Introduction
Ulcerative colitis (UC) and Crohn's disease (CD) are
the most common types among chronic intestinal diseases (1). UC is a chronic relapsing-remitting
inflammatory disorder that affects the colon and rectum. Unlike CD,
UC is a mucosal disease that always affects the rectum, and can
spread up to the cecum with a continuous retrograde distribution
(2). Although it is widely accepted
that UC derives from the presence of a susceptibility gene, mucosal
immunological disorders, colony dysbiosis and environmental risk
factors, the exact underlying etiology remains unknown (3). UC may occur from infancy onwards,
however, the risk increases greatly in early adulthood, slightly
decreasing thereafter (4). In the
past few decades, the incidence of UC has increased, particularly
in developing nations. The major clinical manifestations of UC
include abdominal pain, diarrhea, vomiting and weight loss in the
patients' daily life (5,6). The hallmark clinical symptom of UC is
bloody diarrhea (7). Furthermore, UC
alters the absorption of water and electrolytes, and causes
diarrhea in conjunction with the decline of colon tissue
contraction.
One of the key mechanical contributions of colon
tissues in vivo is to resist tensile forces to maintain
structural stability and biological functionality. Therefore,
previous studies have characterized the biomechanical properties of
colon tissues (8–12). The colon wall is subject to tensile
loading and finite strains, and in general, presents a nonlinear
and anisotropic mechanical response (8,10,13).
This is primarily owing to collagen fiber distribution within the
microstructure of its subcomponents (14). Notably, the mechanical properties of
biological soft tissue may be altered significantly in response to
pathological changes (15). However,
the potential effects of UC on the mechanical properties and
microstructure of the colon wall remain largely unclear, and
relevant quantitative mechanical data are lacking in the
literature. Previous research has presented solid evidence that
mice in a dextran sodium sulfate (DSS)-treated colitis model
exhibit weight loss, bloody diarrhea, mucosal disorders and
inflammation, resembling human UC (16–19).
Given this aforementioned information, a biomechanical analysis of
the colon wall in a mouse model of colitis induced by DSS may
advance our understanding of changes in the complex
structure-function association of tissues in response to colitis,
particularly in the early stages of the disease.
In the present study, uniaxial tensile tests and
histological investigations were performed on tissue samples from
control and experimental colitis mice. The study aimed to clarify
the intrinsic differences in ultimate tensile strength between
these two tissue types, indicating the potential effects of colitis
as a single pathological factor on the mechanical properties and
microstructure of the colon wall.
Materials and methods
Animals
A total of 20 healthy 8-week-old C57BL/10J wild-type
mice (weight, 25–35 g; 10 male and 10 female) were used in the
present study. Mice were purchased from the Model Animal Research
Center of Nanjing University (Nanjing, China), and were housed in
the Experimental Animal Center at Tongji University (Shanghai,
China). The animals were acclimatized to laboratory conditions
(24±1°C, 12-h light/dark cycle, 55% humidity and ad libitum access
to food and water) for two weeks prior to experimentation. All
experimental procedures conformed to the International Guidelines
for the Care and Use of Laboratory Animals and were approved by the
Animal Ethics Committee of Tongji University School of Medicine
(Shanghai, China).
Experimental colitis and animal
groups
The mice were randomly assigned into two groups
(n=10 in each), including the control and DSS (experimental colitis
group). Animals in the control group animals consumed
ddH20 orally, while colitis was induced in the DSS group
by the oral administration of 4% DSS (MW=36,000–50,000; cat. no.
160110; MP Biomedicals, Solon, OH, USA) for 7 days continuously, as
described by Li et al (20).
At 7 days after the induction of colitis, stool consistency and the
presence of occult blood was examined and documented daily.
Colitis score
Colonic damage was assessed by the macroscopic score
(MS), based on three parameters as follows: i) Weight loss; ii)
colon length shortening; and iii) occult blood. Each parameter was
scored with between 0 and 4 points. The MS was the sum of the three
scores, ranging between 0 (healthy) and 12 (maximal). Scoring was
conducted as reported previously by Li et al (20) and Kimball et al (21).
Specimen preparations
All animals were euthanized by cervical dislocation
on day 8 after colitis induction and the entire gut was carefully
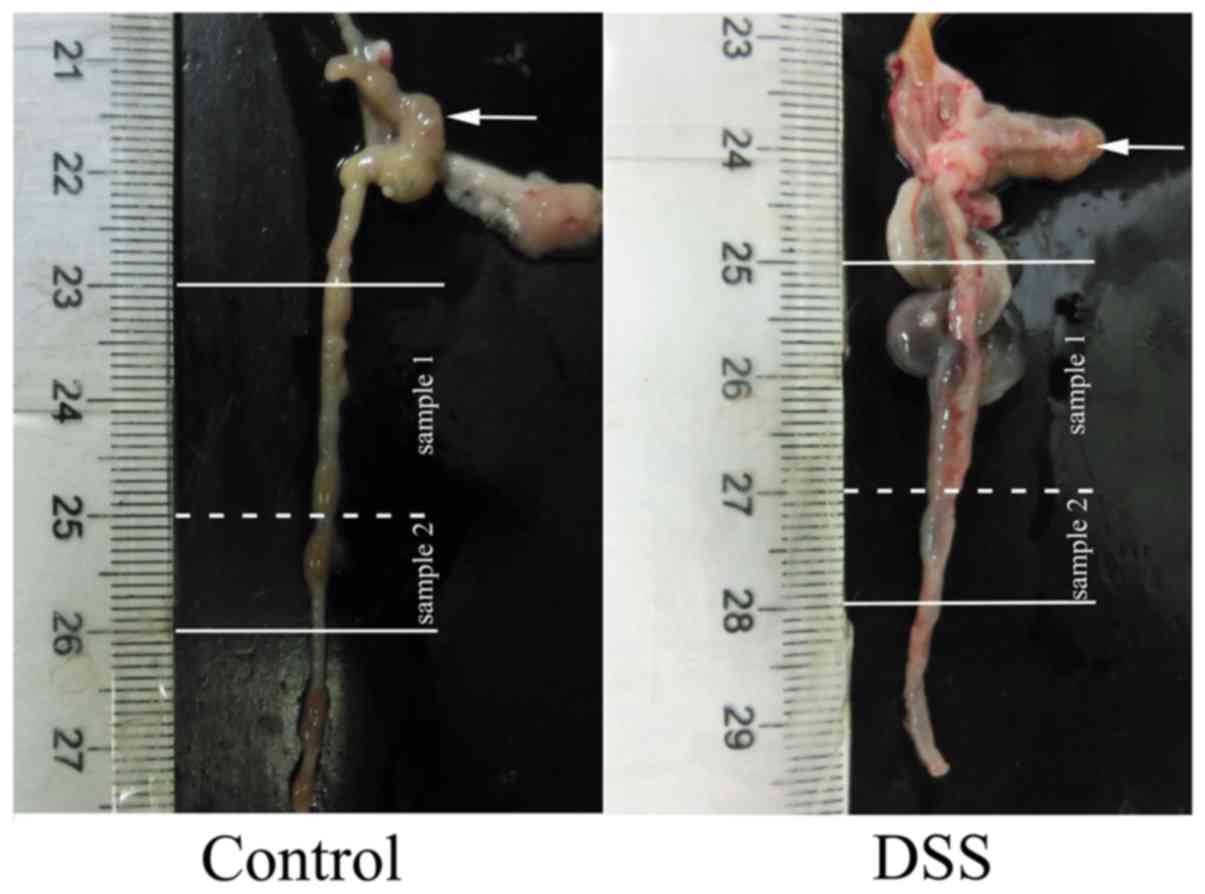
dissected. The colon, starting at 10 mm away from the cecum, was
cut out at a length of 30 mm and weighed. Next, the colon was then
dissected into two portions, including specimens of 20 mm (sample
1) for use in mechanical tests and 10 mm (sample 2) for
histological analysis (Fig. 1).
Sample 1 was stored at −80°C for the biomechanical tests, while
sample 2 was immediately fixed in 4% paraformaldehyde for paraffin
embedding. In total, 10 control and 10 DSS tissue samples were
collected. The thicknesses of colon walls were measured by a
micrometer.
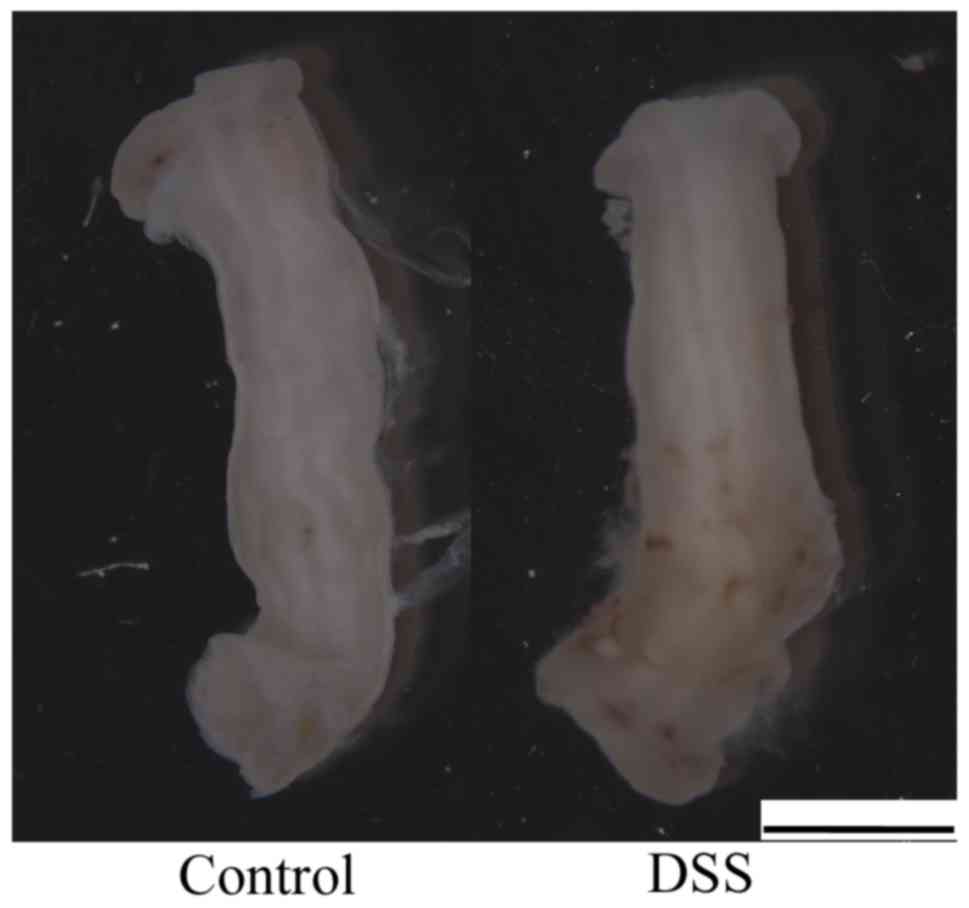
For biomechanical tests, the cleaned colon was cut
open axially (Fig. 2). Using a
scalpel, a rectangular tissue specimen of 20×4 mm (lengthxwidth)
was further extracted. The circumference of the mouse colon is
4.5–5.0 mm; therefore, a uniaxial tensile test could not be
performed using a circumferential strip. In the present study, all
rectangular strips were obtained from the axial direction.
Sandpaper was mounted at the two ends of the prepared strip
specimens with super-adhesive gel in order to facilitate defined
clamping in the tensile testing machine and to prevent slippage
during testing. In addition, two black straw chips (used as gauge
markers for measuring deformation) were glued transversely in
parallel onto the middle part of the specimens to function as gauge
markers for the axial deformation measurements. The strip specimens
were left to equilibrate in phosphate-buffered physiological saline
with ethylene glycol tetraacetic acid at 37°C for ~15 min prior to
mechanical testing.
For histological investigations, the fixed colon
tissue was thoroughly washed with phosphate-buffered physiological
saline. The samples were embedded in paraffin and cut into 5-µm
sections on a Leica RM2126 microtome (Leica Microsystems, Shanghai,
China). The sections were deparaffinized and then stained with
hematoxylin and eosin (cat. nos. 842 and 837; Anatech Ltd., Battle
Creek, MI, USA), and Masson staining (cat. no. HT15; Sigma-Aldrich;
Merck, Darmstadt, Germany).
Mechanical testing
Uniaxial tensile tests were performed on a
computer-controlled, screw-driven, high-precision tensile testing
device adapted for small biological specimens (XJ810-10N; Xiangjie
Instrument Technology Co., Ltd., Shanghai, China). The entire test
was conducted in a Perspex container filled with 0.9% physiological
saline solution maintained at 37.0±1.0°C by a heater-circulation
unit (Ecoline E 200; Lauda, Lauda-Königshofen, Germany).
Preconditioning was achieved by conducting five loading and
unloading cycles at a constant extension rate of 0.5 mm/min to
reach 75 kPa (which is the first Piola-Kirchhoff stress) for each
test. After preconditioning was completed, the sample thickness was
measured by means of a video extensometer, as described in earlier
studies (22,23). Starting from the sixth tensile cycle,
the strain was increased with the same extension rate of 0.5 mm/min
until tissue rupture occurred. The rupture forces and associated
stretches were documented.
Data analysis
The ultimate tensile stresses were computed
according to the following formula: σult =
fruptureλult/WT, where frupture is
the rupture force, λult is the ultimate stretch, and W
and T are the width and the thickness of the specimen in the
unloaded configuration, respectively. The λult was
defined as l/L, where l and L are the gauge lengths in the loaded
(state at rupture) and unloaded configurations, respectively. In
addition, the maximum tangential modulus (MTM), defined as the
slope of the non-linear stress-stretch curve at the maximum load in
preconditioning, was calculated and further averaged to assess the
stiffness of the colon tissue under the same stress state (i.e.,
the first Piola-Kirchhoff stress PLL = 75 kPa).
Statistical analysis
Experimental data are summarized as the mean ±
standard deviation. The Shapiro-Wilk and the Kolmogorov-Smirnov
tests were used to determine data normality. Comparisons of
quantitative values between the control and colitis groups were
performed using the Student's t-test and χ2 test.
Differences with a P-value of <0.05 were determined as
statistically significant. Statistical analyses were performed
using SPSS 21.0 statistical software (IBM Corp., Armonk, NY,
USA).
Results
Mouse model observations
The DSS group began to develop diarrhea at day 3,
and the following day, occult blood was observed in the stools of
the mice. At day 5, all DSS-treated mice experienced diarrhea and
hematochezia. By contrast, the control group presented no abnormal
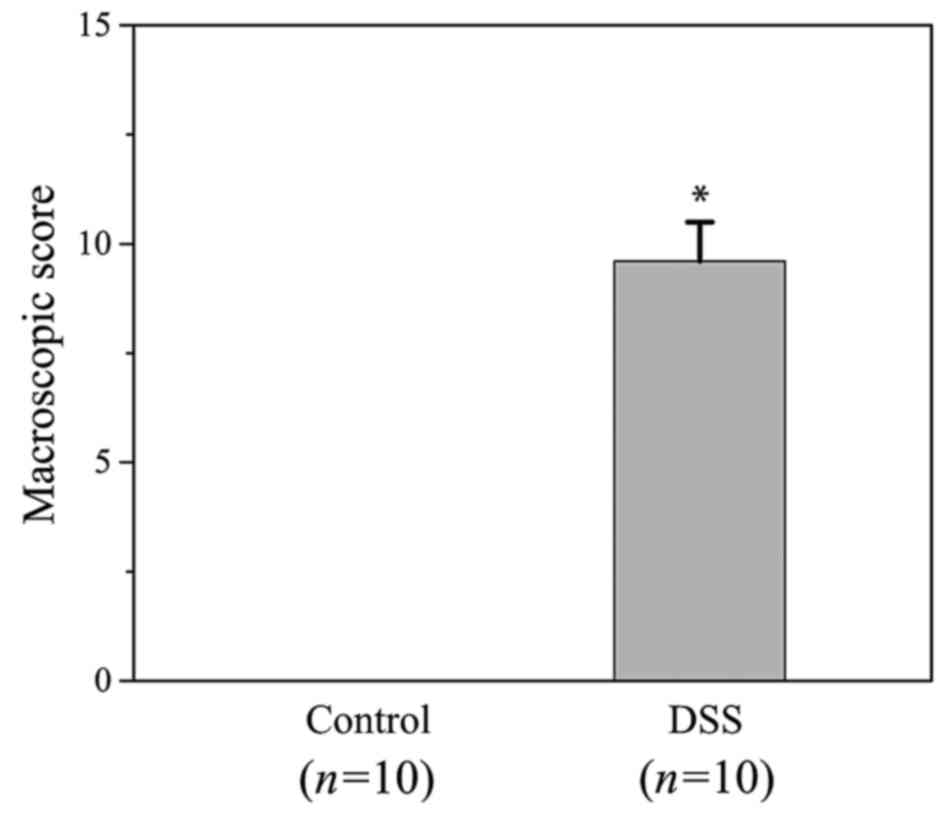
indications. The total MS for the DSS group was 9.6±0.9 (Fig. 3). However, the MS of the control
group was 0. This demonstrated that successive challenges with DSS
induced evident experimental colitis in the DSS group, with
significant pathological alterations, including weight loss, colon
length shortening and occult blood.
Thicknesses of the colon wall
The mean thicknesses of the colon walls for the
control and the DSS groups were 90±10 and 110±30 µm, respectively,
which were significantly different (P=0.08). In general, the colon
wall in the DSS group was thicker as compared with that of the
control group, possibly due to tissue edema.
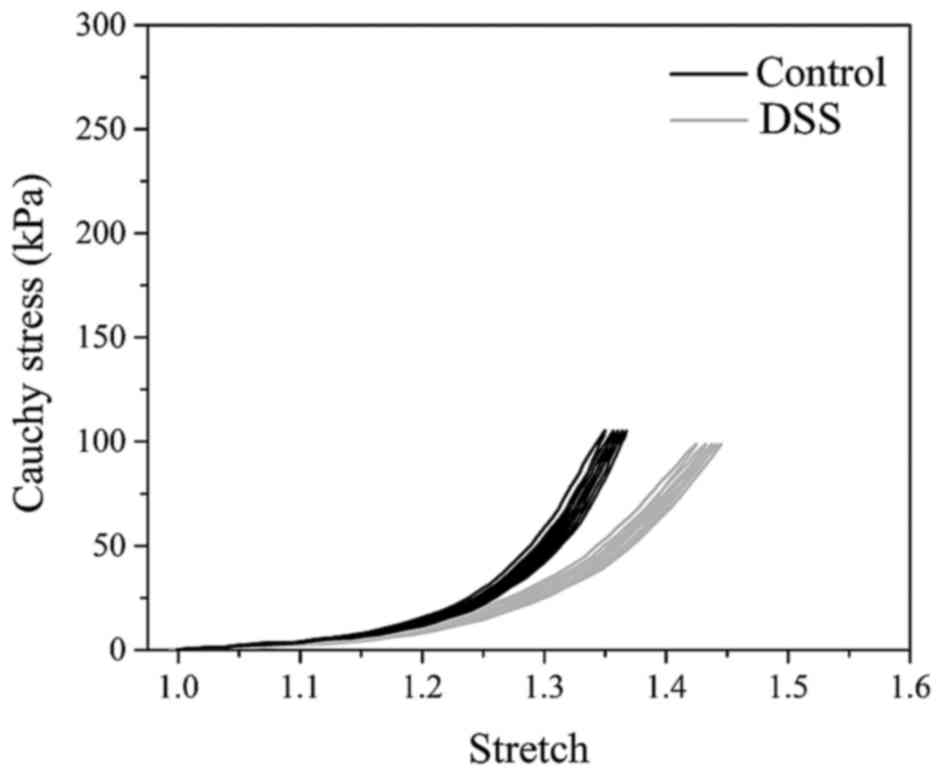
Biomechanical tests
The preconditioning behavior of the colon tissue in
the control and DSS groups is shown in Fig. 4, in terms of the Cauchy stress
against the stretch. Each sample has five loading-unloading cycles.
The tensile stress-stretch curves were highly nonlinear.
Subsequently, the Shapiro-Wilk and the Kolmogorov-Smirnov tests
demonstrated that the quantified ultimate tensile stress, stretch
and MTM values were normally distributed. Differences in ultimate
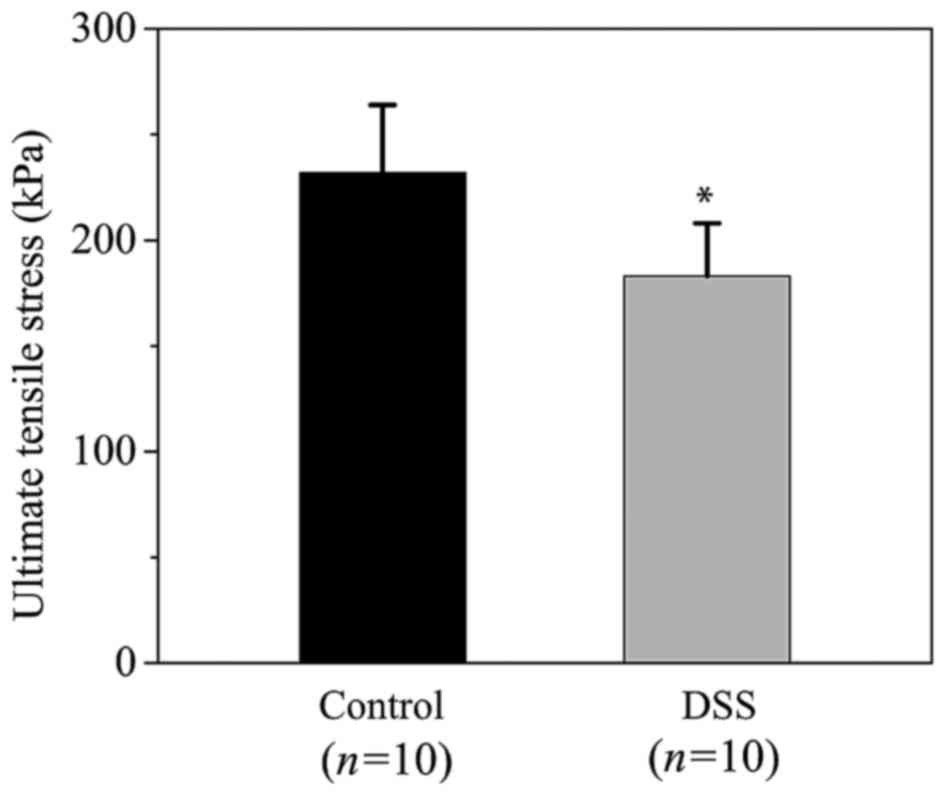
tensile stresses are demonstrated in Fig. 5. The ultimate tensile stresses were
232±33 and 183±25 kPa in the control and the DSS groups,
respectively (P=0.001). This indicated that the ultimate tensile
strength of the colon tissue was significantly decreased in the DSS
group compared with the control group. In addition, the ultimate
stretches at rupture for the control and DSS groups (not shown in
figure) were 1.43±0.04 and 1.51±0.06, respectively (P=0.006). Thus,
the rupture stretch for the DSS group was significantly higher
compared with that of the control group. Statistical comparison of
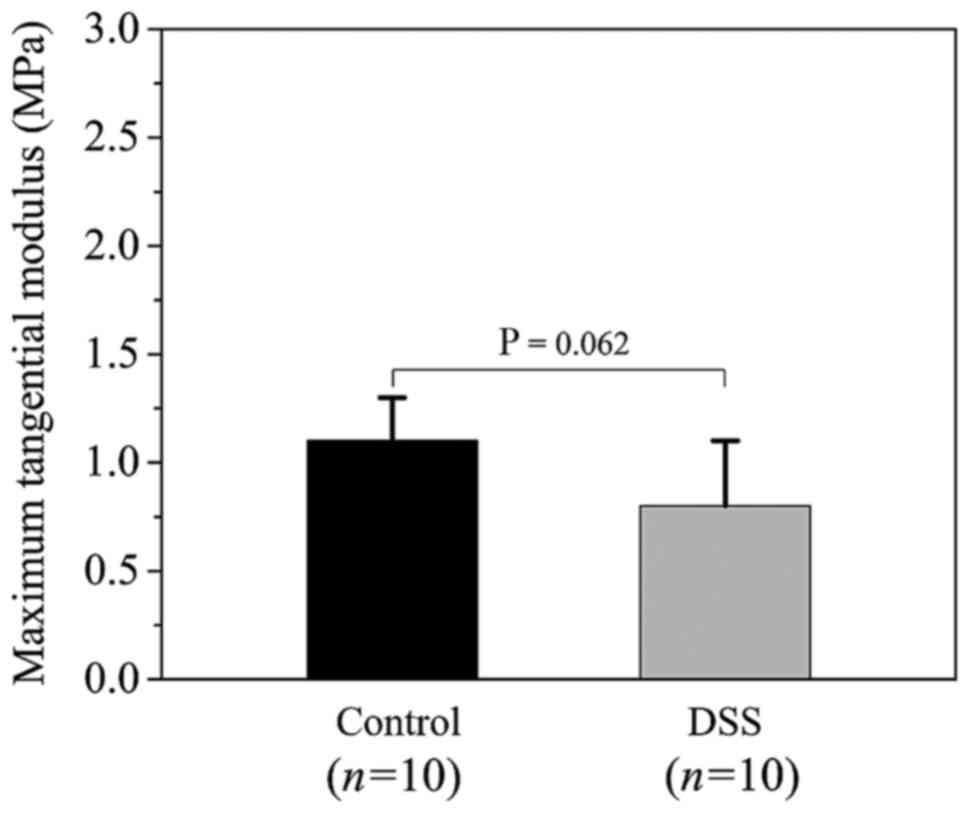
the MTM values between the control and DSS groups is presented in
Fig. 6. The MTM values were 1.1±0.2
and 0.8±0.3 MPa for the control and the DSS groups, respectively
(P=0.062), suggesting that there was no statistically significant
difference in tissue stiffness of the colon wall between the two
groups.
Histology
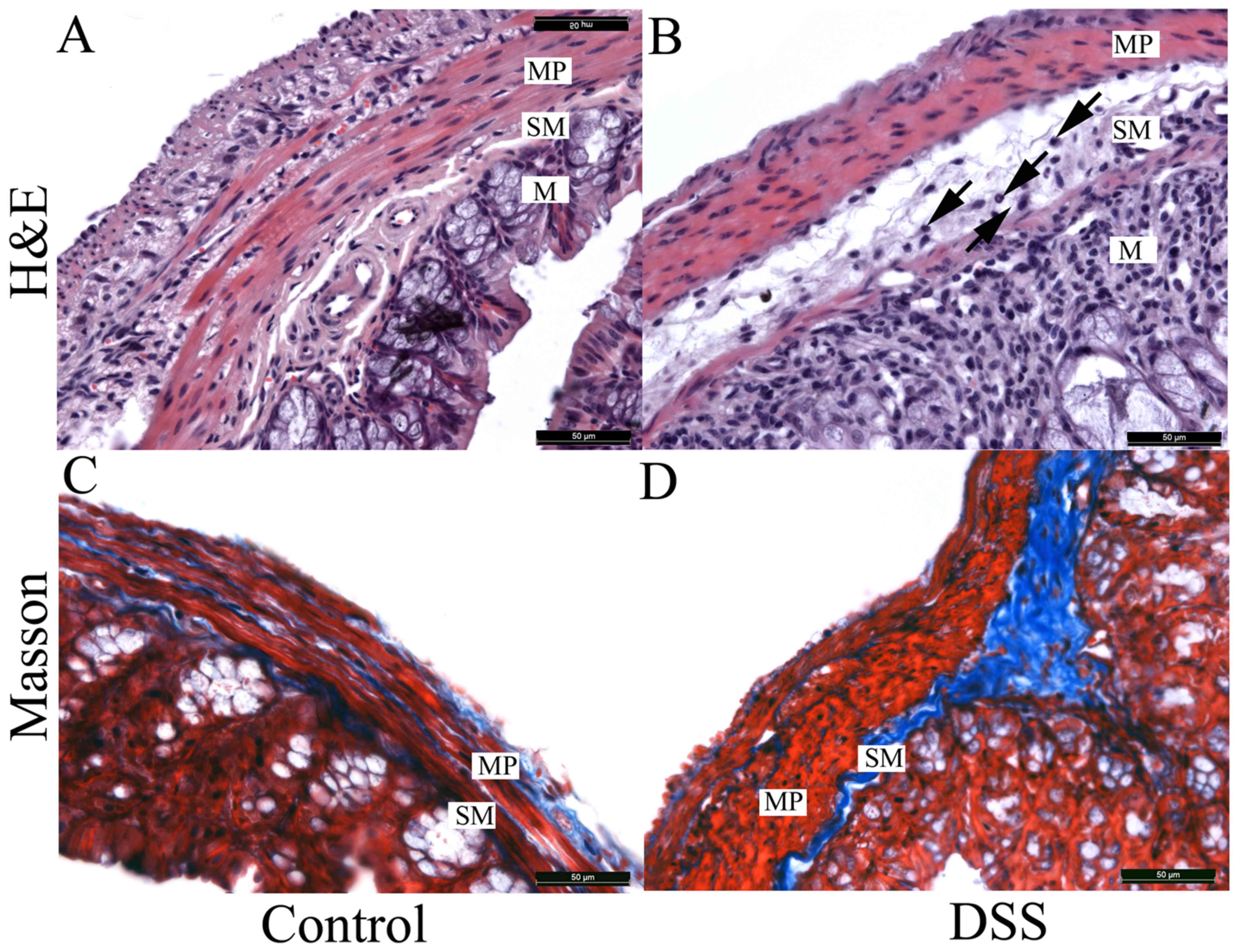
The microstructural characteristics of colon tissues
in the two groups are demonstrated in Fig. 7A. Compared with the control group, a
series of significant pathological changes were observed in the
microstructure of the DSS group (Fig.
7B). In the mucosa, numerous inflammatory cells had infiltrated
into the stroma and the structure of gland, to a certain extent,
was destroyed. A similar phenomenon was also observed within the
submucosa, with inflammatory cells infiltrating into the stroma,
leading to a significant submucosa edema (arrows in Fig. 7B). However, inflammatory cells were
not present in the muscularis propria. The entire thickness of the
colon wall was increased in the DSS group, partly due to the edema
of the submucosa.
In the control group, Masson staining revealed that
the collagen fibers were tightly packed into bundles that
surrounded the smooth muscle cells in the muscularis propria
(Fig. 7C). By contrast, collagen
fibers (blue) in the DSS-treated submucosa (Fig. 7D) demonstrated hyperplasia, and the
fiber alignment was disordered. Notably, a large number of collagen
fibers in the DSS-treated muscular layer were disrupted and fiber
bundles were thinner when compared with the control group (Fig. 7C and D).
Discussion
In the present study, biomechanical tests and
histological investigations were performed using a mouse colitis
model to elucidate alterations in the mechanical properties and
microstructure of colon tissues in response to early-stage of
experimental colitis. In particular, it was attempted to establish
a correlation between the mechanical data and structural
characteristics.
Previous studies have induced UC using a variety of
methods, including acetic acid, carrageenan, DSS,
dinitrochlorobenzene and others agents (5,18). Among
these protocols, the oral administration of DSS is regarded as the
most effective method to generate UC since it is easy to repeat and
has a high success rate. Thus, DSS is specifically appropriate for
investigations of UC pathogenesis. The current results confirmed
that uptake of 4% DSS in drinking water for 7 days caused colitis
with typical manifestations, including colonic epithelia damage and
submucosal edema, along with bloody stool and shortened colon,
followed by body weight loss.
Under uniaxial tensile loadings, colon walls show
pronounced nonlinear mechanical behavior with small hysteresis. The
stress-stretch curves are characterized by an initially flat region
and a subsequent steep region, which is typical for collagen-rich
biological soft tissues (24,25).
Biomechanical analysis in the present study confirmed that
DSS-induced colitis has strong effects on the mechanical properties
of colon tissues. The ultimate tensile strength of DSS-treated
colon tissues was significantly lower in comparison with that of
the control samples. This may be attributed to notable changes in
microstructural characteristics under pathological conditions. As a
load-bearing component, the muscular layer is responsible for the
mechanical behavior of the intact colon wall when subjected to
tensile loading (8,10,13,26).
Histological analysis in the present study revealed that a large
number of collagen fibers were disrupted in the DSS-treated
muscular layer, contributing to a lower ultimate tensile strength
and eventual tissue rupture. Furthermore, thinner fiber bundles in
the DSS-treated samples may have a higher propensity to cause
tissue defects on the microscale level during extension and further
affect ultimate tensile strength. Experimental data in the current
study demonstrated that the DSS-treated tissues were, in general,
more extensible as compared with the control tissues at the same
stress level. This may partially be explained by the pronounced
hyperplasia of collagen fibers in the DSS-treated submucosa.
Notably, hyperplasia of collagen may directly or indirectly
influence adhesion, alignment and stretches of fiber bundles, and,
therefore, serve a key role in tissue remodeling during
pathological processes. In particular, fiber realignment within the
DSS-treated submucosa may lead to a more disorganized
microstructure, and, thus, will induce changes in the mechanical
anisotropy of the colon wall. These factors potentially alter the
extensibility of tissues. Although there was no statistically
significant difference in tissue stiffness of the colon wall
between the control and DSS groups in the present study, the mean
MTM value for the DSS group was decreased when compared with the
control group. This indicates that the colon wall is more
compliant, rather than stiff, when it is initially susceptible to
experimental colitis.
Histopathological analysis has demonstrated that
intestinal fibrosis is a common complication of DSS-induced colitis
and results in a stiff fibrotic colon that cannot perform
peristalsis or resorb fluids, which are the major functions of the
large bowel (18,27,28).
Therefore, intestinal fibrosis frequently leads to abdominal pain
and diarrhea. It should be emphasized that fibrosis mainly affects
the submucosa layer. In the present study, however, a pronounced
fibrosis of colon wall was not observed in the early stages, which
was in accordance with previous studies (27,28).
In general, the quantified values of ultimate
tensile strength and stretch were lower in the current study as
compared with mechanical data obtained in previous studies
(8,12,13,29). The
discrepancy may be, in part, due to the experimental methods and
sample types used herein. In the present study, a uniaxial tensile
test was conducted to the intact colon wall subsequent to cutting
it open. However, previous experimental investigations (8,13,29) used
a pressure-inflation protocol to quantitatively determine
mechanical properties. These methods represent two different
deformations of the colon wall. In addition, the majority of
previous studies (8,13,29) used
rat colon or large intestine for mechanical tests, whereas the
present experiments used mouse colon tissues.
The current study had certain limitations. Firstly,
due to the small sample size of mouse colon, uniaxial tensile tests
based on the circumferential strip specimen could not be performed.
Therefore, it was not possible to measure the ultimate tensile
strength and stretch of the colon wall in the circumferential
direction. Secondly, the mouse colitis model represents a
pathological state of the colon wall in the early stages of
experimental colitis. This comparative study can only interpret
changes in mechanical properties and microstructural
characteristics of the colon wall with respect to early
experimental colitis. As a single pathological factor, early
experimental colitis has been demonstrated to alter the mechanical
properties and microstructure of colon walls.
In conclusion, the ultimate tensile strength of the
colon wall was significantly decreased during early-stage colitis.
In response to the same tensile loads, the colon was more
extensible as compared with that in the controls. However, there
was no statistically significant difference in tissue stiffness
between the DSS and control groups. Compared with the
microstructure of the healthy colon wall, it was observed that a
large number of collagen fibers were disrupted within the
DSS-treated muscular layer. Furthermore, hyperplasia was identified
in the DSS-treated submucosa, causing a disorganized microstructure
within the colon wall. These findings provide an additional
perspective into the effects of DSS-induced colitis on the
mechanical properties and microstructure of the colon wall, and may
be helpful for the interpretation of UC pathogenesis in future
studies.
Acknowledgements
The present study was supported by grants from the
Program for Young Excellent Talents at Tongji University (grant no.
1500219086) and the Fundamental Research Funds (grant nos.
1500219085 and 1500219095) for the Central Universities, China. The
authors are also grateful for funding provided by the National
Natural Science Foundation of China (grant nos. 81100091 and
31571181) and the Shanghai Municipal Commission of Health and
Family Planning (grant no. 20134Y33).
References
|
1
|
Baumgart DC and Carding SR: Inflammatory
bowel disease: Cause and immunobiology. Lancet. 369:1627–1640.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Di Sabatino A, Biancheri P, Rovedatti L,
Macdonald TT and Corazza GR: Recent advances in understanding
ulcerative colitis. Intern Emerg Med. 7:103–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Adams SM and Bornemann PH: Ulcerative
Colitis. Am Fam Physician. 87:699–705. 2013.PubMed/NCBI
|
|
4
|
Herrinton LJ, Liu L, Lewis JD, Griffin PM
and Allison J: Incidence and prevalence of inflammatory bowel
disease in a Northern California managed care organization,
1996–2002. Am J Gastroenterol. 103:1998–2006. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rijnierse A, Nijkamp FP and Kraneveld AD:
Mast cells and nerves tickle in the tummy: Implications for
inflammatory bowel disease and irritable bowel syndrome. Pharmacol
Ther. 116:207–235. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choi SY, Hur SJ, An CS, Jeon YH, Jeoung
YJ, Bak JP and Lim BO: Anti-inflammatory effects of Inonotus
obliquus in colitis induced by dextran sodium sulfate. J Biomed
Biotechnol. 2010:9435162010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
da Silva BC, Lyra AC, Rocha R and Santana
GO: Epidemiology, demographic characteristics and prognostic
predictors of ulcerative colitis. World J Gastroenterol.
20:9458–9467. 2014.PubMed/NCBI
|
|
8
|
Watters DA, Smith AN, Eastwood MA,
Anderson KC and Elton RA: Mechanical properties of the rat colon:
The effect of age, sex and different conditions of storage. Q J Exp
Physiol. 70:151–162. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watters DA, Smith AN, Eastwood MA,
Anderson KC, Elton RA and Mugerwa JW: Mechanical properties of the
colon: Comparison of the features of the African and European colon
in vitro. Gut. 26:384–392. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Egorov VI, Schastlivtsev IV, Prut EV,
Baranov AO and Turusov RA: Mechanical properties of the human
gastrointestinal tract. J Biomech. 35:1417–1425. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rosen J, Brown JD, De S, Sinanan M and
Hannaford B: Biomechanical properties of abdominal organs in vivo
and postmortem under compression loads. J Biomech Eng.
130:0210202008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Howes MK and Hardy WN: Material properties
of the post-mortem colon in high-rate equibiaxial elongation.
Biomed Sci Instrum. 48:171–178. 2012.PubMed/NCBI
|
|
13
|
Gao C and Gregersen H: Biomechanical and
morphological properties in rate large intestine. J Biomech.
33:1089–1097. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Carniel EL, Gramigna V, Fontanella CG,
Stefanini C and Natali AN: Constitutive formulations for the
mechanical investigation of colonic tissues. J Biomed Mater Res A.
102:1243–1254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Humphrey JD: Cardiovascular Solid
Mechanics. Cells, Tissues and Organs. Springer-Verlag; New York:
2002
|
|
16
|
Okumura R, Kurakawa T, Nakano T, Kayama H,
Kinoshita M, Motooka D, Gotoh K, Kimura T, Kamiyama N, Kusu T, et
al: Lypd8 promotes the segregation of flagellated microbiota and
colonic epithelia. Nature. 532:117–121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Elinav E, Strowig T, Kau AL, Henao-Mejia
J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon
JI and Flavell RA: NLRP6 inflammasome regulates colonic microbial
ecology and risk for colitis. Cell. 145:745–757. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hao XP, Lucero CM, Turkbey B, Bernardo ML,
Morcock DR, Deleage C, Trubey CM, Smedley J, Klatt NR, Giavedoni
LD, et al: Experimental colitis in SIV-uninfected rhesus macaques
recapitulates important features of pathogenic SIV infection. Nat
Commun. 6:80202015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou X, Cao L, Jiang C, Xie Y, Cheng X,
Krausz KW, Qi Y, Sun L, Shah YM, Gonzalez FJ, et al: PPARα-UGT axis
activation represses intestinal FXR-FGF15 feedback signalling and
exacerbates experimental colitis. Nat Commun. 5:45732014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li YY, Yuece B, Cao HM, Lin HX, Lv S, Chen
JC, Ochs S, Sibaev A, Deindl E, Schaefer C and Storr M: Inhibition
of p38/Mk2 signaling pathway improves the anti-inflammatory effect
of WIN55 on mouse experimental colitis. Lab Invest. 93:322–333.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kimball ES, Wallace NH, Schneider CR,
D'Andrea MR and Hornby PJ: Vanilloid receptor 1 antagonists
attenuate disease severity in dextran sulphate sodium-induced
colitis in mice. Neurogastroenterol Motil. 16:811–818. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Holzapfel GA, Sommer G, Gasser CT and
Regitnig P: Determination of the layerspecific mechanical
properties of human coronary arteries with nonatherosclerotic
intimal thickening and related constitutive modelling. Am J Physiol
Heart Circ Physiol. 289:H2048–H2058. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tong J, Sommer G, Regitnig P and Holzapfel
GA: Dissection properties and mechanical strength of tissue
components in human carotid bifurcations. Ann Biomed Eng.
39:1703–1719. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Holzapfel GA: Nonlinear Solid Mechanics. A
Continuum Approach for Engineering. 1st. John Wiley & Sons;
Chichester: 2000
|
|
25
|
Holzapfel GA, Gasser TC and Ogden RW: A
new constitutive framework for arterial wall mechanics and a
comparative study of material models. J Elasticity. 61:1–48. 2000.
View Article : Google Scholar
|
|
26
|
Gregerson H: Biomechanics of the
Gastrointestinal TractNew Prospectives in Motility Research and
Diagnostics. Springer-Verlag; London: 2003
|
|
27
|
Quigley EM: What we have learned about
colonic motility: Normal and disturbed. Curr Opin Gastroenterol.
26:53–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bassotti G, Villanacci V, Mazzocchi A,
Castellani D, Giuliano V, Corsi S and Morelli A: Colonic propulsive
and postprandial motor activity in patients with ulcerative colitis
in remission. Eur J Gastroenterol Hepatol. 18:507–510. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sokolis DP, Orfanidis IK and Peroulis M:
Biomechanical testing and material characterization for rat large
intestine: Regional dependency of material parameters. Physiol
Meas. 32:1969–1982. 2011. View Article : Google Scholar : PubMed/NCBI
|