Introduction
Hepatic fibrosis (HF) is a serious health problem
worldwide. The deregulated repairing reaction of liver tissues
after chronic hepatic injuries is the pathological basis of HF. The
continuous damage of liver tissue due to HF may ultimately result
in hepatic cirrhosis, which is a major causes of liver
disease-associated mortality (1).
Using an aggressive method to treat the primary disease and HF is
effective in preventing and even reversing HF and hepatic cirrhosis
(2). Taohongsiwu decoction, is a
traditional Chinese medicinal recipe that contains multiple Chinese
herbs. For instance, peach keruel (3) contains amygdalin, and thus could
inhibit the synthesis (4) and
secretion of collagens I, III, and IV, as well as laminin.
Carthamus tinctorius and hy-droxysafflor flavin A have been
suggested to inhibit the activation and transition of hepatic
stellate cells (HSCs) (5), and their
anti-lipid peroxidation effects could also reduce the degree of
hepatocyte swelling and cirrhosis, thus improving the repair of
damaged liver tissues (6). Herbs in
the genus Angelica (7) are
purported to inhibit the proliferation of fibroblasts, reduce the
synthesis of collagens, and protect the rat models of HF induced by
carbon tetrachloride (CCl4). Total glucosides of peony
(8) may inhibit the expression of
nuclear factor κB and transforming growth factor-β1 (TGF-β1) in the
fibrotic liver tissues of rats. Ligustrazine (tetramethylpyrazine)
(9) could inhibit the TGF-β1-induced
expression of connective tissue growth factor gene, thus blocking
the synthesis of collagen I and resulting in anti-fibrosis effects
in the liver. However, the mechanisms involved in the anti-HF
effects of Taohongsiwu decoction are still unclear. In the present
study, rat models of HF induced by CCl4 were used to
comprehensively investigate the anti-HF effects of Taohongsiwu
decoction and the underlying mechanisms, and provide evidence for
the clinical application of Taohongsiwu decoction.
Materials and methods
Animals
Healthy male Sprague-Dawley rats (specific
pathogen-free grade) with the body weight of 200–220 g were
obtained from the Anhui Experimental Animal Center [SCXK (Wan)
2011–002]. The rats were acclimated for 2 weeks before the
experiments.
Reagents
Taohongsiwu decoction contains peach keruel (9 g),
Carthamus tinctorius (6 g), Ligusticum wallichii (6
g), radix Rehmanniae preparata (12 g), Angelica (9
g), and radix Paeoniae alba (9 g). All these drugs were
purchased from the Anhui Xiehecheng Pharmaceutical Co., Ltd.
(Bozhou, China). The catalogue numbers of the drugs were as
follows: No. 13121001 for peach keruel, no. 13091001 for
Carthamus tinctorius, no. 1310702 for Ligusticum
wallichii, no. 13120101 for radix Rehmanniae preparata,
no. 14021101 for Angelica, and no. 13072001 for radix
Paeoniae alba. Ferulic acid (no. 110773-201313) and
hydroxysafflor yellow (no. A111637-200906) were purchased from the
Chinese National Institutes for Food and Drug Control (Beijing,
China). CCl4 (analytically pure grade, no. 20091216) was
obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai,
China). Lu Hua 5S Pressing First-Class peanut oil (no. 20130521)
was obtained from the Shandong Luhua Group, Co., Ltd. (Shandong,
China). Colchicine tablet (no. 130511) was obtained from
Xishuangbanna Banna Pharmaceutical Co., Ltd. (Jinghong, China).
Aspartate aminotransferase (AST; no. C010-1), alanine
aminotransferase (ALT; no. C009-1), and ALB (no. A028-1) were
obtained from Nanjing Jiancheng Biological technology Co., Ltd.
(Nanjing, China). Hyaluronic acid (HA), Laminin protein (LN),
procollagen III (PCIII) and collagen IV (IV-C) radioimmunoassay
kits (no. 20140507) were obtained from the Beijing Sinouk Institute
of Biological Technology (Beijing, China). A Masson staining kit
(no. MST-8003/8004) was provided by Maixin Biological Technology
Development Co., Ltd. (Fuzhou, China). An electrochemiluminescence
(ECL) kit (no. NL178395) was provided by Thermo Fisher Scientific,
Inc. (Waltham, MA, USA). Actin (no. 140829), goat-anti-mouse IgG
(no. 112971) and goat-anti-rabbit IgG (no. 107015) were obtained
from Beijing Zhongshan Jinqiao Biotechnology Co., Ltd. (Beijing,
China). Primers were synthesized by Invitrogen (Thermo Fisher
Scientific, Inc., Carlsbad, CA, USA). The primer sequences for the
fluorescent quantitation of the rat β-actin were as follows:
Forward, 5′-CCCATCTATGAGGGTTACGC-3′ and reverse,
5′-TTTAATGTCACGCACGATTTC-3′, and the length of the product was 150
bp. The primer sequences for the rat TGF-β1 were as follows:
Forward, 5′-CAGAGAAGAACTGCTGTGTACGG-3′ and reverse,
5′-CAGACAGAAGTTGGCATGGTAGC-3′, and the length of the product was
104 bp. A RevertAid first strand cDNA synthesis kit (no. 00145205)
was obtained from Thermo Fisher Scientific, Inc.
Equipment
High-performance liquid chromatography (HPLC;
Agilent Technologies, Santa Clara, CA, USA) was used in the present
study. An ultraviolet spectrophotometer (UV-1800) was bought from
the Shanghai Jinhua Scientific Instruments Co., Ltd. (Shanghai,
China). A full-electrodynamic advanced upright microscope was
brought from the Olympus Corporation (Tokyo, Japan). A γ-911
full-automatic radioimmunity analyzer was brought from the China
University of Science and Technology Industry Corporation. An EPS
300 electrophoresis apparatus was brought from the Tanon Science
& Technology Co., Ltd. (Shanghai, China). A fluorescent
quantitative polymerase chain reaction (PCR) system (PIKOREAL 96)
was brought from the Thermo Fisher Scientific, Inc.
Preparation and measurement of
Taohongsiwu decoction
Appropriate volumes of the drugs were obtained and
immersed in two volumes of water for 30 min. Then, 10 volumes of
water were added, boiled and then filtered. Eight volumes of water
were added to the residues, boiled and filtered. The liquid
obtained by both the filtration were combined and concentrated to a
concentration of 1 g/ml crude drug. Exactly 5 ml of the extraction
was added to an evaporating dish, evaporated dry, and then 50%
methanol was added to obtain a final volume of 10 ml. The liquid
was mixed then filtered, and the filtered liquid was further
filtered through a 0.45-µm microfiltration membrane to obtain the
solution for the experiment. Gradient elution was performed with
the mobile phase of acetonitrile (A) and 0.1% phosphoric acid
solution (B). The flow velocity used was 1.0 ml/min, the column
temperature was 40°C, the sample size was 20 µl and the determine
wavelengths were as follows: 0–11 min, 290 nm; 11–27 min, 320 nm;
27–45 min, 230 nm; and 45–80 min, 270 nm. An HPLC instrument
(Agilent Technologies) was used for measuring the concentration of
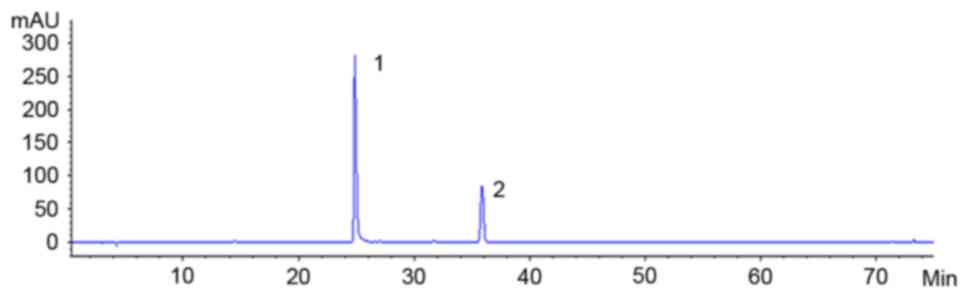
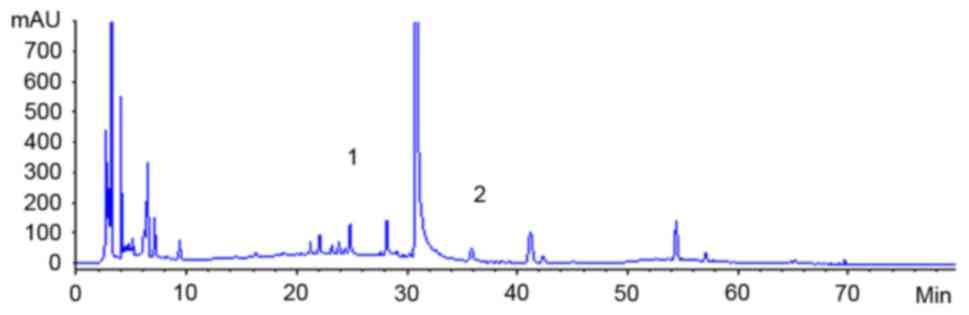
the liquid (Figs. 1 and 2).
Inducing rat model of HF (10)
Male Sprague-Dawley rats with the body weight of
200–220 g were obtained, and 50% CCl4 of peanut oil
solution (0.1 ml/kg, freshly prepared) was subcutaneously injected
to the back of the rats (twice/week) for 12 continuous weeks to
induce the rat model of HF.
Grouping and drug administration
Rats were randomly divided into model, colchicine (2
mg/kg), Taohongsiwu-high (17 g/kg), Taohongsiwu-moderate (8.5
g/kg), and Taohongsiwu-low (4.25 g/kg) groups with 10 rats in each
group. Another 10 rats were included as the control group. Since
week 7 after the model induction, the intragastric administration
of the drugs was performed with the dose of 10 ml/kg (once per day)
for 6 continuous weeks, while the same volume of distilled water
was administered for the rats in the model and control groups. The
rats were deprived of food but not water for 12 h after the last
drug administration; chloral hydrate (300 mg/kg body weight) was
used to anesthetize the rats. Then, blood was collected from the
abdominal aorta, and serum was separated. Part of the liver of the
rats was also collected to measure the parameters.
Measuring serum indexes
Blood was collected from the abdominal aorta of the
rat, placed at room temperature for 1 h, then centrifuged at 825 ×
g for 10 min to separate the serum. The serum level of ALB and
activities of AST and ALT were measured by colorimetry, and serum
levels of HA, LN, PCIII and IV-C were measured by
radioimmunoassay.
Pathological examinations of the liver
tissues
For each rat, part of the liver was obtained, fixed
with 10% formalin, embedded with paraffin, and sliced. Then,
hematoxylin and eosin (H&E) and Masson staining were performed
for pathological examinations.
Measuring Col I levels in the liver
tissues
Col I levels in the liver tissues were measured by
western blot analysis. In brief, liver tissues were collected and
weighed. A total of 100 mg liver tissue was obtained, then 1 ml
radioimmunoprecipitation assay cell lysis buffer (containing 1 mM
phenylmethylsuofonyl fluoride) was added to lyse the cells. The
mixture was then centrifuged at 13,200 × g for 10 min, and the
supernatant was collected. Proteins were separated by 10% SDS-PAGE
and transferred onto a nitrocellulose filter membrane (Merck KGaA,
Darmstadt, Germany). Then the protein-loading membrane was
immediately placed into the western washing buffer for 5 min to
remove the transmembrane buffer. The western blocking buffer (5%
skim milk powder) was then added, and the membrane was blocked at
room temperature for 2 min with gentle shake. Primary rabbit
antibody for Collagen I (1:500, 8% separation gel) was added, the
membrane was incubated at 4°C overnight with gentle shaking. Then
Tris-Buffered Saline and Tween 20 (TBST) was used to wash the
membrane three times for 5 min each time. The horseradish
peroxidase-labeled secondary antibody (1:10,000) was added, the
membrane was blocked at room temperature for 2 h; then washing
buffer (TBST) was used to wash the membrane three times for 10 min
each time. The ECL kit was used to measure the protein level. Image
J software (National Institutes of Health, USA) was used to analyze
the gray values.
Measuring α-SAM expression in the
liver tissues
Immunohistochemistry was used to measure the α-SMA
expression in the liver tissues. In brief, the liver tissues were
embedded with paraffin, continuously sliced (thickness, 5 µm) and
deparaffinated with xylene. Then, gradient ethanol hydration was
performed, and the slices were washed with distilled water and
incubated in 3% H2O2 deionized water mixture
for 15 min to end the activity of endogenous peroxidase. Phosphate
buffered saline (PBS; pH 7.4) was used to wash the slices three
times for 3 min each time, then normal goat serum (Boster
Biological Technology, Pleasanton CA, USA) working buffer used as a
blocking solution was added and incubated for 15 min. The liquid
was discarded, and the slices were not washed. The appropriately
diluted primary antibody was added, and the slices were incubated
at 4°C overnight. Next, the slices were washed again with PBS (pH
7.4) three times for 5 min each time, after which biotinylated
secondary antibody was added, incubated at 37°C for 15 min, washed
with PBS (pH 7.4) three times for 5 min each time, developed with
diaminobenzidine for 2 min, and then water was added to incubate
for 5 min to end the reaction. The slices were blotted dry and
mounted with neutral balsam. The light microscope was used for the
observation, under which α-SMA-positive cells appeared brown
yellow. Six slices were selected from each group, and at least five
non-overlapping, complete visual fields were randomly selected in
each slice under high magnitude. Image-Pro Plus 6.0 software (Media
Cybernetics, Inc., Silver Spring, MD, USA) was used to measure the
average optical density and the integrated optical density in each
visual field, and the mean values were calculated for the
statistical analysis.
Measuring TGF-β1 mRNA expression in
the liver tissues
Fluorogenic quantitative PCR was used to measure the
TGF-β1 mRNA expression in the liver tissues. The processes were as
follows. A total of 50–100 mg liver tissues were obtained, cut into
small pieces, ground in liquid nitrogen, and then 1 ml TRIzol was
added and homogenized. Then the homogenate was centrifuged at 4°C,
13,200 × g for 10 min to remove the insufficiently lysed tissues
and fat. Chloroform (0.2 ml) was added, violently mixed for 15 sec,
and placed at room temperature for 3 min. Then another
centrifugation at 4°C, 13,200 × g was performed for 15 min, and the
supernatant (~500 µl) was transferred to another Eppendorf (EP)
tube. Isopropanol (0.5 ml) was added, mixed gently, placed at room
temperature for 10 min, centrifuged at 4°C, 13,200 × g for 10 min,
and the supernatant was discarded. A total of 1 ml of 75% ethanol
[prepared with diethylpyrocarbonate (DEPC) water] was then added,
after which the mixture was centrifuged at 4°C, 13,200 × g for 5
min, and the supernatant was discarded. Then the EP tube was placed
at room temperature for 30 min to allow the RNA dry. DEPC (20–50
µl) water was added, heated to 55°C to help dissolve the RNA, and
then stored at −80°C until use.
Fluorogenic quantitative PCR
analysis
Total RNA (3 µg) and 1 µl of 10 µM oligo (3dT) were
added into a 0.2-ml EP tube, and then DEPC water was added to
obtain a final volume of 12 µl. The tube was mixed gently and
centrifuged transiently. The tubes were heated to 65°C for 5 min on
the PCR instrument, and then placed in an ice bath for 3 min. A
total of 4.0 µl of 5X reaction buffer, 2 µl of 10 mM dNTP mix, 1 µl
of RNase inhibitor, and 1 nl of RevertAid M-MuLV Reverse
Transcriptase were added into each EP tube for the reaction (at
42°C for 60 min and then at 70°C for 5 min). The reaction liquid
was the cDNA, and was stored at −80°C until use.
The reaction system was as follows: cDNA template, 1
µl; RNase free water, 2 µl; forward and reverse primers (10 µM), 1
µl each; and 2X SYBR Green mixture, 5 µl. The reaction conditions
were as follows: 95°C for 5 min, 95°C for 10 sec, and 60°C for 30
sec for 40 cycles. A relative quantification method was used for
analysis, and the index used for the analysis was
2−∆∆Cq.
Statistical analysis
SPSS 11.0 software (SPSS, Inc., Chicago, IL, USA)
was used for the statistical analysis. All the data are presented
as the mean ± standard deviation, and compared with one-way
analysis of variance followed by least-significant difference
t-test.
Results
Concentrations of hydrosafflow yellow
and ferulic acid in Taohongsiwu decoction
HPLC examinations showed that the concentration of
ferulic acid and hydrosafflow yellow in Taohongsiwu decoction was
0.12 and 0.57 mg/ml, respectively.
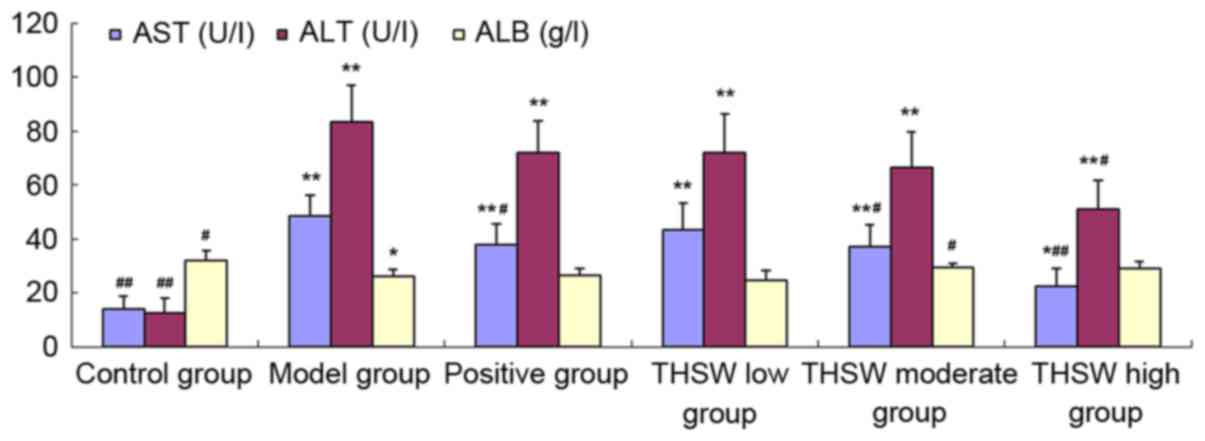
Effects of Taohongsiwu decoction on
the serum levels of AST, ALT, and ALB of the rat models of HF
As shown in Table I
and Fig. 3, the AST and ALT levels
increased significantly in all the groups of rat models, while the
ALB level decreased significantly compared with the control group.
After the drugs were administered for 6 continuous weeks, the AST
level in the colchicine, Taohongsiwu-high, and Taohongsiwu-moderate
groups decreased significantly, and the differences with the model
group were statistically significant; while the ALB level in the
Taohongsiwu-moderate group increased significantly, and the
difference with the model group was statistically significant.
 | Table I.Effects of Taohongsiwu decoction on
the serum levels of AST, ALT and ALB of the rat models of hepatic
fibrosis. |
Table I.
Effects of Taohongsiwu decoction on
the serum levels of AST, ALT and ALB of the rat models of hepatic
fibrosis.
| Group | N | AST (U/l) | ALT (U/l) | ALB (g/l) |
|---|
| Control | 10 |
13.91±5.01a |
12.69±5.35a | 31.93±3.59 |
| Model | 9 |
48.42±7.65b |
83.31±13.85b |
26.17±2.44c |
| Positive | 8 |
37.78±7.71b,d |
71.97±11.66b | 26.43±2.56 |
| Taohongsiwu |
|
|
|
|
| Low | 9 |
43.49±9.94b |
72.23±14.05b | 24.77±3.69 |
| Moderate | 9 |
37.01±8.05b,d |
66.53±13.39b |
29.40±1.54d |
| High | 10 |
22.48±6.62a,d |
51.16±10.73b,d | 28.99±2.79 |
Effects of Taohongsiwu decoction on
the serum levels of LN, PIII, IV-C and HA of the rat models of
HF
As shown in Fig. 4,
the serum levels of LN, PCIII, IV-C and HA increased in all the
groups of rat models, among which the increase of the IV-C and HA
levels was statistically significant compared with the control
group (P<0.05). The levels of all the four indexes improved
after the drug administration, among which the IV-C and HA levels
in the Taohongsiwu-high group, and the IV-C level in the
Taohongsiwu-moderate group decreased significantly, and the
differences with the model group were statistically significant
(P<0.05).
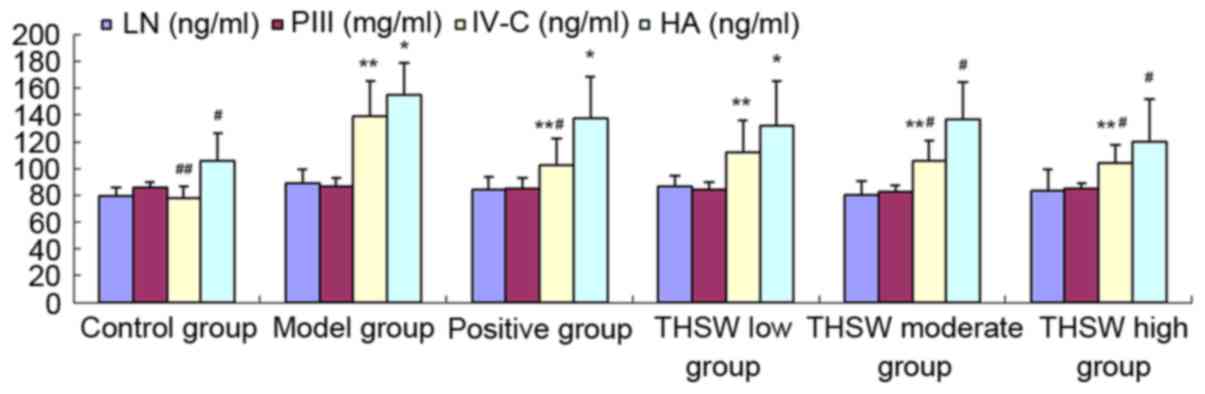 | Figure 4.Effects of Taohongsiwu decoction on
the serum levels of LN, PIII, IV-C, and HA of the rat models of
hepatic fibrosis. *P<0.05, **P<0.01 vs. control group;
#P<0.05, ##P<0.01, vs. model group. LN,
laminin; PIII, procollagen III; IV-C, procollagen IV; HA,
hyaluronic acid; THSW, Taohongsiwu. |
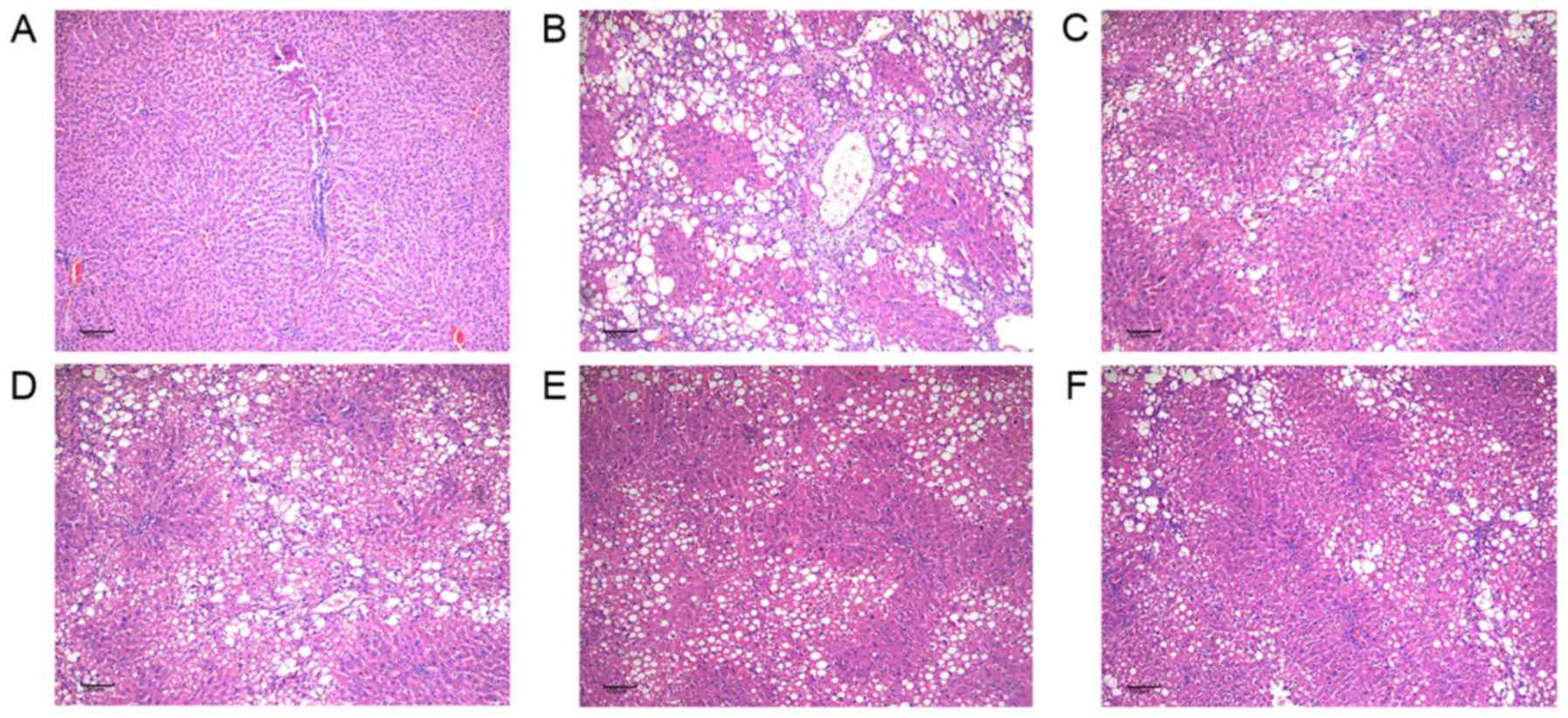
Effects of Taohongsiwu decoction on
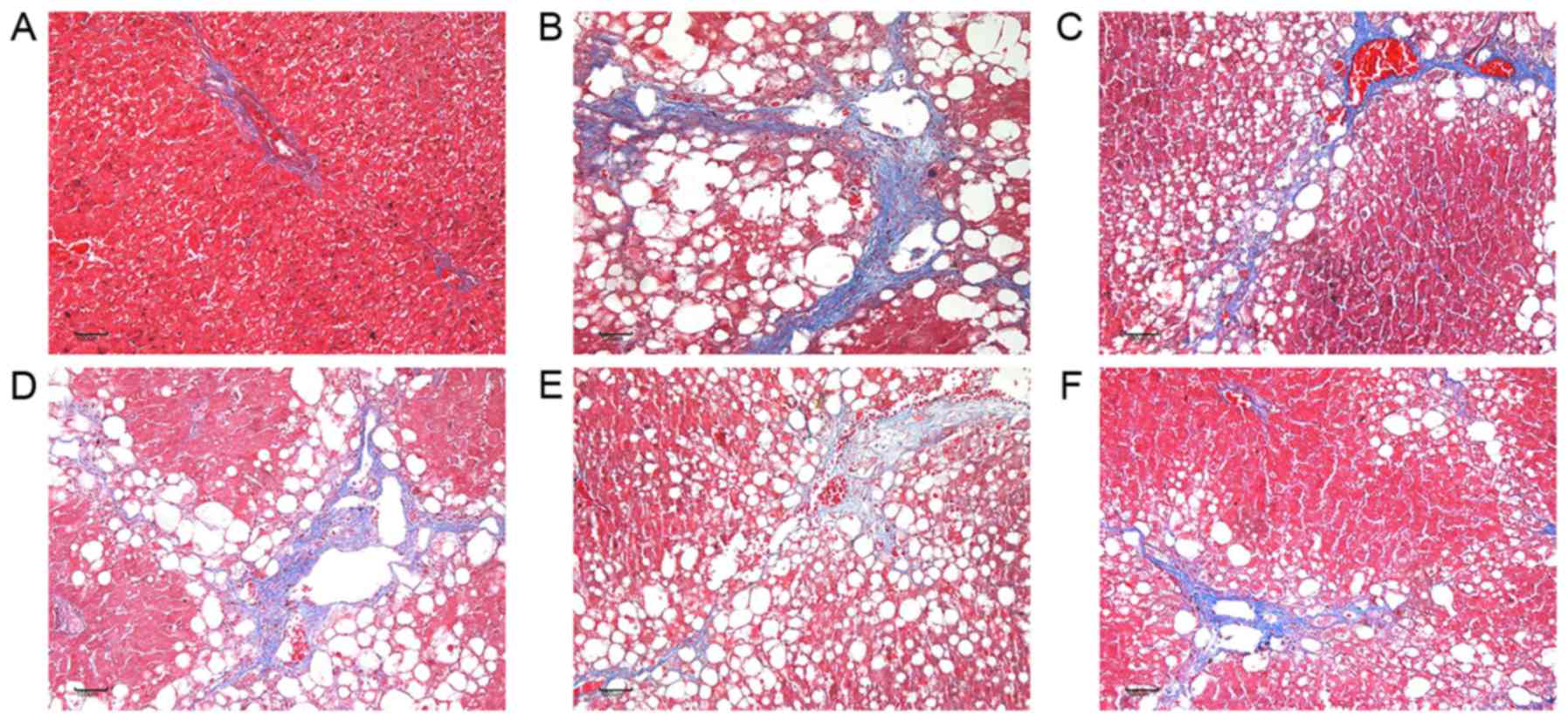
the pathological changes of the liver tissues of the rat models of
HF
The effects of Taohongsiwu decoction on the
pathological changes of the liver tissues of the rat models of HF
are shown in Figs. 5 and 6. H&E staining showed that the hepatic
lobules in the control group were with complete structure, and the
hepatic cords arranged regularly; while no inflammatory cell
infiltration in the portal area of the hepatic lobules or fibrotic
tissue hyperplasia was found. For the rats in the model group, the
structure of the hepatic lobules were found damaged, the
arrangement of the hepatic cords was irregular, the hepatic cells
were with diffused vacuolar degenerations and necrosis. The portal
area expanded and presented with large amounts of fibrotic tissue
hyperplasia, which separated the hepatic lobules into several
hepatocyte clusters of different sizes. The damages of the hepatic
lobules in the control group and the groups treated with drugs were
evidently decreased, with limited inflammatory cell infiltration in
the liver tissues observed. Furthermore, the swelling of the liver
cells and the degree of fatty degeneration were evidently lesser
than in the model group.
Masson staining showed that the liver tissues in the
control group presented with limited collagen deposition at the
venous walls and the bile duct walls in the portal area. For the
rats in the model group, extensive fibrotic tissue hyperplasia was
found in the interstitial tissues; the collagens increased and
enlarged, and diffused in the liver tissues to separate the hepatic
lobules into several pseudolobules of different sizes. While the
collagens in the liver tissues of the rats treated with Taohongsiwu
decoction were evidently decreased, the fibrous septum was short
and narrow, and no pseudolobule was observed.
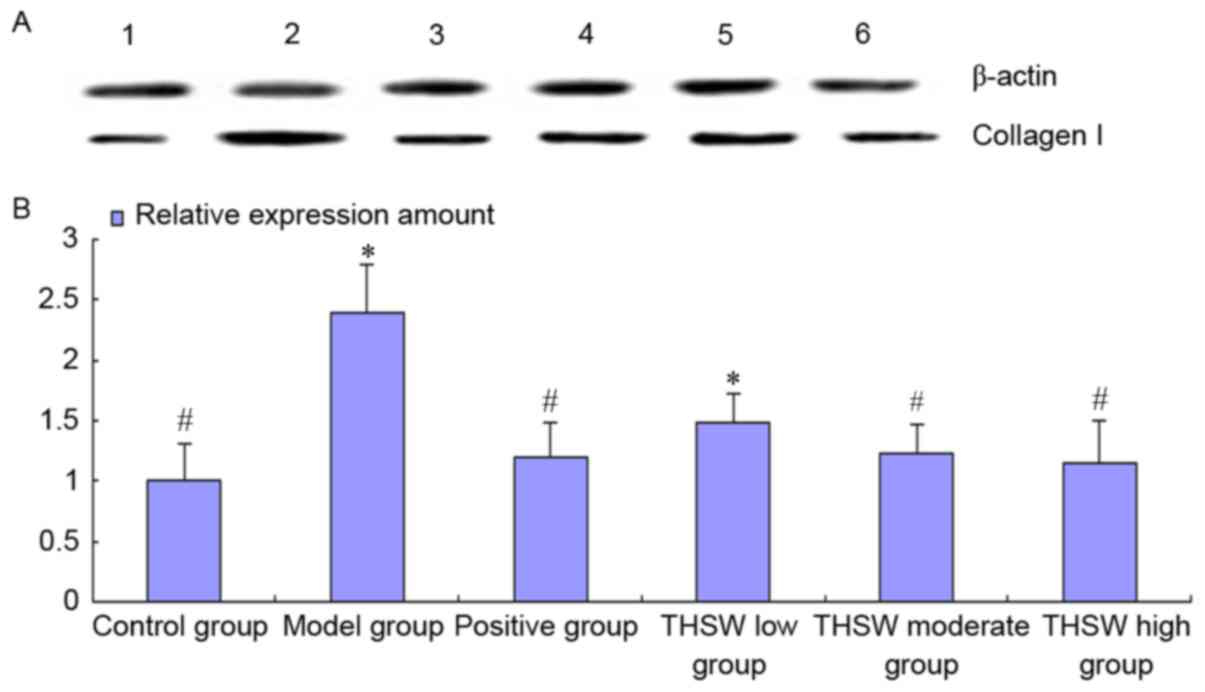
Effects of Taohongsiwu decoction on
the Col I level in the liver tissues of the rat models of HF
As shown in Fig. 7,
the collagen level in the liver tissues increased in all the rats
at 12 weeks after the model induction, and the level in the model
and Taohongsiwu-low groups was significantly different from the
control group. After treatment for 6 weeks, the collagen level in
the liver tissues of the rats decreased, and the collagen level in
the positive, Taohongsiwu-high, and Taohongsiwu-moderate groups was
significantly reduced compared with the model group
(P<0.05).
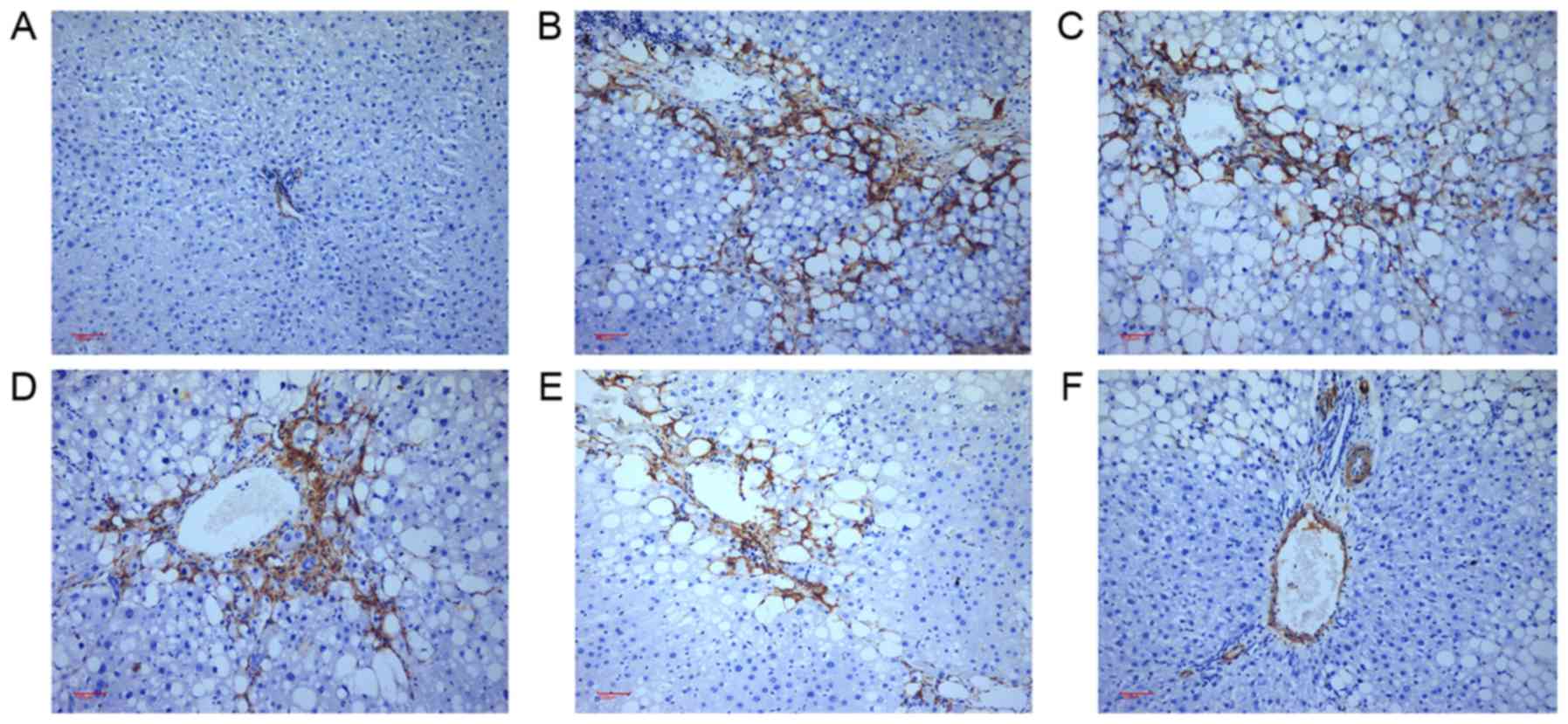
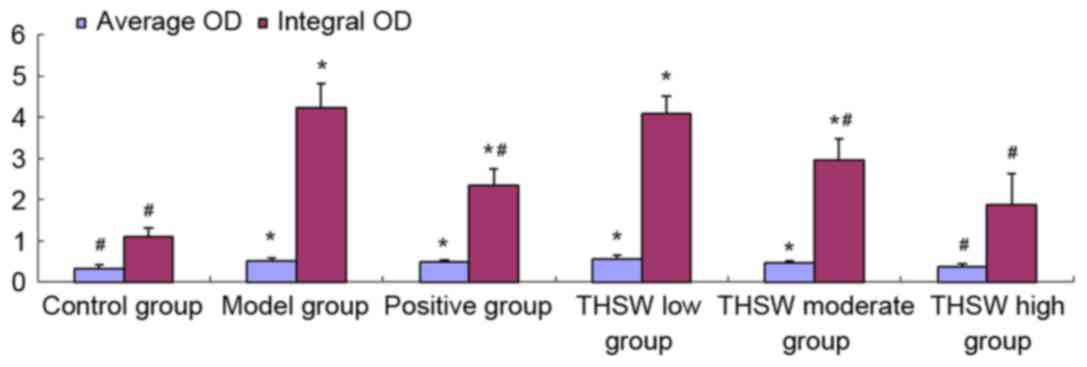
Effects of Taohongsiwu decoction on
α-SMA expression in the liver tissues of the rat models of HF
As shown in Figs. 8
and 9, α-SMA-positive tissues in the
liver tissues are shown as brown yellow. Limited α-SMA expression
was detected at the vascular walls of the liver tissues in the
control group. For the rats in the model group, the expression of
α-SMA was found not only at the vascular walls, but also widely
spread at the portal area, fibrous septum, and the adjacent hepatic
sinusoids. After treated with the drugs for six continuous weeks,
the expression of α-SMA in the liver tissues decreased
significantly, especially in the positive, Taohongsiwu-high, and
Taohongsiwu-moderate groups; the α-SMA expression level in these
three groups was evidently lower compared with the model group
(Fig. 9).
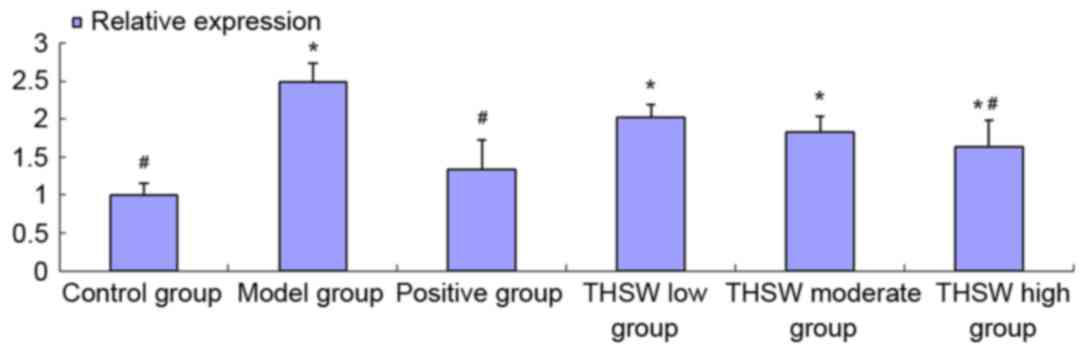
Effects of Taohongsiwu decoction on
TGF-β1 mRNA expression in the liver tissues of the rat models of
HF
As shown in Fig. 10,
the expression of TGF-β1 mRNA increased significantly in the all
the model groups, and the difference with the control group was
statistically significant (P<0.05). After treatment with the
drugs for six continuous weeks, the TGF-β1 mRNA expression
decreased, particularly in the colchicine and Taohongsiwu-high
groups, in which the expression was significantly decreased
compared with the model group (P<0.05).
Discussion
HF is an inevitable stage of the progression of
chronic liver diseases into cirrhosis, and is caused by the
imbalanced proliferation and degeneration of fibrosis, which could
cause the overdeposition of fibrillar connective tissues in the
liver (11). Currently, no specific
drug that could cure liver cirrhosis is available. However,
previous results have shown that HF is reversible, and preventing
the deposition and promoting the degeneration of extracellular
matrix is one of the effective methods in anti-fibrosis and
treating liver cirrhosis (12).
The development and progression of HF is a complex
systemic pathophysiological process, which involves multiple
cytokines and the network of intracellular signaling molecules. The
activation of HSC has been acknowledged as the central factor in
developing HF (13). When the
hepatocytes are damaged by ethanol, viral infection, or drugs, the
hepatocytes occur the inflammatory reaction and stimulate the
secretion of cytokines (such as TGF-β1, PDGF and VEGF) by Kupffer
cells, liver sinusoidal endothelial cells and macrophages will
occur (14). The stimuli will cause
phenotype transformation of the HSC from quiescent phenotype to
myofibroblast (MFB)-like phenotype that express α-SMA, which could
be further activated and transformed to MFB. MFB phenotype HSC to
secrete large quantities of extracellular matrix including collagen
I and III, HA, and laminin; reduce the activity of collagenase and
the degeneration of collagen, resulting in the imbalance of the
production and degeneration of extracellular matrix, formation of
fibrosis, and a large expression of α-SMA protein, which has been
considered as an important marker of the activation of HSC
(15). The activated HSC could also
secrete cytokines including TGF-β1 and tumor necrosis factor-α, and
further promote activation of themselves. Therefore, TGF-β1 is
crucial for the activation, transformation, differentiation and
regulation of HSCs. TGF-β1 has been proposed to be a potential
factor promoting the fibrosis of HSC, and the level of TGF-β1 may
indicate the degree of inflammation, necrosis and fibrosis in
chronic HF tissues (16).
The mechanisms of the currently used drugs for the
treatment of HF include inhibition of liver inflammation and immune
response, anti-oxidative damages, inhibition of the activation of
HSC, inhibition of the synthesis of extracellular matrix, and
acceleration of the degeneration of extracellular matrix. Previous
studies have suggested that Taohongsiwu decoction treatment may be
effective against acute liver damages (17), and may inhibit the activation of HSC
(18). The active ingredients in
Taohongsiwu decoction include amygdalin, ligustrazine and
hydrosafflow yellow that could reduce liver damages, inhibit HSC
activation, improve the structure of liver tissues, inhibit the
deposition of collagen and delay the progression of liver fibrosis
(19–21). The findings of the present study
suggest that Taohongsiwu decoction may improve the liver function
of the rat models of HF, reduce the collagen deposition in the
serum and liver tissues, and inhibit the expression of α-SMA
protein and TGF-β1 mRNA. These findings suggest that Taohongsiwu
decoction treatment exhibits effective protective effects against
HF, and the underlying mechanisms could be associated with liver
protection and inhibition of TGF-β and HSC activation. Further
studies are needed to further investigate the precise underlying
mechanisms.
Acknowledgements
The present study was supported by the Anhui
Province College Students Innovative and Entrepreneurial Training
Project (grant no. AH201310369054).
References
|
1
|
Imani F, Motavaf M, Safari S and Alavian
SM: The therapeutic use of analgesics in patients with liver
cirrhosis: A literature review and evidence-based recommendations.
Hepat Mon. 14:e235392014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun M and Kisseleva T: Reversibility of
liver fibrosis. Clin Res Hepatol Gastroenterol. 39 Suppl 1:60–63.
2015. View Article : Google Scholar
|
|
3
|
Xu LM, Liu P, Liu C, Hong JH, Lu G, Xue
HM, Zhu JL and Hu YY: Observation on the action of extractum semen
Persicae on anti-fibrosis of liver. Zhongguo Zhong Yao Za Zhi.
19491–494. (512)1994.(In Chinese). PubMed/NCBI
|
|
4
|
Wang YQ: Effect and molecular mechanism of
peach kernel extract on liver fibrosis. J Med Theor Pract.
15:2015–2016. 2014.(In Chinese).
|
|
5
|
Zhang Y, Guo J, Dong H, Zhao X, Zhou L, Li
X, Liu J and Niu Y: Hydroxysafflor yellow A protects against
chronic carbon tetrachloride-induced liver fibrosis. Eur J
Pharmacol. 660:438–444. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang CY, Liu Q, Huang QX, Liu JT, He YH,
Lu JJ and Bai XY: Activation of PPARγ is required for
hydroxysafflor yellow A of Carthamus tinctorius to attenuate
hepatic fibrosis induced by oxidative stress. Phytomedicine.
20:592–599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang WQ, Wang BX and Shao WW: Effect of
Angelica on the collagen content of hepatic tissue in liver
fibrosis model rats induced by carbon tetrachloride. Hei Long Jiang
Yi Yao. 26:252003.(In Chinese).
|
|
8
|
Lu JT, Sun WY and Liu H: Effects of total
glucosides of paeony on protein expression of NF-kappaB and TGF-β1
in hepatic tissue of rats with immunological hepatic fibrosis. Chi
Pharmacological Bulletin. 24:588–592. 2008.
|
|
9
|
Lu B, Yu L, Li S, Si S and Zeng Y:
Alleviation of CCl4-induced cirrhosis in rats by
tetramethylpyrazine is associated with downregulation of leptin and
TGF-beta1 pathway. Drug Chem Toxicol. 33:310–315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang Q, Li Y, Zhang S, Huang R, Zheng L,
Wei L, He M, Liao M, Li L, Zhuo L and Lin X: Effect and mechanism
of methyl helicterate isolated from Helicteres angustifolia
(Sterculiaceae) on hepatic fibrosis induced by carbon tetrachloride
in rats. J Ethnopharmacol. 143:889–895. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu T, Wang P, Cong M, Zhang D, Liu L, Li
H, Zhai Q, Li Z, Jia L and You H: Matrix metalloproteinase-1
induction by diethyldithiocarbamate is regulated via Akt and
ERK/miR222/ETS-1 pathway in hepatic stellate cells. Biosci Rep.
36(pii): e003712016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi JH, Hwang YP, Park BH, Choi CY, Chung
YC and Jeong HG: Anthocyanins isolated from the purple-fleshed
sweet potato attenuate the proliferation of hepatic stellate cells
by blocking the PDGF receptor. Environ Toxicol Pharmacol.
31:212–219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han M, Liu X, Liu S, Su G, Fan X, Chen J,
Yuan Q and Xu G: 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces
hepatic stellate cell (HSC) activation and liver fibrosis in C57BL6
mouse via activating Akt and NF-κB signaling pathways. Toxicol
Lett. 273:10–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marrone G, Shah VH and Gracia-Sancho J:
Sinusoidal communication in liver fibrosis and regeneration. J
Hepatol. 65:608–617. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li S, Wang L, Yan X, Wang Q, Tao Y, Li J,
Peng Y, Liu P and Liu C: Salvianolic acid B attenuates rat hepatic
fibrosis via downregulating angiotensin II signaling. Evid Based
Complement Alternat Med. 2012:1607262012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qu Y, Zong L, Xu M, Dong Y and Lu L:
Effects of 18α-glycyrrhizin on TGF-β1/Smad signaling pathway in
rats with carbon tetrachloride-induced liver fibrosis. Int J Clin
Exp Pathol. 8:1292–1301. 2015.PubMed/NCBI
|
|
17
|
Ju MY, Tang YZ and Ji YH: Effect of
Taohong Siwu decoction on acute liver injury in model rats induced
CCl4. Shandong J Tradit Chin Med. 751–752. 2012.(In Chinese).
|
|
18
|
Luo LQ and Chen DB: Effect of Taohong siwu
decoction on the proliferation of hepatic stellate cell in rats
with hepatic fibrosis. Yi Chun Xue Yuan Xue Bao. 36:69–72. 2014.(In
Chinese).
|
|
19
|
Wu X, Zhang F, Xiong X, Lu C, Lian N, Lu Y
and Zheng S: Tetramethylpyrazine reduces inflammation in liver
fibrosis and inhibits inflammatory cytokine expression in hepatic
stellate cells by modulating NLRP3 inflammasome pathway. IUBMB
Life. 67:312–321. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li XM, Hu YY and Duan XH: Uniform designed
research on the active ingredients assembling of Chinese medicine
prescription for anti-liver fibrosis. Zhongguo Zhong Xi Yi Jie He
Za Zhi. 30:58–63. 2010.(In Chinese). PubMed/NCBI
|
|
21
|
Jiang S, Shi Z, Li C, Ma C, Bai X and Wang
C: Hydroxysafflor yellow A attenuates ischemia/reperfusion-induced
liver injury by suppressing macrophage activation. Int J Clin Exp
Pathol. 7:2595–2608. 2014.PubMed/NCBI
|