Introduction
The syndrome of intra-vitreous bleeding in
association with subarachnoid hemorrhage was first described by
French ophthalmologist Albert Terson in 1900 (1). Terson syndrome now encompasses any
intraocular hemorrhage associated with intracranial hemorrhage and
elevated intracranial pressures (2–4), which
often results in significant morbidity and subsequent decreased
quality of life. Vitrectomy has been proposed to have a role in
treating Terson syndrome, and numerous studies have reported good
visual recovery following surgery (5–8).
However, those studies were predominantly related to conventional
20G vitrectomy. Moreover, this technique has several complicating
issues such as iatrogenic retinal breaks, entry site breaks and
lengthy duration.
25G-transconjunctival sutureless vitrectomy (TSV) is
a recently developed minimally invasive vitrectomy surgery system
that has introduced a new approach to vitreoretinal surgery and
shown advantages for some vitreoretinal surgeries (9–11). The
advantages include decreased operative times in certain cases and
decreased postoperative inflammation, early postoperative
rehabilitation, improved patient comfort and minimal conjunctival
damage (12). Comparison between 20G
and 25G vitrectomy revealed that the time saved was localized to
the ‘initial’ and ‘final’ steps of the procedure (13). Damage to the conjunctiva was also
minimalized with a transconjunctival, sutureless approach (14). This may be of clinical significance
in patients requiring glaucoma filtration surgery in the future.
This benefit is also relevant to the limited number of patients
undergoing multiple vitreoretinal procedures. Comparison of 25G-TSV
wounds to conventional 20G wounds in the same patient revealed a
much faster healing rate of 15 days as opposed to 6–8 weeks, using
ultrasound biomicroscopy to assess the injury (15).
Recently, use of a wide-angle viewing system in
vitrectomy surgery has become popular, as this option can easily
provide a panoramic view of the surgical field (16). Two types of the wide-angle viewing
system exist, with both contact and non-contact types available.
The non-contact type is more popular because of the stability of
the image against the tilt of the eyeball and the ease of
manipulation (17). Resight
non-contact wide-angled lenses are a new kind of operative viewing
system. The simple operative procedure, wide observation, right
stereo visual, non-contact wide-angle viewing system in cooperation
with 25G vitrectomy may have the advantage of minimally invasive
vitrectomy. The aim of this study was to compare the outcomes and
therapeutic efficacy of 25G vitrectomy for Terson syndrome under
Resight non-contact wide-angle lenses with the therapeutic efficacy
of standard 20G vitrectomy.
Materials and methods
Materials
Between July 2011 and October 2013, we reviewed the
records of 20 patients (28 eyes) diagnosed with Terson syndrome who
had undergone 25G vitrectomy under Resight non-contact wide angle
lenses (study group) and the records of 20 matched patients (27
eyes) that underwent standard 20G vitrectomy (control group).
Patients in the control group were matched in terms of age, gender
and cause of hemorrhage (Table I).
This was a retrospective study that was approved by the ethics
committee of Second Affiliated Hospital of Nanchang University
[approval ID: 2011 (013); Nanchang, China]. Written consents were
waived by the Ethics Committee.
 | Table I.Patient characteristics and outcomes
by type of vitrectomy performed (25G vs. 20G vitrectomy) for Terson
syndrome. |
Table I.
Patient characteristics and outcomes
by type of vitrectomy performed (25G vs. 20G vitrectomy) for Terson
syndrome.
| Parameter | 25G study group | 20G control
group | P-value |
|---|
| Patients (n) | 20 | 20 |
|
| Gender |
|
|
|
| Male | 11 | 11 | – |
|
Female | 9 | 9 | – |
| Age (years) | 40.0±6.6 | 40.2±7.1 | 0.963 |
| Cause of
hemorrhage |
|
|
|
| CA | 3 | 3 | – |
| TA | 10 | 10 |
|
| CH | 2 | 2 |
|
| SSH | 5 | 5 |
|
| Follow-up
(months) | 10.5 (8–14) | 11 (7–15) | 0.858 |
| Disease eyes (n) | 28 | 27 |
|
| Left/Right | 13/15 | 12/15 | 0.883 |
| Pre-operative
BCVA | 1.05 (0.70–1.70) | 1.10 (0.70–1.70) | 0.986 |
| Final BCVA | 0.26 (0–0.60) | 0.22 (0–0.70) | 0.845 |
| Pre-operative IOP
(mmHg) | 15 (10–20) | 14.9 (10.5–20.5) | >0.05 |
| Operation times
(min) | 13.5 (10–16) | 42 (30–55) | <0.001 |
| Postoperative IOP at
day 1 (mmHG) | 13.5 (10–20) | 20 (10–35) | <0.001 |
| Normal IOP at day 1
(10–21 mmHg) | 100% (28/28) | 59.3% (16/28) | <0.001 |
| Intraoperative
complication of retinal break | 0% (0/28) | 14.8% (4/28) | 0.111 |
Preoperative examinations
All patients underwent history consultation, an
Early Treatment Diabetic Retinopathy Study examination was
performed (18) and the
best-corrected visual acuity (BCVA) (19) was recorded as LogMAR (20) visual examination. Intraocular
pressure (IOP), ophthalmology B-scan ultrasonography and fundus
photography were performed before the operation and IOP test was
repeated at day 1 post operation. B-scan ultrasonography and fundus
photography were performed to evaluate the posterior segment.
Vitreous hemorrhage occurred in 24 and 24 eyes in study and control
groups, respectively. The other 4 and 3 eyes were macular
hemorrhage. The normal range for intraocular pressure was defined
as between 10 and 21 mmHg.
Surgical methods for 25G and 20G
vitrectomy
All surgeries were conducted by the same experienced
doctor under local anesthesia (4 ml; 2% lidocaine) and using a
CONSTELLATION 25G system (Alcon, Inc., Hünenberg, Switzerland)
while Resight non-contact wide-angle lenses (Carl Zeiss Meditec AG,
Jena, Germany) were used to observe fundus. The surgical method
used was standardized three channels vitrectomy. Three channels
were made with three 25G catheter needles on 4-mm site away from
the limbus in the supertemporal, supernasal and infratemporal
quadrant. The infusion pressure was maintained at 25 mmHg, the
illumination level at H4, the suction pressure of 400 mmHg and the
cutting rate at 5,000 rpm. The vitrectomy consisted of core
vitrectomy, the creation of a posterior vitreous detachment,
peripheral vitrectomy and vitreous base shaving. The epiretinal
membrane was peeled with 25G forceps. For eyes with epiretinal
macular hemorrhage, the central vitreous body was cut first, then
the epiretinal macular hemorrhage was removed by suction pressure.
Exchange of intraocular fluid was performed in eyes with local
retinal detachment. Finally, the three cannulas were removed. Eight
eyes were tamponaded with Octafluoropropane C3F8 (Tianjin Jingming
New Technology Development Co., Ltd., Tianjin, China); the other 20
eyes were not tamponaded with any material. Sclerotomies were
sutured with 7–0 VICRYL (Ethicon US, LLC, Cincinnati, OH, USA),
conjunctival peritomies were sutured with 8–0 VICRYL.
The 20G vitrectomy was performed using the standard
20G Accurus system (Alcon, Inc.). The surgery procedure was
according to the routine operating methods (9). Nine eyes were tamponaded with C3F8 and
4 eyes tamponaded with silicone oil.
Statistical analysis
Statistical analysis was performed using SAS,
version 9.2 (SAS Institute, Cary, NC, USA). Data are expressed as
the mean ± standard deviation or median (range), and Student's
t-test was used to compare the difference between two groups or
pre- vs. post-operation. The Chi-square test or Fisher's exact test
was used to calculate the probability value for the comparison of
dichotomous variables. A two-sided P-value of <0.05 was
considered statistically significant.
Results
Patient characteristics and
pre-operative data
The patient characteristics for the 25G vitrectomy
(study) group and the 20G vitrectomy (control) group are shown in
Table I. All patients recovered from
a coma after neurosurgery or neurological treatment. The average
interval between diagnosis of Terson syndrome and vitreous surgery
was 6 weeks (range, 4–8 weeks). For patients that underwent the 25G
vitrectomy, the preoperative visual acuity of LogMAR was 1.05
(range, 0.70–1.70), and for those who underwent the 20G vitrectomy
it was 1.10 (range, 0.70–1.70). The intraocular pressure of all
eyes was normal.
Operation time
The average operative time was 14 min (range, 10–16
min) for the 25G (study) group and it was 42 min (range, 30–55 min)
for the 20G (control) group. The difference in the operative times
between the two groups was statistically significantly different
(P<0.001).
Postoperative visual acuity
Amongst the 25G (study) group, the preoperative
visual acuity of LogMAR was 1.05 (range, 0.70–1.70) while
postoperative visual acuity of LogMAR was 0.26 (range, 0–0.60);
these pre- and post-operative values were significantly different
(P<0.001). Amongst the 20G (control) group, the pre-operative
visual acuity of LogMAR was 1.10 (range, 0.70–1.70), while
postoperative visual acuity of LogMAR was 0.22 (range, 0.00–0.70);
these pre- and post-op values were also significantly different
(P<0.001). Significant visual improvement occurred in all eyes
following vitrectomy.
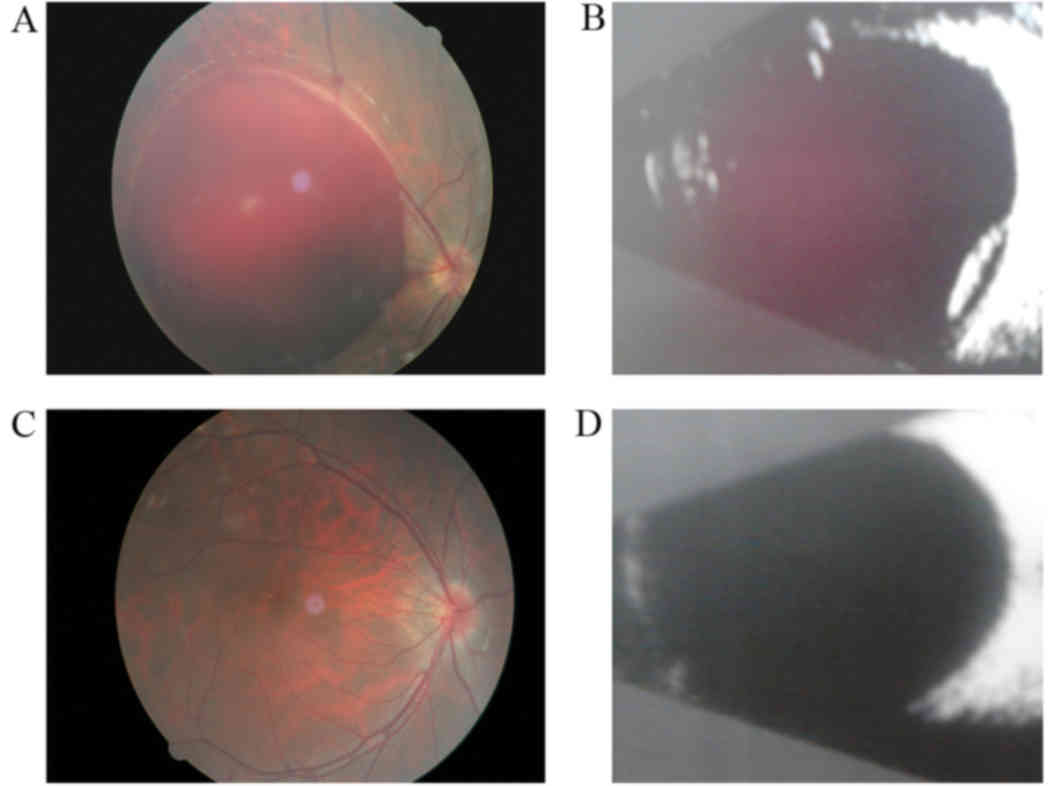
Intraoperative fundus
In all eyes that underwent 25G vitrectomy,
incomplete posterior vitreous detachment (PVD) was found in 18 eyes
(64.3%), epiretinal membrane in 8 eyes (28.6%), macular epiretinal
hemorrhage in 5 eyes (17.8%). In all eyes that underwent 20G
vitrectomy PVD was found in 17 eyes (63%), epiretinal membrane in 9
eyes (33%) and macular epiretinal hemorrhage in 5 eyes (19%). There
was not a significant difference between the two groups (Fig. 1).
Complications
Postoperativly, amongst the 25G vitrectomy group,
cataracts occurred in 2 eyes (7.1%), the intraocular pressure was
normal at day 1 in all 28 eyes and retinal detachment and
hemorrhage did not occur in any eyes. Amongst the patients that
underwent the 20G vitrectomy (control group), cataracts occurred in
3 eyes (11%) intraocular pressure was normal at day 1 in 16 eyes
(59.3%) and retinal detachment and hemorrhage in 3 eyes
(11.1%).
Discussion
In the present study, we compared the outcomes and
therapeutic effects of 20 patients that underwent the 25G
vitrectomy under Resight non-contact wide-angle lenses with a group
of 20 matched patients that underwent the standard 20G vitrectomy
operation (control group). A total of 28 eyes with Terson syndrome
underwent 25G vitrectomy and 27 underwent 20G vitrectomy using
Resight non-contact wide-angle lenses. A significant therapeutic
effect was achieved in both groups and the visual acuity was
improved in all eyes after vitrectomy. The mean operative time was
significantly shorter in the study group 14 min (range, 10–16 min)
compared with 42 min (range, 30–55 min) in the control group. The
postoperative intraocular pressure was within the normal range at
day 1 in 100% of eyes in the study group and 59% in the control
group. Slight inflammatory reaction occurred in all eyes in study
group postoperatively, while several eyes in control group had
inflammatory reaction a more severe, but this result comes from
subjective feelings without objective indexes.
Vitrectomy has been shown to be effective at
improving BCVA in previous studies in the majority of patients
(7). For example visual acuity of
20/40 or better has been achieved in 76–93% of patients (7,21,22).
Variations in improving BCVA are apparently related to patient age
and time elapsed between hemorrhage and surgery (7,23).
However, the majority of studies have used a 20G system. The
results of the present study suggest that 25G vitrectomy produced
similar improvements in visual acuity in patients with Terson
syndrome to procedures involving 20G.
The main advantages suggested by these results of
the 25G system over the standard 20G system are significantly
shorter operation time and higher numbers of eyes with an
intraocular pressure within the normal range one day after the
procedure. A shorter operation time would be expected to decrease
the cost of the procedure and is likely to be a major benefit to
both the patient and the surgical team. However, there is some
evidence that increased intraocular pressure after vitroretinal
surgery might increase the risk of developing secondary glaucoma
(24). Intraocular pressure often
increases immediately after vitrectomy (25), and this was the case in this study.
However, all of the eyes in the study group were within the normal
range at day one but only 59% of those in the control group.
Usually the increased intraocular pressure after vitrectomy is
transient and easily managed (25).
Long-term follow-up is needed to evaluate the risk to the eyes of
this difference between the groups.
Despite no statistical difference in intraoperative
complications between the groups, the result that there were no
cases of retinal break in the study group but there were four in
the control group suggests that a larger study is needed to fully
evaluate the differences in complications between the groups. The
higher number of retinal breaks suggests that the control group is
likely to be at greater risk of retinal detachment, although the
true risk this poses will have to be evaluated by long-term
follow-up. The occurrence of retinal detachment with or without
proliferative viteroretinopathy in Terson syndrome has been
described previously (26). Retinal
detachment is one of the more common postoperative complications
reported in 9 and 32% of eyes in other studies where Terson
syndrome was treated by vitrectomy (7,22).
However, none of the eyes in this study exhibited retinal
detachment and vitreous hemorrhage after vitrectomy, which may be
due to the small number of cases. Other common complications
include cataracts, and in the present study cataracts occurred in
two eyes tamponaded with C3F8 in the study group, a rate of 7%.
Visual acuity improves after phacoemulsification (7). The development of nuclear sclerosis
following vitreous surgery is a well-documented complication
(22). It has been noted that 7–10%
of patients developed cataracts after vitrectomy for Terson
syndrome, and other studies have reported rates of 16, 20 and 32%
(7,22,23).
In the present study, the epiretinal membrane was
observed in 8 eyes (28.6%) in the 25G virectomy group and in 9 eyes
(33%) in the 20G vitrectomy group. Other investigations have also
observed the occurrence of the membrane in Terson syndrome
(7,21,22,27). It
is likely that some gaps developed in the internal limiting
membrane during the evolution of a dome-shaped hemorrhage, and
later the proliferation of glial cells penetrate these gaps
resulting in the epiretinal membrane. Another reason could be that
the release of growth factors in the hemorrhage stimulates cell
migration and proliferation.
The present study included some limitations. As a
retrospective study the patients were not randomly allocated into
groups rather they were assessed according to the treatment they
received, this may have introduced some bias into the results. The
study size was relatively small; a larger sample from multiple
centers might provide statistical evidence of a difference in the
rates of complications between the groups. The patients should be
followed up for a longer period of time, this would provide more
details about the true rate of complications that might occur many
months after the procedure, and whether the visual improvements are
long term.
This study suggests that, in comparison with 20G
vitrectomy, 25G vitrectomy for Terson syndrome provides similar
improvements in visual acuity but with shorter operation time and
better intraocular pressure at day one. These results suggest that
25G vitrectomy should be considered for the treatment of Terson
syndrome.
Acknowledgements
The authors thank the staff members of this trial,
their colleagues and all the study staff for their efforts in
collecting and ensuring the accuracy and completeness of all the
data.
References
|
1
|
Kuhn F, Morris R, Witherspoon CD and
Mester V: Terson syndrome. Results of vitrectomy and the
significance of vitreous hemorrhage in patients with subarachnoid
hemorrhage. Ophthalmology. 105:472–477. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fountas KN, Kapsalaki EZ, Lee GP, Machinis
TG, Grigorian AA, Robinson JS, Vergados I and Theodosiadis PG:
Terson hemorrhage in patients suffering aneurysmal subarachnoid
hemorrhage: Predisposing factors and prognostic significance. J
Neurosurg. 109:439–444. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stienen MN, Lücke S, Gautschi OP and
Harders A: Terson haemorrhage in patients suffering aneurysmal
subarachnoid haemorrhage: A prospective analysis of 60 consecutive
patients. Clin Neurol Neurosurg. 114:535–538. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rheinboldt M, Francis K, Parrish D, Harper
D and Blase J: Terson syndrome in conjunction with ruptured
intracranial aneurysm and penetrating intracranial injury: A review
of two cases. Emerg Radiol. 21:215–218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kapoor S: Terson syndrome: An often
overlooked complication of subarachnoid hemorrhage. World
Neurosurg. 81:e42014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Errera MH, Barale PO, Ounnoughene Y, Puech
M and Sahel JA: 25-Gauge transconjunctival vitrectomy in a case of
bilateral epiretinal membrane associated with a Terson syndrome. J
Fr Ophtalmol. 32:268–272. 2009.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garweg JG and Koerner F: Outcome
indicators for vitrectomy in Terson syndrome. Acta Ophthalmol.
87:222–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murjaneh S, Hale JE, Mishra S, Ling RH and
Simcock PR: Terson's syndrome: Surgical outcome in relation to
entry site pathology. Br J Ophthalmol. 90:512–513. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gallemore R, Thomas E and Boyer DS:
Minimally Invasive Vitreoretinal Surgery. Review of Οphthalmology.
9:11–16. 2002.
|
|
10
|
Fujii GY, De Juan E Jr, Humayun MS,
Pieramici DJ, Chang TS, Awh C, Ng E, Barnes A, Wu SL and
Sommerville DN: A new 25-gauge instrument system for
transconjunctival sutureless vitrectomy surgery. Ophthalmology.
109:1807–1813. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujii GY, De Juan E Jr, Humayun MS, Chang
TS, Pieramici DJ, Barnes A and Kent D: Initial experience using the
transconjunctival sutureless vitrectomy system for vitreoretinal
surgery. Ophthalmology. 109:1814–1820. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen E: 25-Gauge transconjunctival
sutureless vitrectomy. Curr Opin Ophthalmol. 18:188–193. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang CJ, Chang YH, Chiang SY and Lin LT:
Comparison of clear corneal phacoemulsification combined with
25-gauge transconjunctival sutureless vitrectomy and standard
20-gauge vitrectomy for patients with cataract and vitreoretinal
diseases. J Cataract Refract Surg. 31:1198–1207. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagpal M, Wartikar S and Nagpal K:
Comparison of clinical outcomes and wound dynamics of sclerotomy
ports of 20, 25, and 23 gauge vitrectomy. Retina. 29:225–231. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhengyu S, Fang W, Ying F and Qinghua Q:
The experimental research of rabbit's sclerotomy sites undergoing
transconjunctival sutureless vitrectomy. Curr Eye Res. 32:647–652.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aras C, Ucar D, Koytak A and Yetik H:
Scleral buckling with a non-contact wide-angle viewing system.
Ophthalmologica. 227:107–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park SW, Kwon HJ, Kim HY, Byon IS, Lee JE
and Oum BS: Comparison of scleral buckling and vitrectomy using
wide angle viewing system for rhegmatogenous retinal detachment in
patients older than 35 years. BMC Ophthalmol. 15:1212015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Writing Committee for the Diabetic
Retinopathy Clinical Research Network, ; Fong DS, Strauber SF,
Aiello LP, Beck RW, Callanan DG, Danis RP, Davis MD, Feman SS,
Ferris F, et al: Comparison of the modified early treatment
diabetic retinopathy study and mild macular grid laser
photocoagulation strategies for diabetic macular edema. Arch
Ophthalmol. 125:469–480. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pei-Pei W, Shi-Zhou H, Zhen T, Lin L, Ying
L, Jiexiong O, Wen-Bo Z and Chen-Jin J: Randomised clinical trial
evaluating best-corrected visual acuity and central macular
thickness after 532-nm subthreshold laser grid photocoagulation
treatment in diabetic macular oedema. Eye (Lond). 29:313–322. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mason JO III, Nixon PA and White MF:
Intravitreal injection of bevacizumab (avastin) as adjunctive
treatment of proliferative diabetic retinopathy. Am J Ophthalmol.
142:685–688. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sharma T, Gopal L, Biswas J, Shanmugam MP,
Bhende PS, Agrawal R, Shetty NS and Sanduja N: Results of
vitrectomy in Terson syndrome. Ophthalmic Surg Lasers. 33:195–199.
2002.PubMed/NCBI
|
|
22
|
Ritland JS, Syrdalen P, Eide N, Vatne HO
and Øvergaard R: Outcome of vitrectomy in patients with Terson
syndrome. Acta Ophthalmol Scand. 80:172–175. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gnanaraj L, Tyagi AK, Cottrell DG,
Fetherston TJ, Richardson J, Stannard KP and Inglesby DV: Referral
delay and ocular surgical outcome in Terson syndrome. Retina.
20:374–377. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tranos P, Asaria R, Aylward W, Sullivan P
and Franks W: Long term outcome of secondary glaucoma following
vitreoretinal surgery. Br J Ophthalmol. 88:341–343. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Costarides AP, Alabata P and Bergstrom C:
Elevated intraocular pressure following vitreoretinal surgery.
Ophthalmol Clin North Am. 17:507–512. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Velikay M, Datlinger P, Stolba U, Wedrich
A, Binder S and Hausmann N: Retinal detachment with severe
proliferative vitreoretinopathy in Terson syndrome. Ophthalmology.
101:35–37. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yokoi M, Kase M, Hyodo T, Horimoto M,
Kitagawa F and Nagata R: Epiretinal membrane formation in Terson
syndrome. Jpn J Ophthalmol. 41:168–173. 1997. View Article : Google Scholar : PubMed/NCBI
|