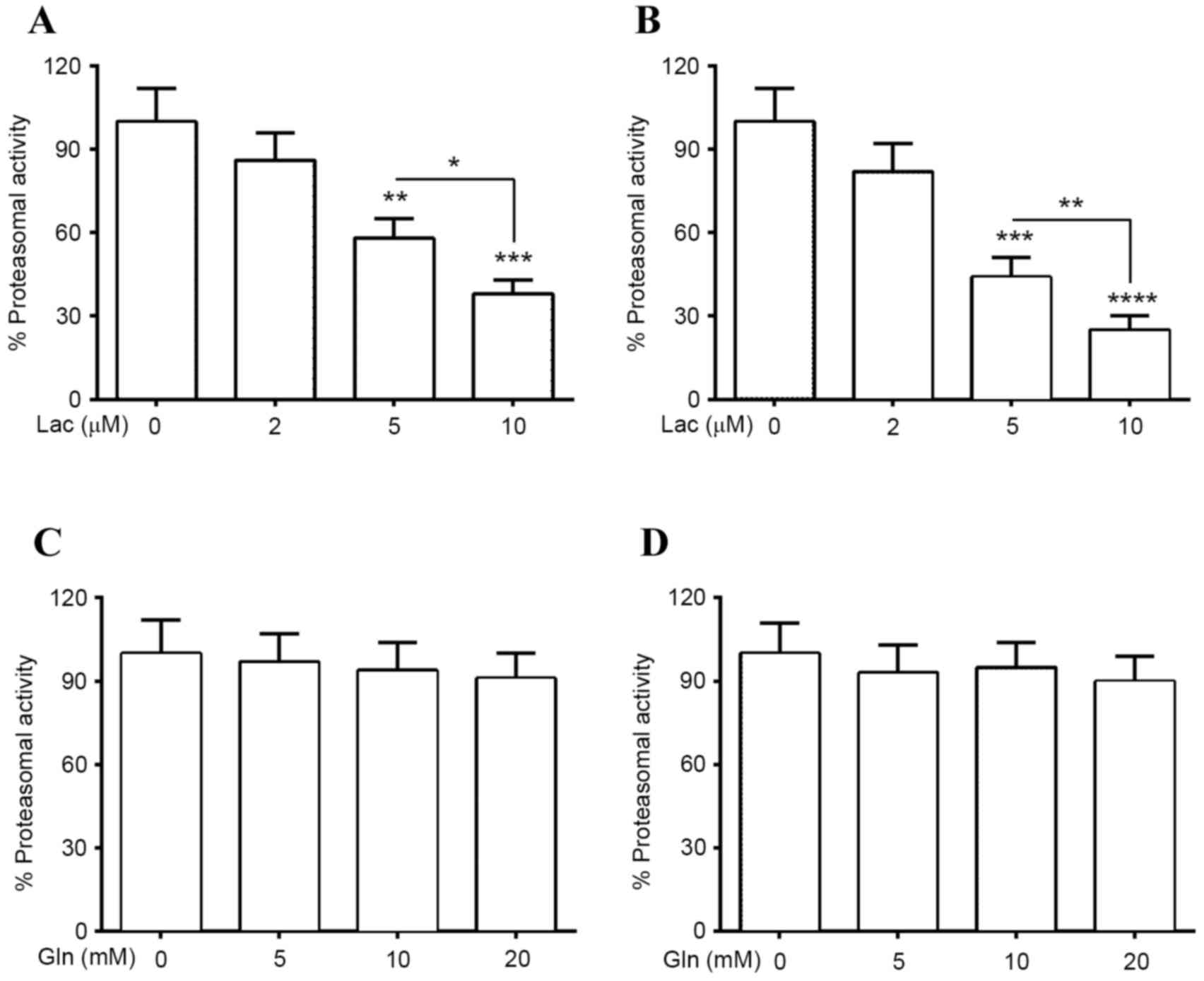 | Figure 3.Proteasomal activities in PC12
(α-Syn+) cells treated with Lac and/or Gln. Proteasomal activity in
(A) PC12 (α-Syn+) or (B) PC12 (con) cells treated with 0, 2, 5 or
10 µM Lac for 24 h. Proteasomal activity in (C) PC12 (α-Syn+) or
(D) PC12 (con) cells treated with 0, 5, 10 or 20 mM Gln for 24 h.
Each value was relative to the proteasomal activity in PC12
(α-Syn+) cells treated without Lac or Gln for 24 h. Data was
presented as mean ± standard deviation of four independent results
*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 vs. 0 µM
Lac. PC12 (α-Syn+), α-Syn-overexpressed PC12; α-Syn, α-Synuclein;
Lac, lactacystin; Gln, glutamine; con, control. |
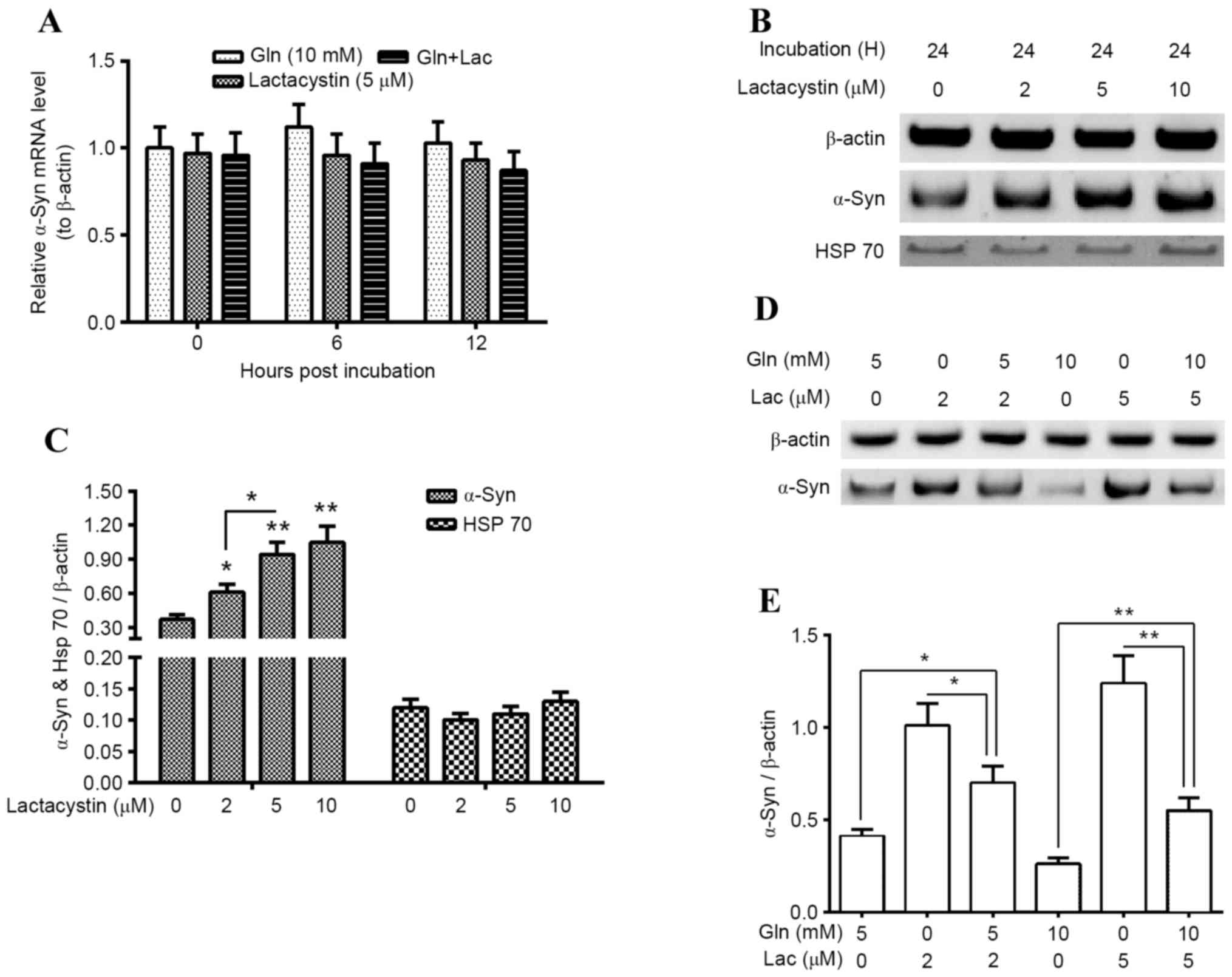
α-Syn degradation in PC12 (α-Syn+) cells, which were
treated with Gln and/or Lac, was subsequently evaluated. It was
indicated that α-Syn mRNA was not significantly altered following
treatment with 10 mM Gln, 5 µM Lac or both agents (10 mM Gln and 5
µM Lac; Fig. 4A). However, western
blotting demonstrated that proteasomal activity inhibition by 2, 5
and 10 µM Lac significantly upregulated α-Syn accumulation in the
PC12 (α-Syn+) cells (P<0.05 for 2 µM; P<0.01 for 5 and 10
µM), in a dose-dependent manner (P<0.05 between 2 and 5 µM;
Fig. 4B and C). However, Lac
treatment for 24 h did not significantly regulate Hsp70 expression
in PC12 (α-Syn+) cells (Fig. 4C).
Furthermore, it was demonstrated that there is a reduction in
Lac-induced α-Syn accumulation following Gln treatment. As
presented in Fig. 4D and E, 5 mM Gln
significantly reduced 2 µM Lac-induced α-Syn accumulation
(P<0.05) and 10 mM Gln significantly reduced the 5 µM
Lac-induced α-Syn accumulation (P<0.01; Fig. 4E). These findings suggest that the
upregulation of Hsp70 by Gln may inhibit the proteasomal
inhibitor-induced α-Syn accumulation in PC12 (α-Syn+) cells.
Discussion
It has been recognized that hypofunction of
molecular chaperones is associated with aberrant α-Syn aggregation
in PD (19,20) and that Hsp promotion is effective at
promoting α-Syn degradation; Hsp70, in particular, has been
confirmed to prevent α-Syn aggregation in PD (20,21).
Previous studies have demonstrated that Hsp70 is subjected to
transcriptional regulation upon different stresses and is regulated
by a variety of molecules (32–34). It
has previously been demonstrated that Gln enhances Hsp70 expression
(26,27,35). Gln
mediates cellular protection against heat-stress injury to the
lungs by promoting HSF-1 expression (26) or enhancing HSF-1
phosphorylation/activation (36). In
the present study, Gln-regulated upregulation of Hsp70 expression
was observed in PC12 cells at both the mRNA and protein levels in a
dose- and time-dependent manner. The Gln-regulation of Hsp70
expression was demonstrated to be HSF-1-dependent via HSF-1
knockdown. HSF-1-specific siRNA was able to block HIF-1 expression
and blunt Gln-promoted Hsp70 upregulation. These findings suggest
that the Gln-promoted Hsp70 upregulation is HSF-1-dependent.
It has been suggested that in both sporadic and
familial PD, proteasomal impairment is associated with the abnormal
accumulation of α-Syn in PD pathogenesis (30,31). The
α-Syn accumulation and the proteasomal inactivation may be
associated with either the oxidative or the nonoxidative mode of
dopamine toxicity (37) and
accumulated α-Syn subsequently induces toxicity in different cell
lines or on isolated brain mitochondria (38,39). The
present study demonstrated that Gln treatment facilitated α-Syn
degradation but did not significantly regulate α-Syn mRNA
expression in PC12 (α-Syn+) cells, which overexpressed α-Syn. This
suggests that Gln may be a promising agent to ameliorate α-Syn
accumulation in PC12 (α-Syn+) cells. In addition, it was determined
that although the regulatory role of Gln on α-Syn accumulation was
independent of proteasomal activity, it was able to reverse the
inhibition of α-Syn degradation, which was mediated by the
proteasomal inhibitor Lac.
In conclusion, the present study demonstrated that
Gln is able to promote Hsp70 in pheochromocytoma PC12 cells in an
HSF-1-dependent manner and that Gln-promoted Hsp70 facilitates
α-Syn degradation proteasome-independently. These findings suggest
that Gln may be a promising reagent to prevent α-Syn aggregation in
PD.
Acknowledgements
This study was supported by grants from the Natural
Science Foundation of Heilongjiang Province (grant no. H201434),
the Scientific Foundation of the First Affiliated Hospital of
Harbin Medical University (grant no. 2011BS13), the Heilongjiang
Postdoctoral Financial Assistance (grant no. LBH-Z11096), the
International S&T Cooperation Program of China (grant no.
2014DFA31630) and the National Nature Science Foundation of China
(grant no. 81571646).
References
|
1
|
Bertram L and Tanzi RE: The genetic
epidemiology of neurodegenerative disease. J Clin Invest.
115:1449–1457. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wirdefeldt K, Adami HO, Cole P,
Trichopoulos D and Mandel J: Epidemiology and etiology of
Parkinson's disease: A review of the evidence. Eur J Epidemiol. 26
Suppl 1:S1–S58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Irizarry MC, Growdon W, Gomez-Isla T,
Newell K, George JM, Clayton DF and Hyman BT: Nigral and cortical
Lewy bodies and dystrophic nigral neurites in Parkinson's disease
and cortical Lewy body disease contain alpha-synuclein
immunoreactivity. J Neuropathol Exp Neurol. 57:334–337. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spillantini MG, Crowther RA, Jakes R,
Hasegawa M and Goedert M: Alpha-Synuclein in filamentous inclusions
of Lewy bodies from Parkinson's disease and dementia with lewy
bodies. Proc Natl Acad Sci USA. 95:pp. 6469–6473. 1998; View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jellinger KA: Neuropathology of sporadic
Parkinson's disease: Evaluation and changes of concepts. Mov
Disord. 27:8–30. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ueda K, Fukushima H, Masliah E, Xia Y,
Iwai A, Yoshimoto M, Otero DA, Kondo J, Ihara Y and Saitoh T:
Molecular cloning of cDNA encoding an unrecognized component of
amyloid in Alzheimer disease. Proc Natl Acad Sci USA. 90:pp.
11282–11286. 1993; View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jakes R, Spillantini MG and Goedert M:
Identification of two distinct synucleins from human brain. Febs
Lett. 345:27–32. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartels T, Choi JG and Selkoe DJ:
α-Synuclein occurs physiologically as a helically folded tetramer
that resists aggregation. Nature. 477:107–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mueller A, Ziegler K, Amsharov KY and
Jansen M: Perchloropyracylene and its fusion with C60 by
chlorine-assisted radio-frequency furnace synthesis. Chemistry.
17:11797–11804. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eichner T and Radford SE: A diversity of
assembly mechanisms of a generic amyloid fold. Mol Cell. 43:8–18.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kruger R, Kuhn W, Müller T, Woitalla D,
Graeber M, Kösel S, Przuntek H, Epplen JT, Schöls L and Riess O:
Ala30Pro mutation in the gene encoding alpha-synuclein in
Parkinson's disease. Nat Genet. 18:106–108. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chartier-Harlin MC, Kachergus J, Roumier
C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J,
Hulihan M, et al: Alpha-synuclein locus duplication as a cause of
familial Parkinson's disease. Lancet. 364:1167–1169. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singleton AB, Farrer M, Johnson J,
Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra
A, Nussbaum R, et al: Alpha-Synuclein locus triplication causes
Parkinson's disease. Science. 302:8412003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alvarez-Erviti L, Seow Y, Schapira AH,
Rodriguez-Oroz MC, Obeso JA and Cooper JM: Influence of microRNA
deregulation on chaperone-mediated autophagy and α-synuclein
pathology in Parkinson's disease. Cell Death Dis. 4:e5452013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cuervo AM, Stefanis L, Fredenburg R,
Lansbury PT and Sulzer D: Impaired degradation of mutant
alpha-synuclein by chaperone-mediated autophagy. Science.
305:1292–1295. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alvarez-Erviti L, Rodriguez-Oroz MC,
Cooper JM, Caballero C, Ferrer I, Obeso JA and Schapira AH:
Chaperone-mediated autophagy markers in Parkinson disease brains.
Arch Neurol. 67:1464–1472. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wacker JL, Zareie MH, Fong H, Sarikaya M
and Muchowski PJ: Hsp70 and Hsp40 attenuate formation of spherical
and annular polyglutamine oligomers by partitioning monomer. Nat
Struct Mol Biol. 11:1215–1222. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Periquet M, Fulga T, Myllykangas L,
Schlossmacher MG and Feany MB: Aggregated alpha-synuclein mediates
dopaminergic neurotoxicity in vivo. J Neurosci. 27:3338–3346. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Auluck PK, Chan HY, Trojanowski JQ, Lee VM
and Bonini NM: Chaperone suppression of alpha-synuclein toxicity in
a Drosophila model for Parkinson's disease. Science. 295:865–868.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Klucken J, Shin Y, Masliah E, Hyman BT and
McLean PJ: Hsp70 reduces alpha-synuclein aggregation and toxicity.
J Biol Chem. 279:25497–25502. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luk KC, Mills IP, Trojanowski JQ and Lee
VM: Interactions between Hsp70 and the hydrophobic core of
alpha-synuclein inhibit fibril assembly. Biochemistry.
47:12614–12625. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang R, Zhao J, Zhang J, Liu W, Zhao M, Li
J, Lv J and Li Y: Effect of lysosomal and ubiquitin-proteasome
system dysfunction on the abnormal aggregation of α-synuclein in
PC12 cells. Exp Ther Med. 9:2088–2094. 2015.PubMed/NCBI
|
|
23
|
Weissman AM: Themes and variations on
ubiquitylation. Nat Rev Mol Cell Biol. 2:169–178. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Davies SE, Hallett PJ, Moens T, Smith G,
Mangano E, Kim HT, Goldberg AL, Liu JL, Isacson O and Tofaris GK:
Enhanced ubiquitin-dependent degradation by Nedd4 protects against
α-synuclein accumulation and toxicity in animal models of
Parkinson's disease. Neurobiol Dis. 64:79–87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morrison AL, Dinges M, Singleton KD, Odoms
K, Wong HR and Wischmeyer PE: Glutamine's protection against
cellular injury is dependent on heat shock factor-1. Am J Physiol
Cell Physiol. 290:C1625–C1632. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singleton KD and Wischmeyer PE:
Glutamine's protection against sepsis and lung injury is dependent
on heat shock protein 70 expression. Am J Physiol Regul Integr Comp
Physiol. 292:R1839–R1845. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang J, Zhang Y, Zhao S, Zhang Z, Tong X,
Wei F and Lu Z: Heat shock protein 70 induction by glutamine
increases the alphasynuclein degradation in SHSY5Y neuroblastoma
cells. Mol Med Rep. 12:5524–5530. 2015.PubMed/NCBI
|
|
29
|
Zhang J, Liu B, Li J, Zhang L, Wang Y,
Zheng H, Lu M and Chen J: Hsf and Hsp gene families in Populus:
Genome-wide identification, organization and correlated expression
during development and in stress responses. BMC Genomics.
16:1812015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
McNaught KS, Belizaire R, Jenner P, Olanow
CW and Isacson O: Selective loss of 20S proteasome alpha-subunits
in the substantia nigra pars compacta in Parkinson's disease.
Neurosci Lett. 326:155–158. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McNaught KS, Belizaire R, Isacson O,
Jenner P and Olanow CW: Altered proteasomal function in sporadic
Parkinson's disease. Exp Neurol. 179:38–46. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song Y and Masison DC: Independent
regulation of Hsp70 and Hsp90 chaperones by Hsp70/Hsp90-organizing
protein Sti1 (Hop1). J Biol Chem. 280:34178–34185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fukayama S, Lanske B, Guo J, Kronenberg HM
and Bringhurst FR: Regulation of HSP70 by PTH: A model of gene
regulation not mediated by changes in cAMP levels. Am J Physiol.
271:C121–C129. 1996.PubMed/NCBI
|
|
34
|
Jacquier-Sarlin MR, Jornot L and Polla BS:
Differential expression and regulation of hsp70 and hsp90 by
phorbol esters and heat shock. J Biol Chem. 270:14094–14099. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wischmeyer PE, Kahana M, Wolfson R, Ren H,
Musch MM and Chang EB: Glutamine induces heat shock protein and
protects against endotoxin shock in the rat. J Appl Physiol (1985).
90:2403–2410. 2001.PubMed/NCBI
|
|
36
|
Singleton KD, Serkova N, Beckey VE and
Wischmeyer PE: Glutamine attenuates lung injury and improves
survival after sepsis: Role of enhanced heat shock protein
expression. Crit Care Med. 33:1206–1213. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Banerjee K, Munshi S, Sen O, Pramanik V,
Roy MT and Chakrabarti S: Dopamine cytotoxicity involves both
oxidative and nonoxidative pathways in SH-SY5Y cells: Potential
role of alpha-synuclein overexpression and proteasomal inhibition
in the etiopathogenesis of Parkinson's disease. Parkinsons Dis.
2014:8789352014.PubMed/NCBI
|
|
38
|
Zhou W, Hurlbert MS, Schaack J, Prasad KN
and Freed CR: Overexpression of human alpha-synuclein causes
dopamine neuron death in rat primary culture and immortalized
mesencephalon-derived cells. Brain Res. 866:33–43. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bisaglia M, Greggio E, Maric D, Miller DW,
Cookson MR and Bubacco L: Alpha-synuclein overexpression increases
dopamine toxicity in BE2-M17 cells. BMC Neurosci. 11:412010.
View Article : Google Scholar : PubMed/NCBI
|