Introduction
Depression is a common psychiatric disorder
characterized by a persistent low mood, feeling of helplessness and
suicidal tendencies, which causes enormous personal suffering and
economic loss, as well as sluggish thought patterns and cognitive
function (1). As the underlying
mechanisms of depression are relatively complex and are not
explicit, currently available treatments continue to have
significant limitations, including low response rates and frequent
remission, treatment resistance, severe adverse effects and a
delayed clinical response (weeks to months) (2). Therefore, it is important to probe and
develop more effective anti-depressant medications. It has been
reported that depression is associated with neuronal atrophy and
loss, including reduction in the number of spine synapses, one of
the key points of connection between neurons (3). The leading hypothesis on depression
suggests that neuronal plasticity and impaired synaptic function
are critical for mediating behavioral responses to anti-depressants
(4,5). Studies have indicated that the
impairment of synaptic plasticity in the hippocampus may be a core
factor in the pathophysiology of depression (6,7).
Brain-derived neurotrophic factor (BDNF) is the key
regulator of neuronal plasticity and strongly influences
synaptogenesis, spine formation, neuronal survival and adult
hippocampal neurogenesis (8–10). There is growing evidence that links
BDNF to depression; post-mortem analyses of depressive patients
have found a reduction of BDNF in brain and serum (11,12).
Decreased BDNF mRNA expression in the hippocampus contributes to
depression-like behavior in rats (13), while brain infusion of BDNF in
animals produced anti-depressant-like effects (14). The glycogen synthase kinase-3 (GSK-3)
pathway is thought to have a principal role in long-term depression
and long-term potentiation in the hippocampus (15). In addition, studies including
behavioral assessments indicated that GSK-3β dysregulation promotes
mood disorders (16). The classical
mood stabilizer lithium is a direct inhibitor of GSK-3 (17). Pre-clinical studies on animal models
have shown anti-depressant-like effects of GSK-3β inhibitors
(18,19). These results suggested that
regulating the stimulation of BDNF and suppression of GSK-3β may be
a potential approach for treating depression.
Traditional Chinese Medicine is gaining attention
for its mild nature, its tendency to maintain an individual balance
and its multiple target effect (20). Jieyu chufan capsules (JYCF) consist
of four Chinese herbs: Gardenia jasminoides Ellis (Zhi-Zi),
Forsythia suspensa Vahl (Lian-Qiao), Cortex Magnolia
officinalis (Hou-Pu) and rhizoma Pinelliae ternatae
(Ban-Xia). They are a novel Chinese herbal medicine for the
treatment of depression. The JYCF formula is derived from the
zhi-zi-hou-pu and ban-xia-hou-pu decoctions. The first description
of these two decoctions was recorded in Shan-han-lun and
Jin-gui-yao-lue written by Zhang Zhongjing during the Han Dynasty
(150–219 A.D.) and they have historically been used to relieve
depression symptoms, anxiety disorders, obsessive-compulsive
disorders and hysteria (21–23). Gardenia jasminoides Ellis is
the major active ingredient of JYCF, but the molecular mechanism
underlying these decoctions has remained elusive.
In the present study, the anti-depressant effects of
JYCF were first assessed in a mouse model of unpredictable chronic
mild stress (UCMS)-induced depression. Evidence was provided that
JYCF suppressed the impairment of synaptic density by regulating
BDNF and GSK-3β activity, suggesting that JYCF may potentially be
developed into a drug for the treatment of depression.
Materials and methods
Ethics statement
All animal care and experimental procedures complied
with the National Institute of Health Guidelines for the Care and
Use of Laboratory Animals. All procedures that involved animals
were in compliance with the European Community Council Directive of
November 24, 1986 and approval was granted by the Ethics Committee
of Nanjing Drum Tower Hospital (Nanjing, China; permission no.
2014-085-01). All surgical procedures were performed under sodium
pentobarbital anesthesia and all efforts were made to reduce
suffering.
Animals and groups
A total of 60 healthy adult female C57BL/6 mice
(age, 4–5 weeks; weight, 18–21 g), were provided by the Animal
Experiment Center of Nanjing Drum Tower Hospital (the Affiliated
Hospital of Nanjing University Medical School; Nanjing, China). The
animals were kept in the laboratory (5 per cage) for 2 weeks prior
to experimentation, with free access to food and water. The animal
room had a temperature of 23±1.5°C with 50% relative humidity and a
12-h light/dark cycle (light on from 7:00 am to 7:00 pm). The
behavioral experiments were performed during the light phase. Mice
were randomly divided into five groups with 12 mice in each group
including: Normal control group (NC group), model control group (MC
group), JYCF 1.25 g/kg group, JYCF 2.5 g crude drug/kg group and
JYCF 5 g crude drug/kg group (JYCF groups).
Drugs and reagents
JYCF powder was provided by Shijiazhuang Yiling
Pharmaceutical Factory (Shijiazhuang, China). All medicinal
components of JYCF were ground to superfine (≤10 µm) powder by a
micronizer and prepared as 0.38-g capsules. The NC and MC groups
received no medication, while JYCF was administered at 1.25, 2.5 or
5 g/kg to the three JYCF groups. The outer coating of the capsule
was removed, the drugs were dissolved in distilled water and
intragastrically administered at 10 ml/kg. At the same time, mice
in the NC and MC groups were given 10 ml/kg distilled water. JYCF
or water was administered 1 h prior to each behavioral test to
avoid potential stress induced by gavage. Administration was
continuous during the entire three weeks.
Establishment of the UCMS model
The UCMS model was established following a modified
Willner's method (24,25). Mice of the NC group were treated
normally, while mice in the other four groups were subjected to
UCMS daily between 11:00 am and 12:00 pm for 3 successive
weeks.
Mice were housed individually in cages and exposed
to the following stressors: Food or water deprivation (24 h), damp
sawdust (12 h), forced swimming in cold water (4°C) for 4 min, tail
suspension (5 min), inversion of light/dark cycle (24 h), cage
tilting (45°C, 12 h) and tail nipping (10 times for 5 sec per
time). To prevent familiarization and provide an unpredictable
feature, all stressors were randomly scheduled over a 1-week period
and repeated throughout the 3-week experiment.
Sucrose preference test
The sucrose preference test was used for evaluating
depression-like behavior and was performed as described in previous
studies (26,27). Prior to the test, all mice were given
a free choice between two bottles-one with 1% sucrose solution and
another with tap water-over 24 h. To prevent potential location
preference, the position of the bottles was changed after 12 h.
Mice were deprived of food and water for 24 h prior to the test.
Bottles were weighed prior to each test and then re-weighed one
hour later. The sucrose intake was measured by comparing the weight
of the bottles prior to and after the test.
Forced swimming test
A forced swimming test was used for evaluating
depression-like behavior. A modified protocol was used as described
by Porsolt et al (28). In
brief, 30 min after JYCF injection, mice were forced to
individually swim in a glass cylinder (diameter, 13 cm; height, 19
cm) filled with water (25±1°C) at a depth of 10 cm. The water was
changed after each trial. All animals were forced to swim for 5 min
and the immobility time (including passive swimming) during the
test was recorded. Immobilization and passive swimming were defined
as the mouse floating in the water without struggling, making only
those movements necessary to keep its head above the water.
Observers were blinded to grouping of mice.
Open field test
An open field test was used for evaluating emotional
and ambulatory behavior. Apparatus and methods used were similar to
those previously described (29).
Mice were placed individually in the dark in a wooden box
(100×100×40 cm) with the floor divided into 25 (5×5) squares. The
apparatus was illuminated with a red bulb (50 W) on the ceiling. In
each test, mice were placed in the central area and allowed to
explore freely for 5 min. During the test time, the number of
crossings (squares crossed with all paws) and rearings (raising of
front paws) was recorded. The open field arena was thoroughly
cleaned between tests. The observers were blinded to the treatment
of the mice.
Elevated plus maze test
The elevated plus maze test was used for evaluating
anxiety-associated behavior (30).
The apparatus consisted of two opposing open arms (45×10 cm) and
two closed arms (45×10×38 cm) that extend from a central platform
(10×10 cm), elevated 50 cm above the floor. During testing, the
mice were placed individually on the central platform facing the
open arms of the maze and allowed to explore the maze for 5 min.
Behavior was monitored using a video camera and analyzed with a
computerized tracking system (Ethovision 3.1.16; Noldus IT,
Wageningen, The Netherlands). Time spent in the open and closed
arms (and their edges) was recorded. The anxiety level was measured
by the time spent in the open arm. The observer was blinded to
group classification to avoid bias.
Morris water maze (MWM) test
The MWM test was used for evaluating spatial
learning and memory and was performed as previously described
(31). In brief, during the
acquisition phase trials (days 1–5), mice were allowed to stay on
the platform for 5 sec if they were able to find it within 60 sec.
If they could not, 60 sec was regarded as the escape latency and
mice were allowed to remain on the platform for 20 sec to memorize
the platform location. During the probe trial phase, after removing
the platform, mice were allowed to swim for 60 sec for two tests.
The test was performed in four training trials per day for six
consecutive days. All movements were recorded by a computerized
video system (ANY-maze; Stoelting, Wood Dale, IL, USA). In order to
exclude the influence of artificial factors, the MWM test was
performed by two observers who were blinded to the group
assignments of the mice.
Tissue preparation
After behavioral tests, mice were deeply
anesthetized with intraperitoneal injection of sodium pentobarbital
(50 mg/kg body weight). Bilateral hippocampi were rapidly dissected
and homogenized in lysis buffer (Beyotime Institute of
Biotechnology, Inc., Haimen, China), centrifuged at 12,000 × g for
30 min at 4°C and the supernatants were collected for western blot
assay. Whole mouse brains were removed, washed with 1 X
phosphate-buffered saline and prepared for Golgi silver
staining.
Western blot analysis
Total protein was estimated using a BCA Protein
Quantification kit (Bioworld Technology Inc., St. Louis Park, MN,
USA). Samples were then mixed with 5X loading buffer and boiled for
5 min. Equal amounts of protein extract (30 µg) were separated by
10% SDS/PAGE and then transferred onto polyvinylidene difluoride
membranes. After being blocking with 5% powdered skimmed milk for
1.5 h, membranes were incubated overnight at 4°C with primary
antibodies to GSK-3β (cat. no. 12,456), phosphorylated (p)-GSK-3β
(cat. no. 5,558) or postsynaptic density protein-95 (PSD-95; cat.
no. 3450; all 1:1,000 dilution), p-cyclic adenosine monophosphate
response element binding protein (p-CREB; cat. no. 9,198) or
Synaptophysin (Syn; cat. no. 5,461; 1:500 dilution), CREB (cat. no.
9,197; 1:500 dilution), p-AKT, (cat. no. 4,060; 1:500 dilution),
AKT (all Cell Signaling Technology, Inc., Danvers, MA, USA; cat.
no. 9,272; 1:1,000 dilution) or β-tubulin (Bioworld Technology,
Inc.; cat. no. bs1482; 1:5,000 dilution), or BDNF (Epitomics;
Abcam, Cambridge, UK; cat. no. 3160-1; 1:500 dilution).
Subsequently, membranes were treated with horseradish
peroxidase-conjugated secondary antibodies (Bioworld Technology,
Inc.; cat. no. bs13278; 1:5,000 dilution) and incubated for 2 h at
room temperature. The blots were visualized with chemiluminescence
reagents using an ECL kit (Bioworld Technology, Inc.). Optical
density of the bands was determined using Quantity One Software
(4.62; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Golgi staining
Golgi staining was performed following the
manufacturer's instructions (FD Rapid GolgiStain Kit; FD
NEurotexhnologies, Inc., Ellicott City, MD, USA). In brief, whole
brains were impregnated with silver solution for 2 weeks and then
sectioned at 100 µm on a cryostat. Sections were mounted on
adhesive microscope slides and air-dried at room temperature in the
dark. For synaptic density quantification, the number of synapses
was counted along dendritic segments of equivalent length from the
CA1 region of the hippocampi.
Statistical analysis
All statistical analysis was performed using SPSS
16.0 software (SPSS Inc., Chicago, IL, USA). Values are expressed
as the mean ± standard error of the mean. Differences between mean
values were evaluated using one-way analysis of variance with
post-hoc tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
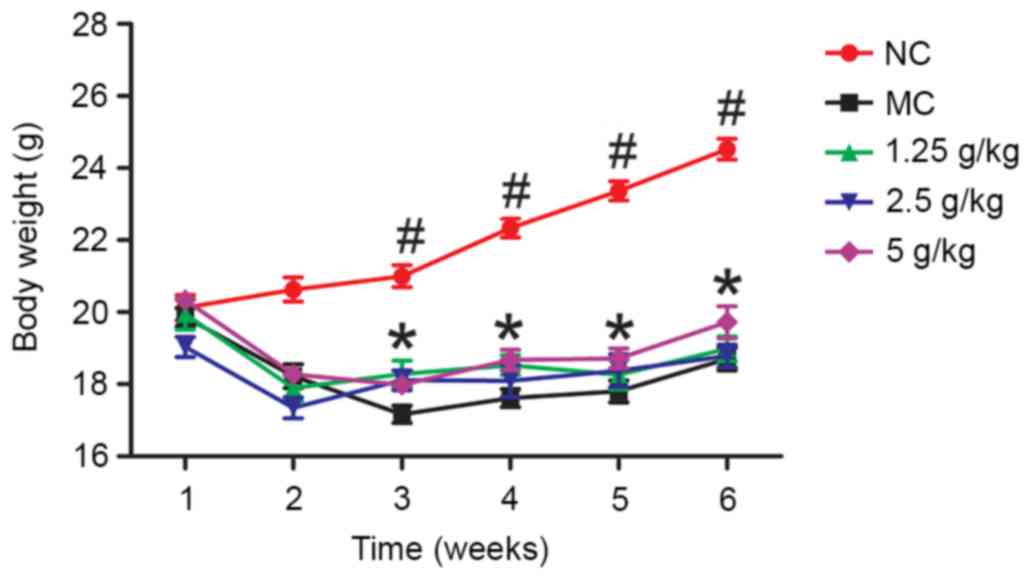
Effects of JYCF on body weight
Fig. 1 shows the body
weight in the five groups during the experimental period. After
week 1, there were no significant differences between the five
groups (P>0.05). After 6 weeks, the body weight in the MC group
was significantly lower than that in the NC group (P<0.01) and
in the 5 g/kg JYCF group (P<0.05).
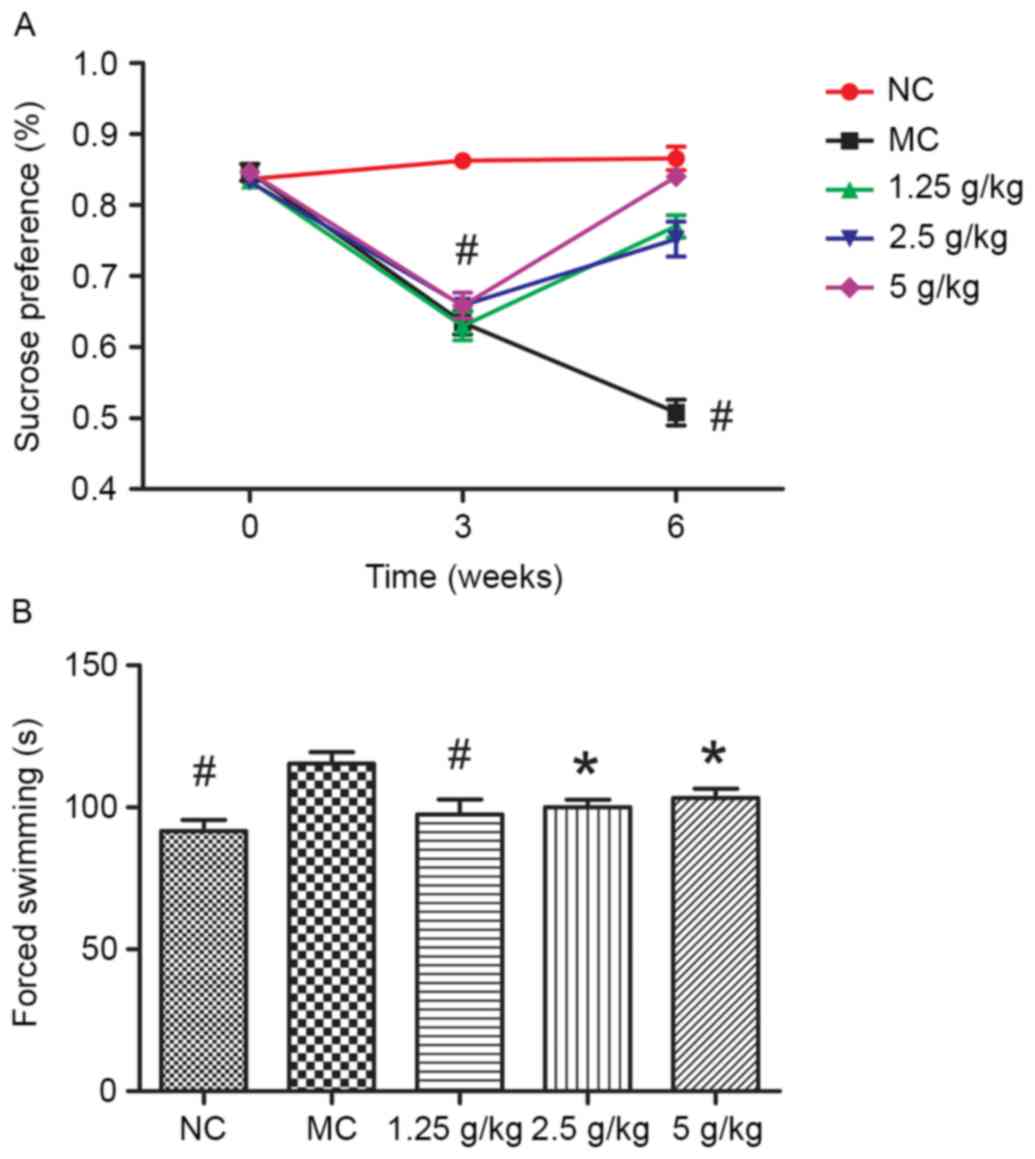
JYCF affects depression-like behavior
in UCMS mice
Fig. 2A shows that
the sucrose preference rate in the MC group was lower than that in
the NC group (P<0.01). Compared with the MC group, after regular
treatment with JYCF, the sucrose intake rates in UCMS-exposed mice
were all increased (P<0.01).
Fig. 2B shows the
results of the forced swimming tests. Compared with the NC group,
the total immobilized time in the MC group was significantly longer
compared with that in the other groups (P<0.05). After regular
treatment with JYCF, the total immobilized time in the UCMS-exposed
mice was significantly reduced (1.25 g/kg, P<0.01; 2.5 and 5
g/kg, P<0.05).
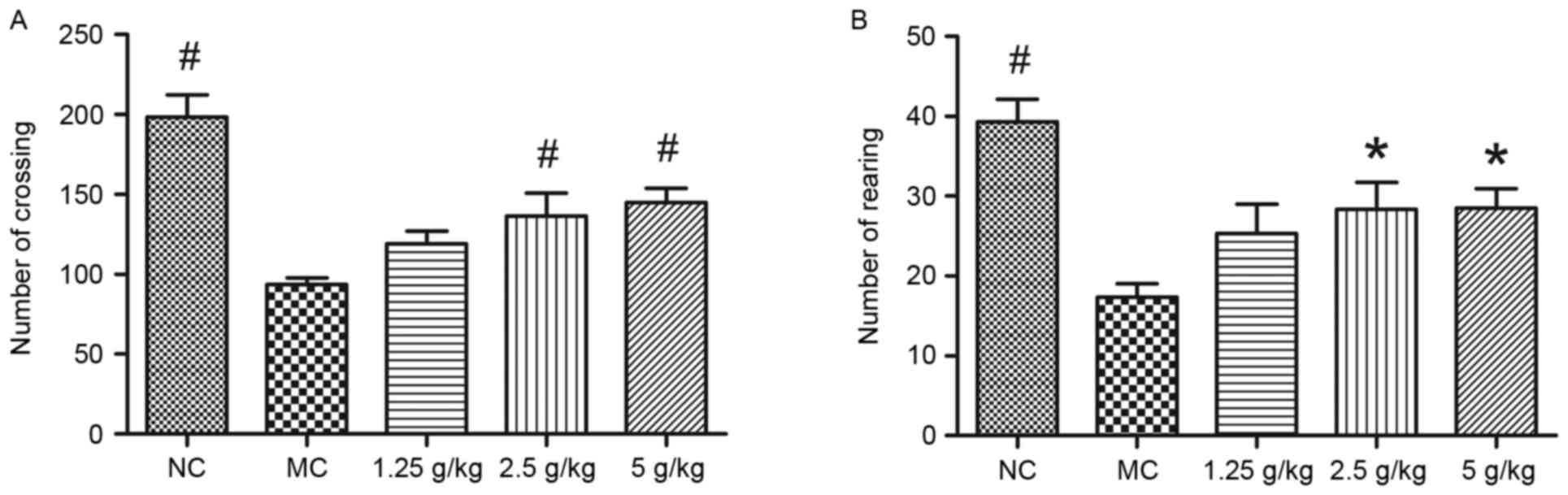
JYCF affects anxiety-like behavior in
UCMS mice
Fig. 3 shows the
results of the open-field test. Compared with the NC group, the
number of square crossings and rearings in the MC group was
significantly decreased, which was significantly inhibited by
treatment with 2.5 and 5 g/kg JYCF (P<0.05). However, there were
no differences in the number of square crossings and rearings
between the MC group and the 1.25 g/kg JYCF group (P>0.05;
Fig. 3A and B).
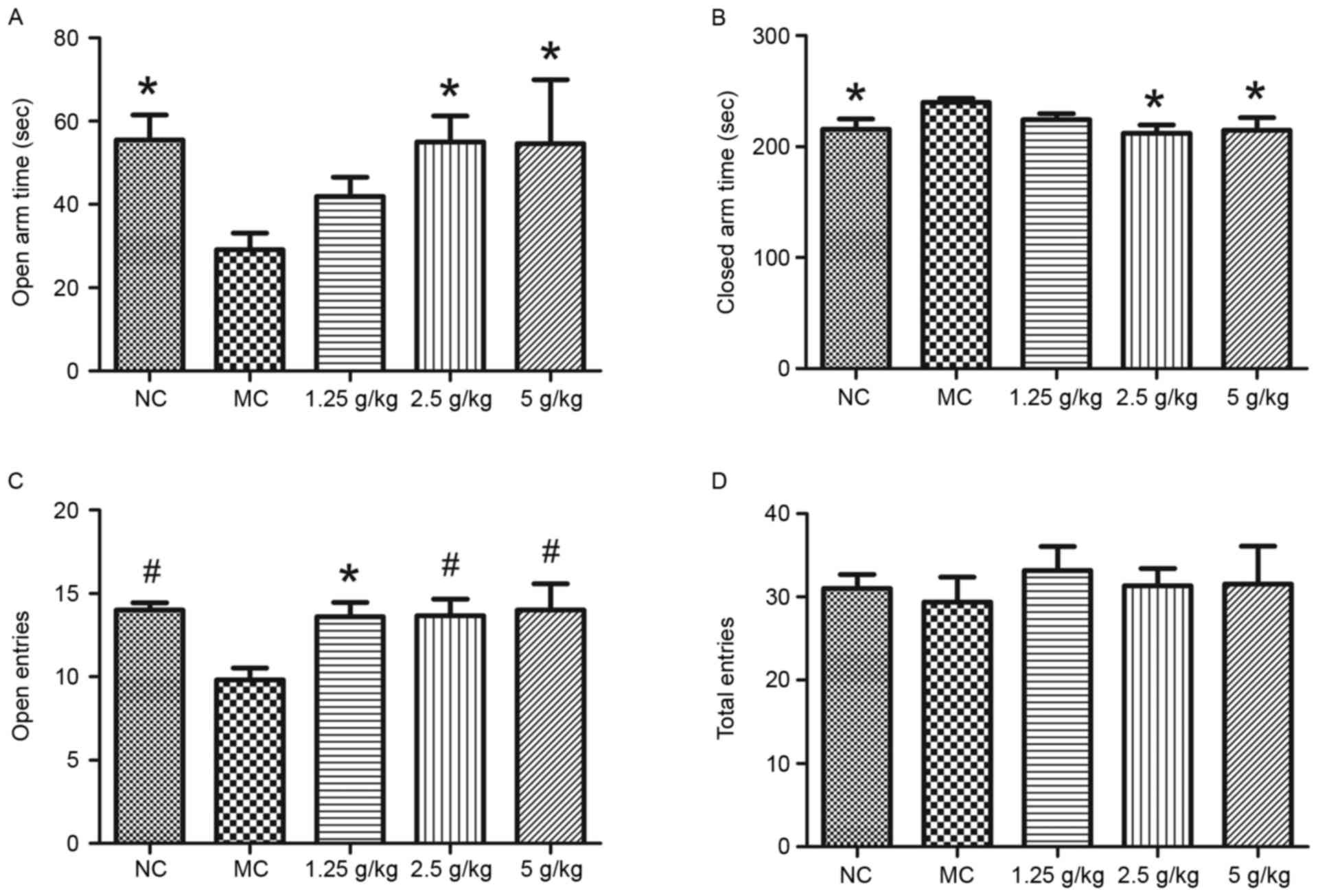
Fig. 4 shows the
results of the elevated plus maze test. Compared with those in the
NC group, mice in the MC group spent significantly less time in the
open arm and more time in the closed arm, which was reversed by
treatment with JYCF at 2.5 and 5 g/kg (P<0.05; Fig. 4A and B). The number of entries in the
open arms in the MC group was also significantly decreased compared
with that in the NC group, which was reversed by JYCF at all of the
concentrations tested (P<0.05; Fig.
4C). The number of total entries was not significantly
different between the five groups (Fig.
4D).
JYCF affects spatial learning and
memory in UCMS mice
Fig. 5 shows the
results of the MWM test. During the acquisition phase trials (days
1–5), the mean escape latency and search distance of MC mice was
significantly increased compared with that in the NC group
(P<0.05). However, compared with the MC group, mice in all JYCF
groups showed a significant improvement in escape latency during
the 5-days training period, and the distance in the 5 g/kg JYCF
group was decreased during the 5-day training period (P<0.05;
Fig. 5A and B). During the probe
trial, the number of platform crossings in the MC group was lower
than that in the NC group and MC mice spent significantly less time
in the target quadrant, which was significantly improved by JYCF at
5 g/kg (P<0.05; Fig. 5C and
D).
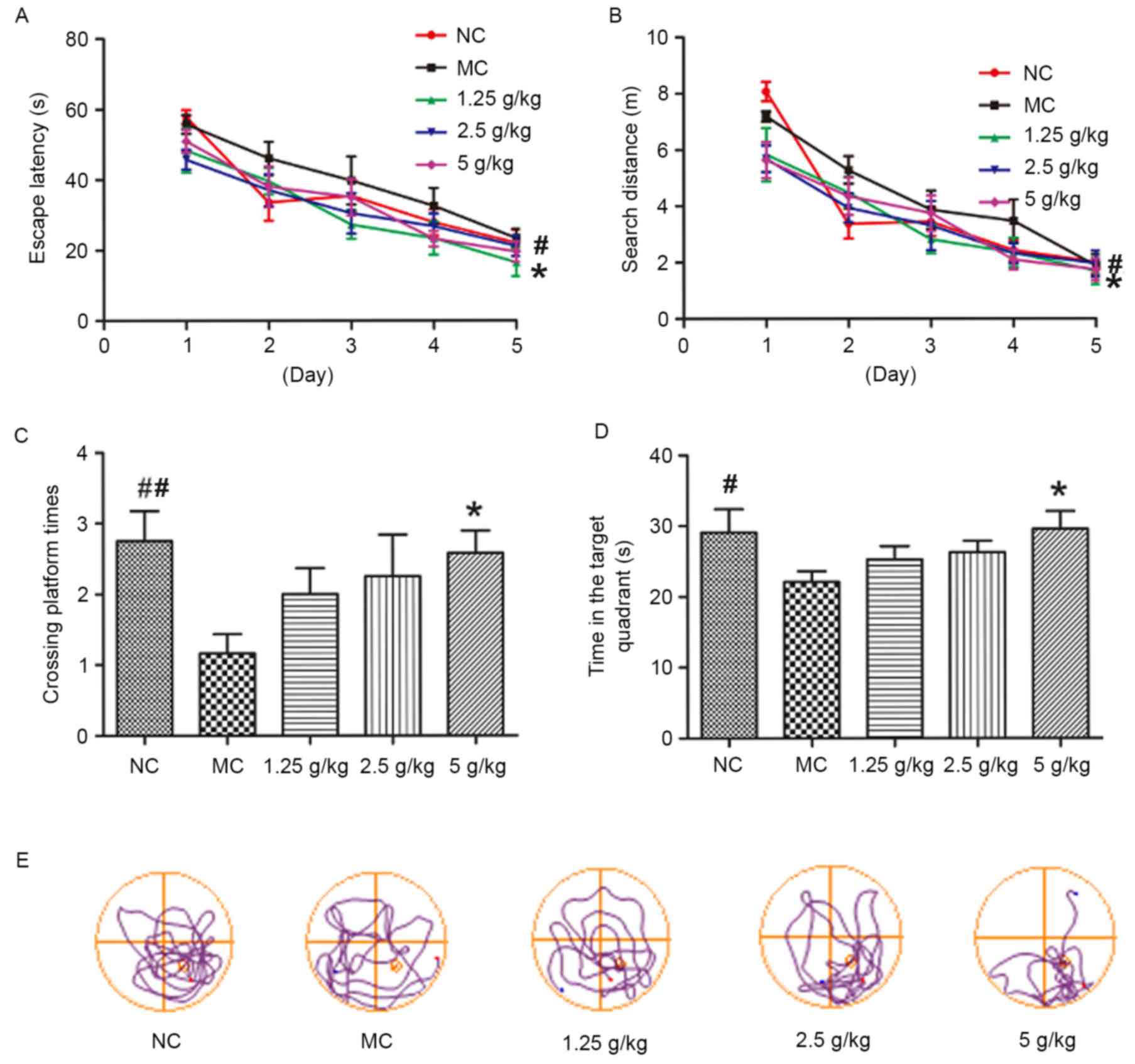 | Figure 5.JYCF improves cognitive impairment in
mice subjected to unpredictable chronic mild stress. (A) Escape
latency for searching the submerged platform in MC group mice was
significantly increased compared with the NC group, and compared
with the MC group, all JYCF groups exhibited a significant decrease
in escape latency. (B) Total distance for reaching the submerged
platform in the MC group mice was significantly increased compared
with the NC group, and the distance was decreased in the 5 g/kg
JYCF group over the 5-day training period compared with the MC
group. (C) 5 g/kg JYCF treatment significantly increased the number
of times the platform of MC mice in the probe trials and (D) time
spent in the target quadrant, as determined in training trials.
Values are expressed as the mean ± standard error of the mean of
three independent experiments. (E) Representative track plots
indicating the path length to find the platform position for each
group are shown. #P<0.05, ##P<0.01 NC
vs. MC group; *P<0.05, **P<0.01 JYCF vs. MC group. NC,
negative control; MC, model control group; 1.25/2.5/5 g/kg, model
mice treated with 1.25/2.5/5 g/kg JYCF; JYCF, Jieyu chufan. |
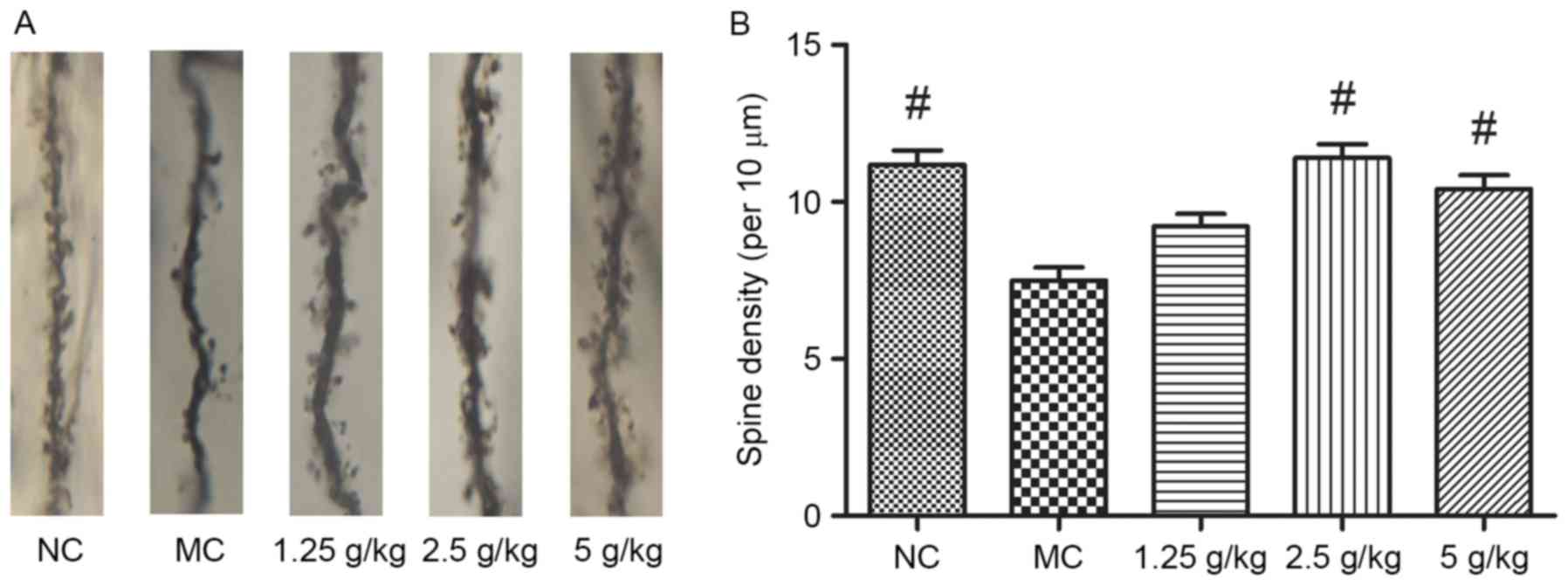
JYCF increases the synaptic density in
the brains of UCMS mice
As shown in Fig. 6,
synaptic density in the hippocampal neurons of MC mice was
significantly decreased after 6 weeks of UCMS (P<0.01). Compared
with the MC group, treatment with 2.5 and 5 g/kg JYCF significantly
increased the synaptic density in the hippocampal neurons of
UCMS-treated mice (P<0.01), while increases in the 1.25 g/kg
JYCF group were not statistically significant (P>0.05).
JYCF increases the expression of
synapsis-associated proteins
To further determine whether JYCF affected
synapsis-associated proteins, the levels of PSD-95, Syn, BDNF and
p-CREB were investigated. As shown in Fig. 7, exposure to UCMS for 6 weeks caused
a significant decrease in the levels of these proteins in the
hippocampi of MC mice as compared to those in the NC group, which
was significantly reversed by treatment with JYCF (2.5 and 5
g/kg).
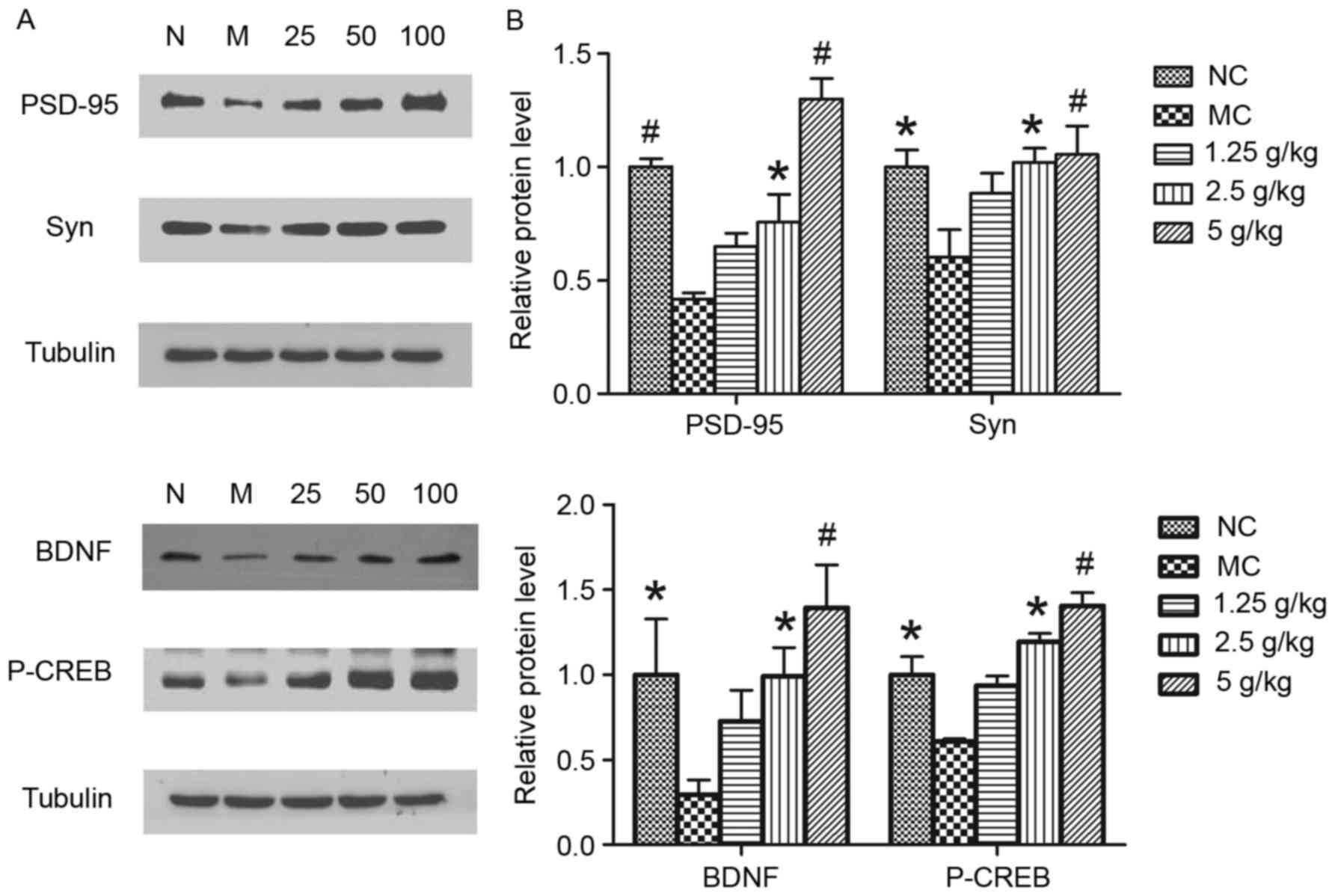 | Figure 7.JYCF treatment increases the
expression of synaptic-associated proteins in mice subjected to
unpredictable chronic mild stress. (A) The protein levels of
PSD-95, Syn, BDNF and P-CREB were determined by western blot
analysis. (B) Quantitative analysis of protein expression levels.
Values are expressed as the mean ± standard error of the mean of
three independent experiments. *P<0.05, #P<0.01
compared with MC group. PSD-95, postsynaptic density protein-95;
Syn, synaptophysin; P-CREB, phosphorylated cyclic adenosine
monophosphate response element binding protein; BDNF, brain-derived
neurotrophic factor; GSK, glycogen synthase kinase; NC, negative
control; MC, model control group; 1.25/2.5/5 g/kg, model mice
treated with 1.25/2.5/5 g/kg JYCF; JYCF, Jieyu chufan. |
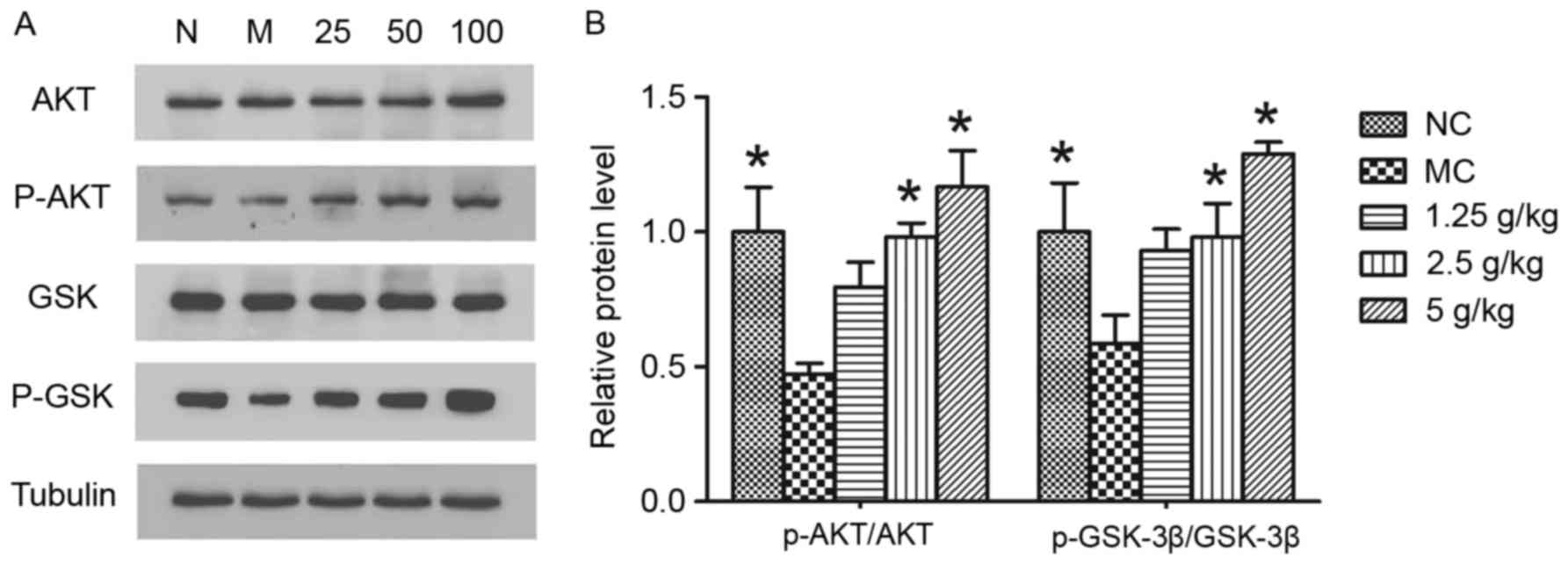
JYCF increases p-Akt and p-GSK-3β
To elucidate the mechanism of JYCF in ameliorating
the impairment of synaptic function, GSK-3β signaling pathways,
which contribute to neuroplasticity, were studied. As shown in
Fig. 8, significant decreases of
p-Akt (P<0.05) and p-GSK-3β (P<0.05) were observed in the
hippocampi of mice in the MC and NC groups. Statistical analysis
revealed that, compared with that in the MC group, JYCF treatment
at 2.5 g/kg or 5 g/kg significantly increased the ratios of
p-Akt/AKT and p-GSK-3β/GSK-3β compared with the MC group
(P<0.05).
Discussion
The present study indicated, for the first time, to
the best of our knowledge, that JYCF produced robust
anti-depressant effects in a rodent depression model and that the
underlying mechanism of this neuroprotection may be the
upregulation of BDNF and p-GSK-3β.
In the present study, administration of JYCF for 3
weeks increased the body weight of mice receiving UCMS. The
preference for sucrose solution gradually recovered and other
behavioral tests, such as the forced swimming, open field, plus
maze and MWM test showed that regular daily administration of JYCF
substantially ameliorated behavioral deficits of mice subjected to
UCMS. These results suggested that JYCF may be developed as a novel
anti-depressant.
In spite of the vast number of studies on
depression, the current understanding of the precise
neurobiological pathogenic mechanisms is limited. Morphometric
magnetic resonance imaging studies have reported a decrease in
hippocampal volume in depression patients (32). Studies on the longitudinal course of
late-life depression showed that hippocampal atrophy is associated
with the severity of depression (33,34).
Studies in animal models of depression found that chronic stress
produces atrophy and dendritic arborization of the CA3 pyramidal
neuron (35). Depression or stress
strongly reduce the hippocampal potential for plasticity (36). An experiment using an animal model of
depression also showed that electroconvulsive shock enhances
neurogenesis in the hippocampus, decreases neuronal synapses and
increases dendritic spine density in various cortical and limbic
structures (37).
Studies on depression and anti-depressive therapies
have revealed that hippocampal neurogenesis has a key role, and
certain anti-depressants block or reverse neuronal deficits in the
prefrontal cortex and the hippocampus (38). Other studies found that depression
reduces neuronal plasticity and impairs synaptic function, which
may be an important target of pharmacological intervention
(39). The anti-psychotic drug
Lurasidone has shown to be effective in a CMS model through the
modulation of synaptic and neuroplastic proteins and PSD-95
(40). UCMS diminished the sucrose
preference and reduced measures of locomotor activity in mice and
decreased the expression of Syn in the hippocampal CA3 region,
while electroconvulsive stimulation improved these depressive
behaviors and increased the mean density of Syn (41).
In the present study, synaptic density and
neurogenesis of pyramidal neurons was decreased in mice subjected
to UCMS, while JYCF administration reversed these changes and
increased synaptic density and neurogenesis. Moreover, JYCF
administration upregulated the expression of PSD-95 and Syn in the
hippocampi of the UCMS mice, suggesting that JYCF modulates
synaptic and neuroplastic proteins.
The results of the present study demonstrated that
JYCF upregulated the protein levels of BDNF in the hippocampus of
UCMS mice. BDNF knockout mice displayed depression-like behavior
and the genetic defect regarding BDNF inhibited the effects of
anti-depressants in mice exposed to UCMS (42). The interaction between CREB and BDNF
is important in signal transduction and has an essential role in
the concept of altered neuroplasticity in depression. The increase
in p-CREB was significantly associated with clinical improvement in
patients treated with anti-depressants or psychotherapy (43). A previous study found that the
activity of CREB in peripheral blood of depression patients is
increased (44). The present study
showed that, the p-CREB was decreased in the MC group and treatment
with JYCF was able to increase the expression of p-CREB in UCMS
mice, which may be helpful in ameliorating depression.
Studies on depression-like behaviors in rodents have
provided evidence that dysregulation of GSK-3 causes increased
activity in specific pathways and promotes susceptibility to mood
disorders (16). GSK3 was originally
identified as a key enzyme of glucose metabolism and as a broadly
influential enzyme that regulates a large group of transcription
factors and transcriptional modulators (45). GSK-3β, the isoform of GSK-3, is
important in numerous intracellular signaling pathways of
neuroplasticity (46).
Overexpression of GSK-3β in the hippocampi of UCMS-treated mice
shows a pre-depression-like behavior (47). Moreover, in CMS-treated mice or
depressive patients, the expression of GSK-3β has been found to be
significantly elevated and after treatment with GSK-3β inhibitors,
and the depressive behavior was reversed (48,49). In
addition, it was reported that the phosphorylation of Akt and
GSK-3β is a direct regulator of cell survival (50), and modulation of the activity of AKT
and GSK-3 signaling may contribute to specific therapeutic effects
for depression (51). In platelets
of depressive patients, decreased phosphorylation of GSK-3β and
increased GSK-3β activity were observed (52). The present study demonstrated
decreased p-GSK-3β expression levels in UCMS and regular
administration of JYCF increased the expression levels of
phosphorylated Akt and GSK-3β and alleviated depression-like
behaviors.
The present study revealed the antidepressant-like
activity of JYCF in a chronic mild stress model and the
anti-depressant-like properties mainly resulted from the modulation
of synaptic structure and function and the mechanisms may be
associated with the upregulation of BDNF and p-GSK-3β activity. The
findings contributed to the understanding of the pathological
effects of synaptic dysfunction in the depressive brain and suggest
that JYCF may be used as a neuroprotective agent against
depression.
Acknowledgements
The present study was supported by the National
Science Foundation of China (grant no. 81471102), Outstanding
Researcher Program of Jiangsu Province (grant no. LJ201101), the
National Natural Science Foundation of Jiangsu Province of China
(grant no. BK2009037), the Six Talent Peak Project of Jiangsu
Province (grant no. 2015-WSN-083) and the Key Project of Nanjing
Medical Science and Technology Development Project (grant no.
ZKX13020).
References
|
1
|
Aldao A, Mennin DS, Linardatos E and
Fresco DM: Differential patterns of physical symptoms and
subjective processes in generalized anxiety disorder and unipolar
depression. J Anxiety Disord. 24:250–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Trivedi MH, Rush AJ, Wisniewski SR,
Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz
B, McGrath PJ, et al: Evaluation of outcomes with citalopram for
depression using measurement-based care in STAR*D: Implications for
clinical practice. Am J Psychiatry. 163:28–40. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rotheneichner P, Lange S, O'Sullivan A,
Marschallinger J, Zaunmair P, Geretsegger C, Aigner L and
Couillard-Despres S: Hippocampal neurogenesis and antidepressive
therapy: Shocking relations. Neural Plast. 2014:7239152014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Duman RS and Aghajanian GK: Synaptic
dysfunction in depression: Potential therapeutic targets. Science.
338:68–72. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wainwright SR and Galea LA: The neural
plasticity theory of depression: Assessing the roles of adult
neurogenesis and PSA-NCAM within the hippocampus. Neural Plast.
2013:8054972013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Masi G and Brovedani P: The hippocampus,
neurotrophic factors and depression: Possible implications for the
pharmacotherapy of depression. CNS Drugs. 25:913–931. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Erickson KI, Miller DL and Roecklein KA:
The aging hippocampus: Interactions between exercise, depression,
and BDNF. Neuroscientist. 18:82–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoshii A and Constantine-Paton M:
Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity,
and disease. Dev Neurobiol. 70:304–322. 2010.PubMed/NCBI
|
|
9
|
Lipsky RH and Marini AM: Brain-derived
neurotrophic factor in neuronal survival and behavior-related
plasticity. Ann NY Acad Sci. 1122:130–143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schmidt HD and Duman RS: The role of
neurotrophic factors in adult hippocampal neurogenesis,
antidepressant treatments and animal models of depressive-like
behavior. Behav Pharmacol. 18:391–418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Castrén E, Võikar V and Rantamäki T: Role
of neurotrophic factors in depression. Curr Opin Pharmacol.
7:18–21. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aydemir C, Yalcin ES, Aksaray S, Kisa C,
Yildirim SG, Uzbay T and Goka E: Brain-derived neurotrophic factor
(BDNF) changes in the serum of depressed women. Prog
Neuropsychopharmacol Biol Psychiatry. 30:1256–1260. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hritcu L and Gorgan LD: Intranigral
lipopolysaccharide induced anxiety and depression by altered BDNF
mRNA expression in rat hippocampus. Prog Neuropsychopharmacol Biol
Psychiatry. 51:126–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Musazzi L, Cattaneo A, Tardito D, Barbon
A, Gennarelli M, Barlati S, Racagni G and Popoli M: Early raise of
BDNF in hippocampus suggests induction of posttranscriptional
mechanisms by antidepressants. BMC Neurosci. 10:482009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bradley CA, Peineau S, Taghibiglou C,
Nicolas CS, Whitcomb DJ, Bortolotto ZA, Kaang BK, Cho K, Wang YT
and Collingridge GL: A pivotal role of GSK-3 in synaptic
plasticity. Front Mol Neurosci. 5:132012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu W, Wang H, Wang Y, Li H and Ji L:
Metabolic factors-triggered inflammatory response drives
antidepressant effects of exercise in CUMS rats. Psychiatry Res.
228:257–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Klein PS and Melton DA: A molecular
mechanism for the effect of lithium on development. Proc Natl Acad
Sci USA. 93:pp. 8455–8459. 1996; View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaidanovich-Beilin O, Milman A, Weizman A,
Pick CG and Eldar-Finkelman H: Rapid antidepressive-like activity
of specific glycogen synthase kinase-3 inhibitor and its effect on
beta-catenin in mouse hippocampus. Biol Psychiatry. 55:781–784.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shapira M, Licht A, Milman A, Pick CG,
Shohami E and Eldar-Finkelman H: Role of glycogen synthase
kinase-3beta in early depressive behavior induced by mild traumatic
brain injury. Mol Cell Neurosci. 34:571–577. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lao Y, Wang X, Xu N, Zhang H and Xu H:
Application of proteomics to determine the mechanism of action of
traditional Chinese medicine remedies. J Ethnopharmacol. 155:1–8.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun Y, Feng F and Yu X: Pharmacokinetics
of geniposide in Zhi-Zi-Hou-Pu decoction and in different
combinations of its constituent herbs. Phytother Res. 26:67–72.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li JM, Kong LD, Wang YM, Cheng CH, Zhang
WY and Tan WZ: Behavioral and biochemical studies on chronic mild
stress models in rats treated with a Chinese traditional
prescription Banxia-houpu decoction. Life Sci. 74:55–73. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma Z, Ji W, Qu R, Wang M, Yang W, Zhan Z,
Fu Q and Ma S: Metabonomic study on the antidepressant-like effects
of banxia houpu decoction and its action mechanism. Evid Based
Complement Alternat Med. 2013:2137392013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Willner P, Towell A, Sampson D, Sophokeous
S and Muscat R: Reduction of sucrose preference by chronic
unpredictable mild stress, and its restoration by a tricyclic
antidepressant. Psychopharmacology (Berl). 93:358–364. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grippo AJ, Beltz TG, Weiss RM and Johnson
AK: The effects of chronic fluoxetine treatment on chronic mild
stress-induced cardiovascular changes and anhedonia. Biol
Psychiatry. 59:309–316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Benelli A, Filaferro M, Bertolini A and
Genedani S: Influence of S-adenosyl-L-methionine on chronic mild
stress-induced anhedonia in castrated rats. Br J Pharmacol.
127:645–654. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Forbes NF, Stewart CA, Matthews K and Reid
IC: Chronic mild stress and sucrose consumption: Validity as a
model of depression. Physiol Behav. 60:1481–1484. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Porsolt RD, Anton G, Blavet N and Jalfre
M: Behavioural despair in rats: A new model sensitive to
antidepressant treatments. Eur J Pharmacol. 47:379–391. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zomkowski AD, Engel D, Gabilan NH and
Rodrigues AL: Involvement of NMDA receptors and L-arginine-nitric
oxide-cyclic guanosine monophosphate pathway in the
antidepressant-like effects of escitalopram in the forced swimming
test. Eur Neuropsychopharmacol. 20:793–801. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Iwamoto Y, Morinobu S, Takahashi T and
Yamawaki S: Single prolonged stress increases contextual freezing
and the expression of glycine transporter 1 and vesicle-associated
membrane protein 2 mRNA in the hippocampus of rats. Prog
Neuropsychopharmacol Biol Psychiatry. 31:642–651. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu L, Wang S, Chen X, Yang H, Li X, Xu Y
and Zhu X: Orientin alleviates cognitive deficits and oxidative
stress in Aβ1-42-induced mouse model of Alzheimer's disease. Life
Sci. 121:104–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ezzati A, Zimmerman ME, Katz MJ and Lipton
RB: Hippocampal correlates of depression in healthy elderly adults.
Hippocampus. 23:1137–1142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Taylor WD, McQuoid DR, Payne ME, Zannas
AS, MacFall JR and Steffens DC: Hippocampus atrophy and the
longitudinal course of late-life depression. Am J Geriatr
Psychiatry. 22:1504–1512. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
André C, Dinel AL, Ferreira G, Layé S and
Castanon N: Diet-induced obesity progressively alters cognition,
anxiety-like behavior and lipopolysaccharide-induced
depressive-like behavior: Focus on brain indoleamine
2,3-dioxygenase activation. Brain Behav Immun. 41:10–21. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lupien SJ, Nair NP, Brière S, Maheu F, Tu
MT, Lemay M, McEwen BS and Meaney MJ: Increased cortisol levels and
impaired cognition in human aging: Implication for depression and
dementia in later life. Rev Neurosci. 10:117–139. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lucassen PJ, Stumpel MW, Wang Q and
Aronica E: Decreased numbers of progenitor cells but no response to
antidepressant drugs in the hippocampus of elderly depressed
patients. Neuropharmacology. 58:940–949. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ampuero E, Rubio FJ, Falcon R, Sandoval M,
Diaz-Veliz G, Gonzalez RE, Earle N, Dagnino-Subiabre A, Aboitiz F,
Orrego F and Wyneken U: Chronic floxetine treatment induces
structural plasticity and selective changes in glutamate receptor
subunits in the rat cerebral cortex. Neuroscience. 169:98–108.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tanti A and Belzung C: Neurogenesis along
the septo-temporal axis of the hippocampus: Are depression and the
action of antidepressants region-specific? Neuroscience.
252:234–252. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rayen I, Gemmel M, Pauley G, Steinbusch HW
and Pawluski JL: Developmental exposure to SSRIs, in addition to
maternal stress, has long-term sex-dependent effects on hippocampal
plasticity. Psychopharmacology (Berl). 232:1231–1244. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Luoni A, Macchi F, Papp M, Molteni R and
Riva MA: Lurasidone exerts antidepressant properties in the chronic
mild stress model through the regulation of synaptic and
neuroplastic mechanisms in the rat prefrontal cortex. Int J
Neuropsychopharmacol. 18(pii): pyu0612014.PubMed/NCBI
|
|
41
|
Li W, Liu L, Liu YY, Luo J, Lin JY, Li X,
Wang B and Min S: Effects of electroconvulsive stimulation on
long-term potentiation and synaptophysin in the hippocampus of rats
with depressive behavior. J ECT. 28:111–117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yulug B, Ozan E, Gönül AS and Kilic E:
Brain-derived neurotrophic factor, stress and depression: A
minireview. Brain Res Bull. 78:267–269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Koch JM, Hinze-Selch D, Stingele K,
Huchzermeier C, Goder R, Seeck-Hirschner M and Aldenhoff JB:
Changes in CREB phosphorylation and BDNF plasma levels during
psychotherapy of depression. Psychother Psychosom. 78:187–192.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pláteník J, Fišar Z, Buchal R, Jirák R,
Kitzlerová E, Zvěřová M and Raboch J: GSK-3β, CREB, and BDNF in
peripheral blood of patients with Alzheimers disease and
depression. Prog Neuropsychopharmacol Biol Psychiatry. 50:83–93.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jope RS: Glycogen synthase kinase-3 in the
etiology and treatment of mood disorders. Front Mol Neurosci.
4:162011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jope RS and Johnson GV: The glamour and
gloom of glycogen synthase kinase-3. Trends Biochem Sci. 29:95–102.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wada A: Lithium and neuropsychiatric
therapeutics: Neuroplasticity via glycogen synthase kinase-3beta,
beta-catenin, and neurotrophin cascades. J Pharmacol Sci.
110:14–28. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang K, Song X, Xu Y, Li X, Liu P, Sun N,
Zhao X, Liu Z, Xie Z and Peng J: Continuous GSK-3β overexpression
in the hippocampal dentate gyrus induces prodepressant-like effects
and increases sensitivity to chronic mild stress in mice. J Affect
Disord. 146:45–52. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Silva R, Mesquita AR, Bessa J, Sousa JC,
Sotiropoulos I, Leão P, Almeida OF and Sousa N: Lithium blocks
stress-induced changes in depressive-like behavior and hippocampal
cell fate: The role of glycogen-synthase-kinase-3beta.
Neuroscience. 152:656–669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Oh DH, Park YC and Kim SH: Increased
glycogen synthase kinase-3β mRNA level in the hippocampus of
patients with major depression: A study using the Stanley
neuropathology consortium integrative database. Psychiatry
Investig. 7:202–207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nayak G and Cooper GM: p53 is a major
component of the transcriptional and apoptotic program regulated by
PI3-kinase/Akt/GSK3 signaling. Cell Death Dis. 3:e4002012.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kitagishi Y, Kobayashi M, Kikuta K and
Matsuda S: Roles of PI3K/AKT/GSK-3/mTOR pathway in cell signaling
of mental illnesses. Depress Res Treat. 2012:7525632012.PubMed/NCBI
|
|
53
|
Pláteník J, Fišar Z, Buchal R, Jirák R,
Kitzlerová E, Zvěřová M and Raboch J: GSK3β, CREB, and BDNF in
peripheral blood of patients with Alzheimer's disease and
depression. Prog Neuropsychopharmacol Biol Psychiatry. 50:83–93.
2014. View Article : Google Scholar : PubMed/NCBI
|