Introduction
Bronchial asthma is a chronic inflammatory airway
disease in which a variety of cells and cytokines are involved,
including eosinophils, macrophages, neutrophils and Th2 cells, in
addition to other inflammatory cytokines and chemokines (1–3).
Although the mechanism of asthma remains unclear, previous studies
have suggested that an imbalance of Th1/Th2 is directly involved in
the inflammatory response of asthma (4,5). The
treatment approaches for asthma that are used clinically include
hormones, β2 receptor agonists and leukotriene receptor
antagonists. However, due to the complex causes and mechanisms of
bronchial asthma, these treatments are not satisfactory, and do not
significantly reduce the symptoms, which include wheezing,
shortness of breath, chest tightness and coughing. Studies have
also identified imbalances in Th1/Th2 and Th17/Treg in patients
with allergic asthma (6,7). Previous evidence (8) has suggested that follicular helper T
(TFH) cells are a specific cluster of differentiation
CD4+ effector T cells subset, that is distinguished from
T helper 1 (Th1) cells and Th2 cells in the tonsils, spleen and
lymph nodes. They specifically maintain the structure and function
of germinal centers (GC) and regulate memory B cell responses
(9,10). There is also evidence indicating that
a TFH imbalance or dysfunction is associated with autoimmune
diseases, including systemic lupus erythematosus, rheumatoid
arthritis and autoimmune thyroiditis (11–13). To
the best of our knowledge, the functions of TFH cells in the
pathogenesis of asthma, however, have not been reported. The
present study hypothesized that TFH cells affect the asthma
pathogenesis via TFH-associated markers.
Materials and methods
Animals
A total of 16 female BALB/c mice (6–8 weeks old,
20–22 g), purchased from the Animal Experiment Center, Xinjiang
Medical University (Xinjiang, China), were housed in microisolator
cages and received a regular diet (access to food and water ad
libitum). The laboratory temperature was 24±1°C, and the relative
humidity was 40–80%. All experimental protocols were approved by
the Animal Ethics Committee of the First Affiliated Hospital of
Xinjiang Medical University.
Reagents
Ovalbumin (OVA) was used as an allergen
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Aluminum hydroxide
gel was used as an adjuvant (Tianjin Pharmaceutical Group Co.,
Ltd., Tianjin, China). Anti-mouse inducible T-cell costimulator
(ICOS)-fluorescein isothiocyanate (FITC) (11–9942), anti-mouse
C-X-C chemokine receptor type 5 (CXCR5)-phycoerythrin (PE)
(85–12-1859-41), anti-mouse CD4-PE Cy5 (15-0042-82), anti-mouse
CD3-PE-allophycocyanin (APC) (85-17-0032-80) antibodies were
purchased from eBioscience (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Red Blood Cell Lysis Buffer was purchased from
Tiandz, Inc. (Beijing, China). Lymphocyte separation medium was
purchased from Westang Bio-Tech, Co., Ltd. (Shanghai, China).
TRIzol, synthesize first-strand cDNA with reverse transcriptase and
Platinum SYBR-Green qPCR SuperMix-UDG were purchased from
Invitrogen (Thermo Fisher Scientific, Inc.). Primers were designed
and synthetized by the Jiancheng Bioengineering Institute of
Nanjing (Nanjing, China). SDS-PAGE electrode buffer was purchased
from BestBio (Shanghai, China), antibodies against B cell lymphoma
6 (BCL-6) (sc-365618), CXCR5 (sc-8178) and ICOS (sc-65285) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
ICOS ligand (ICOSL) antibody (bs-2471R) and interleukin (IL)-21
rabbit polyclonal antibody (bs-2621R) were purchased from Bioss
(Woburn, MA, USA). All other chemicals were of reagent grade.
Model
A total of 16 female BALB/c mice were randomly
divided into two equal groups: Control and asthma group (n=8). As
previously described (14), mice in
the asthma group received an intraperitoneal injection of
sensitizing agent 0.2 ml (containing 100 µg OVA and 1 mg aluminum
hydroxide gel) on days 1 and 15. The asthma group breathed in 1%
OVA for 30 min, starting on day 22, three times per week for 8
weeks. The control group was treated with phosphate-buffered saline
(PBS) instead of OVA. Mice were sacrificed by cervical dislocation
following the last challenge, and blood, lung and spleen specimens
were collected.
Determination of flow cytometry
During the last 2 h of the final challenge, a blood
sample was collected from all mice. The spleen was removed
following sacrifice and 4 ml red blood cell lysis buffer was added
prior to the tissues being ground. The mixtures were filtered and
collected by a 75-µm sieve. Red blood cell lysis buffer was added,
centrifuged (2,120 × g, 5 min, room temperature) and the
supernatant was decanted. Subsequently, 10% RPMI-1640 (1 ml)
(Thermo Fisher Scientific Inc.) was added and mixed with lymphocyte
separation medium. The lymphocyte layer was aspirated, diluted to
10 ml by PBS and washed it to form a cell suspension. The cell
concentration was adjusted with PBS to 1–5×106 cells/ml.
Samples were then stained with 0.5 µl antibodies: Anti-mouse
ICOS-FITC (1:200), anti-mouse CXCR5-PE (1:200), anti-mouse CD4-PE
Cy5 (1:200) and anti-mouse CD3 PE APC (1:100) (away from light at
4°C, 20 min). Cell pellets were resuspended in 500 µl FACS-PBS and
maintained on ice prior to flow cytometry analysis. Flow cytometry
was conducted on a BD FACSCalibur flow cytometer and analyzed using
Cell Quest Pro software version 5.1 (both from BD Biosciences,
Franklin Lakes, NJ, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA (2 µl) was extracted from lung tissue, collected
following sacrifice, using TRIzol reagent RNA isolation reagent.
cDNA was synthesized from 1 µl RNA of each sample using a
synthesize M-MLV first-strand reverse transcription system kit
(C28025-032) according to the manufacturer's protocol. Primers were
designed as follows: 5′-GGAAACCCAGTCAGAGTATTCG-3′ (forward) and
5′-CACATTTGTAGGGCTTTTCTCC-3′ (reverse) for BCL-6;
5′-TCTGCCGTGTCTTTGTCTTCT-3′ (forward) and
5′-GAGCATTGGATTCTTGATGGA-3′ (reverse) for ICOS;
5′-ATCTCGTGGGGATGTTCTGT-3′ (forward) and
5′-GGTTTCCTGTGGGTTCTTTGT-3′ (reverse) for ICOSL;
5′-GACTCCTCTCCATCCACATCA-3′ (forward) and
5′-TAACACCATCCCATCACAAGC-3′ (reverse) for CXCR5;
5′-GGACCCTTGTCTGTCTGGTAG-3′ (forward) and
5′-TGTGGAGCTGATAGAAGTTCAGG-3′ (reverse) for IL-21; and
5′-TGTTACCAACTGGGACGACA-3′ (forward) and 5′-GGGGTGTTGAAGGTCTCAAA-3′
(reverse) for β-actin. RT-qPCR was performed in a 20-µl final
volume containing 0.8 µl cDNA sample, 0.4 µl primer pairs and 10 µl
SYBR-Green PCR Master mix. PCR was performed as follows: Initial
denaturation at 95°C for 2 min, followed by 40 cycles of annealing
at the following temperatures: 53.5°C (ICOS), 55°C (CXCR5), 55.1°C
(ICOSL), 57.6°C (BCL-6), 56°C (IL-21) and 50°C (β-actin) in an ABI
PRISM 7000 (Applied Biosystems; Thermo Fisher Scientific, Inc.)
system sequence detector. Each RNA sample was measured in
triplicate. The specificity of amplified PCR products was evaluated
by a comparative Cq method (15).
The threshold cycle value (Cq value) was calculated from cycle
number at which the PCR product crosses a threshold of detection.
BCL-6, CXCR5, ICOS, ICOSL, IL-21 mRNA expression levels were
normalized against β-actin, as determined by the 2−∆∆Cq
method.
Western blot analysis
The lungs were removed following sacrifice. Tissue
protein (200 µg) was extracted using RIPA lysis buffer (Beijing
Solarbio Science and Technology Co., Ltd., Beijing, China) and the
concentrations were determined using the bicinchoninic acid method.
Proteins (50 µg/well) were separated by 5% SDS-PAGE and transferred
onto a polyvinylidene fluoride membrane. Blocking by 5% skim milk
powder (65°C, 1 h) was performed following denaturation.
Subsequently, the membrane was incubated with primary antibodies
(BCL-6 1:500, ICOS 1:1,000, CXCR5 1:2,000, ICOSL 1:300; 4°C,
overnight) and secondary antibodies (Goat anti-rabbit IgG 1:10,000;
room temperature, 1 h), then developed with the Enhanced
Chemiluminescence Plus Western Blotting Detection system (Amersham,
Cambridge, UK), according to the manufacturer's instructions.
Hematoxylin-eosin staining
The left lung of each mouse was fixed in 10%
formalin for 24 h and embedded in paraffin. Sections of 4–5 µm were
cut, deparaffinized, rehydrated and stained with hematoxylin and
eosin. A pathologist blind to experimental groups evaluated the
changes of lung tissue by using a light microscope.
Immunohistochemistry stain
The left lung tissue was fixed in 10% buffered
formalin for 48 h. Tissue samples, from paraffin-wax-embedded
blocks, were sectioned to be 4–5 µm thick. Following the process of
dewaxing, hydration, closed endogenous peroxidase and antigen
repair, polyclonal IL-21 antibody (rabbit anti-mouse, 1:300) and
universal secondary antibody (PV6000, ZSGB-BIO, Beijing, China)
were applied (1–2 h at room temperature). Slides were observed
using a light microscope at a magnification of ×200 and ×400.
Sections were considered positive according to the color
observation, which is an indication of the antibody-antigen
reaction, and manifested as an intracytoplasm brown coloration in
different areas of the stained tissue section.
Statistical analysis
SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA) was
used to analyse data. Data were presented as the mean ± standard
error of the mean. Comparison between groups was made with analysis
of variance followed by Dunnett's test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Behavioral changes
Mice in the asthma group exhibited symptoms
following the challenge with OVA, including anxiety, frequent nose
scratching, cough, nodding breathing, shortness of breath,
retardation, piloerection and cyanosis. These behavioural
activities were decreased following continuous challenge.
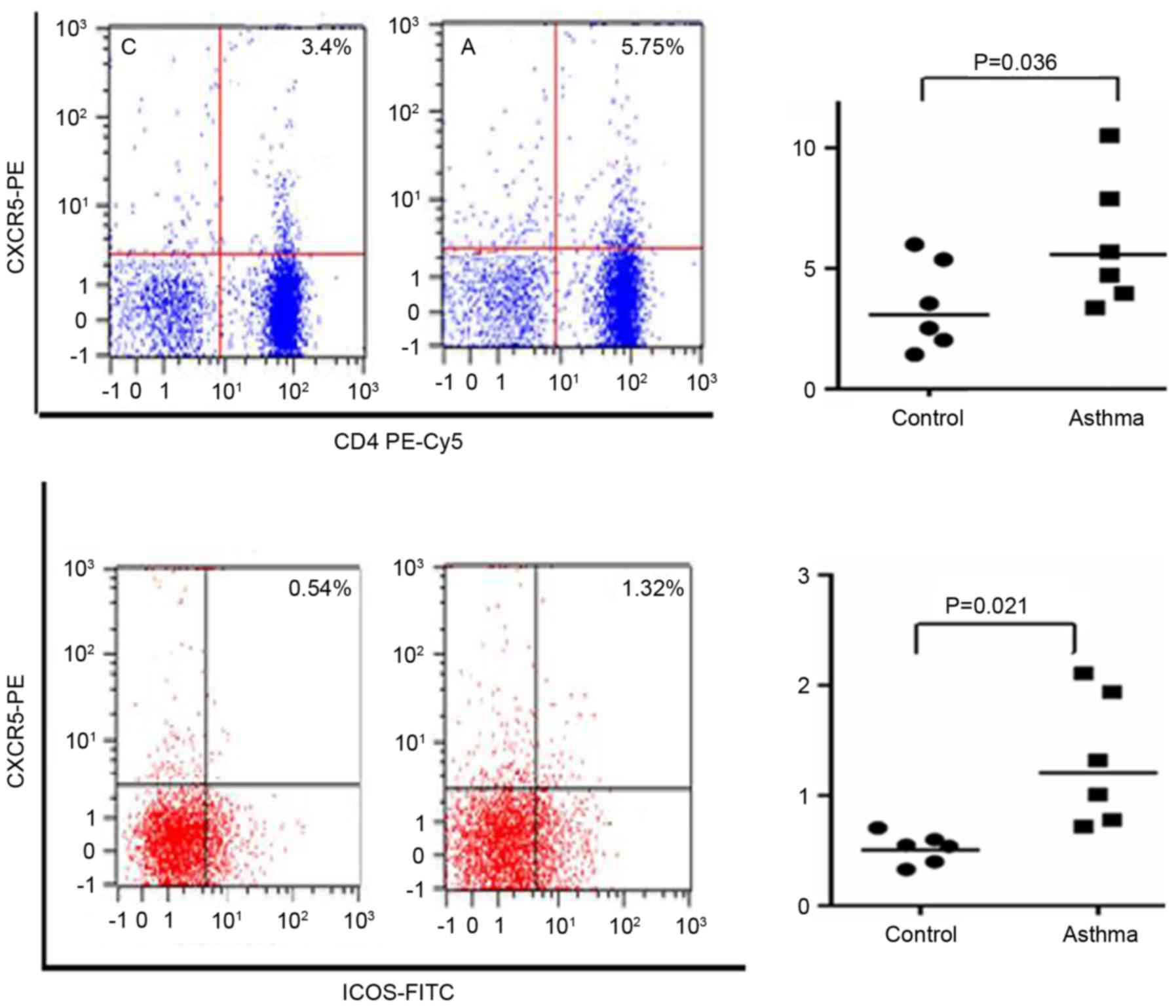
Results of flow cytometry
CXCR5 has been identified as an important transport
molecule in the process of migration and positioning of TFH cells
(16). While ICOS has a role in the
differentiation and maturation of TFH cells. As presented in
Fig. 1, the ratio of
CD4+CXCR5+/CD4+ and
CD4+CXCR5+ICOS+/CD4+CXCR5+
in the spleen tissues was significantly increased in the asthma
group compared with the control group (P<0.05).
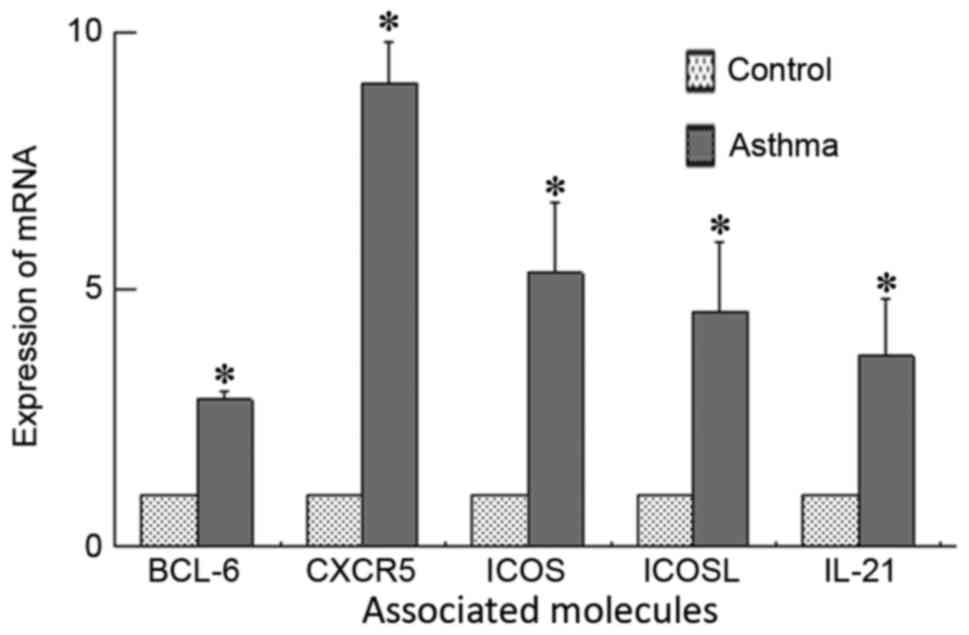
Results of RT-qPCR
Expression levels of BCL-6, ICOS, ICOSL, CXCR5 and
IL-21 mRNA in lung tissue are presented in Fig. 2. A significant increase of BCL-6,
ICOS, ICOSL, CXCR5 and IL-21 mRNA expression was detected in lung
tissue from the asthma group compared with the control group
(P<0.05).
Results of western blot analysis
Proteins within the lung tissues of mice were
extracted and assayed by western blot analysis. As presented in
Fig. 3, the results indicate that
BCL-6, CXCR5, ICOS and ICOSL exhibited positive expression. At the
same time, the amount of protein was increased in the asthma group
compared with the control group.
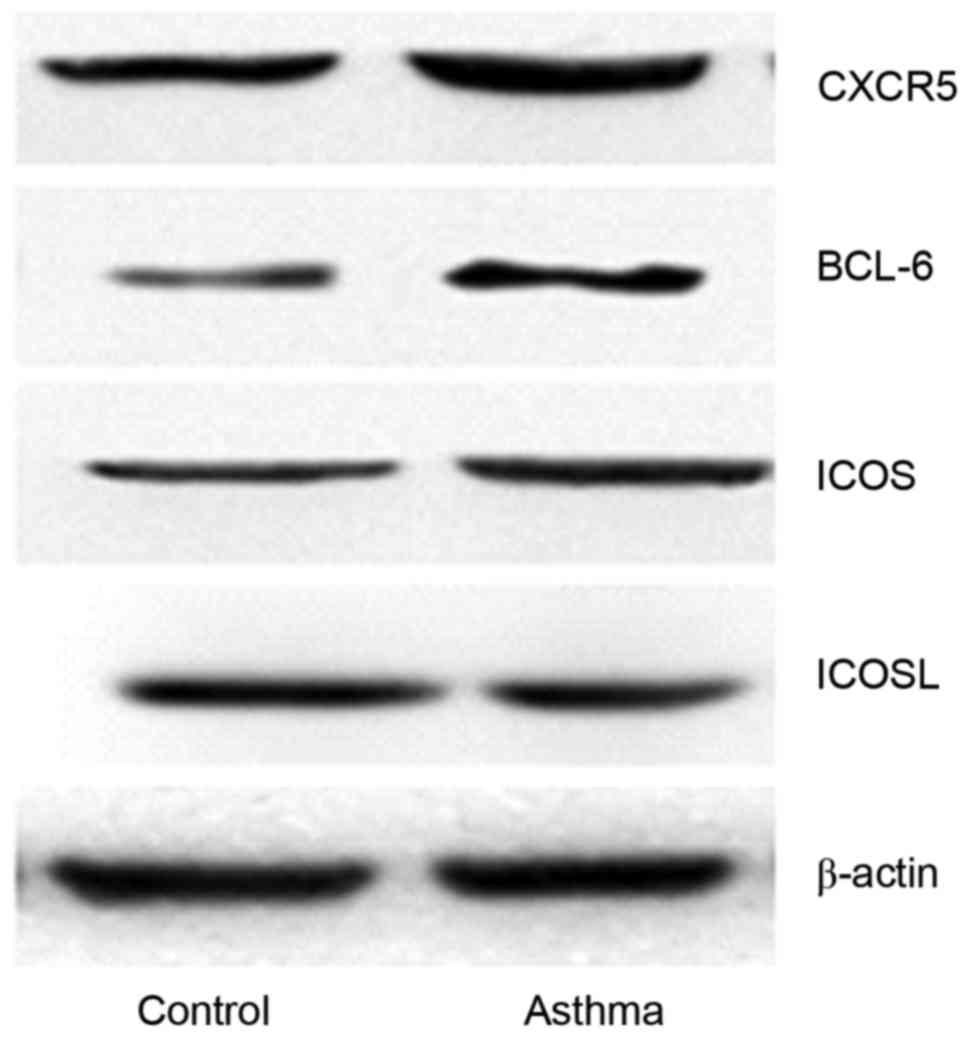 | Figure 3.Western blot analysis to compare the
expression levels of proteins associated with TFH cells (BCL-6,
ICOS, ICOSL, CXCR5 and IL-21) in the control and asthma groups,
with β-actin as the control. TFH, follicular helper T; BCL-6, B
cell lymphoma 6; ICOS, inducible T-cell COStimulator; ICOSL, ICOS
ligand; CXCR5, C-X-C chemokine receptor type 5; IL-21,
interleukin-21. |
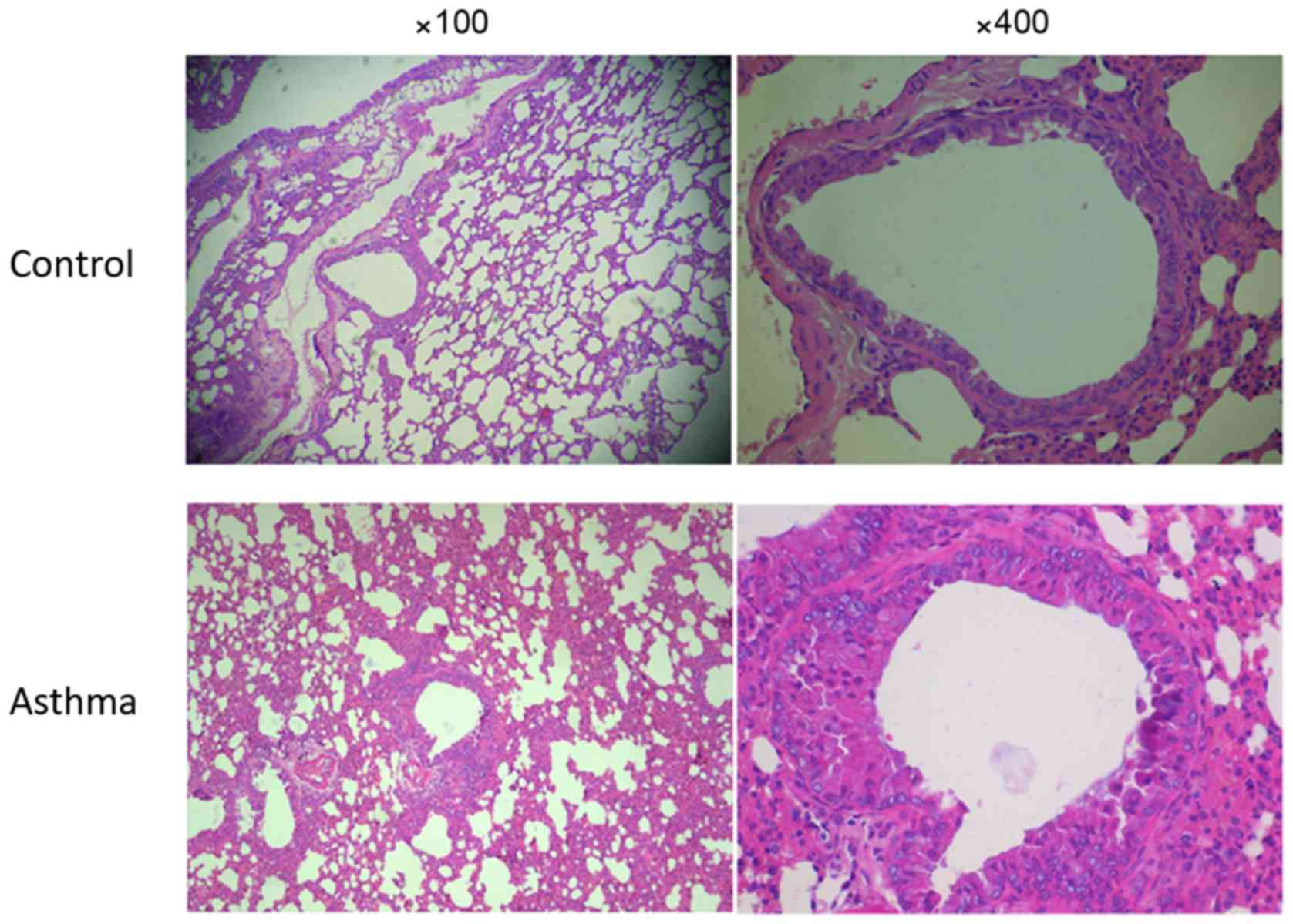
Changes to lung histopathology
As presented in Fig.
4, alveolar samples from mice in the control group exhibited a
normal structure with no histopathological changes observed under
the microscope (Fig. 4). However,
lung tissue obtained from mice in the asthma group exhibited a
marked bronchial epithelial necrosis, thickening of the bronchial
walls and smooth muscle hyperplasia, bronchial lumen irregular and
mucus plug within it.
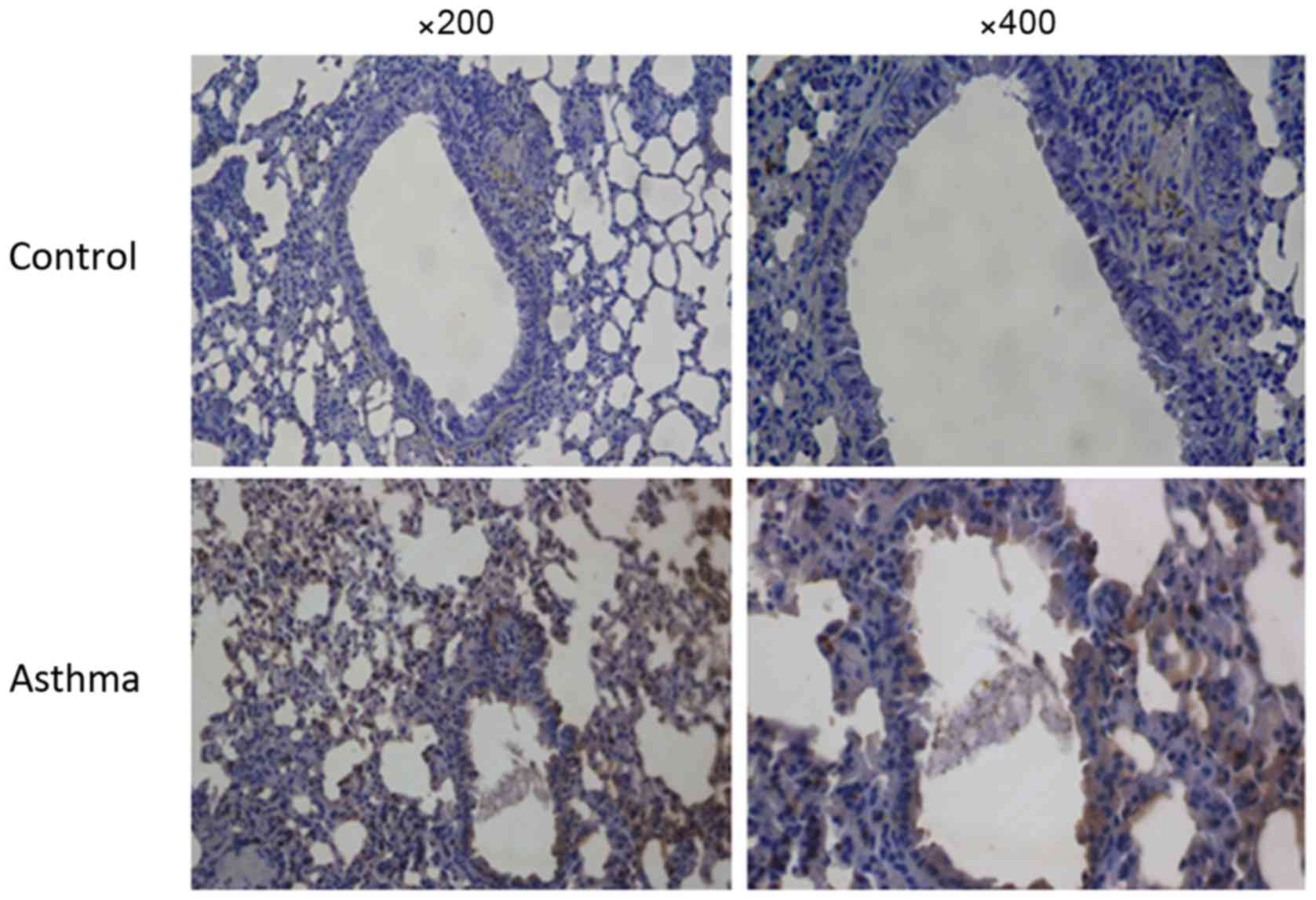
Results of immunohistochemistry
staining
Positive expression of IL-21 in the cytoplasm of
false stratified columnar ciliated epithelium cells is demonstrated
in Fig. 5. It was observed that
there were more positive IL-21 cells in the asthma group, compared
with the control group.
Discussion
Asthma is a type I allergic reaction mediated by
immunoglobulin E. CD4+T helper cell (Th), serve a key
role in the pathogenesis of allergic inflammation (2). It has previously been suggested that
Th1 and Th2 are involved in the inflammatory response of asthma
directly (3). However, studies by
Afshar et al (17) and others
(10–20) have further revealed that Tregs and
Th17 cells are also changed in the pathogenesis of asthma. A
previous study identified novel subsets of CD4+T cells,
named TFH cells, and these were demonstrated to help B cells
produce antibodies (8–10). It was identified that certain
patients who present with autoimmune diseases including systemic
lupus erythematosus, rheumatoid arthritis, autoimmune thyroid
disease and immune-active chronic hepatitis B, demonstrate an
association between an increased in TFH cells and injury severity
of autoantibody and target organs (21,22).
A variety of molecular markers are involved in the
differentiation of TFH cells, including CXCR5, ICOS, ICOSL, BCL-6
and IL-21 (23). TFH cells and
mature B cells are able to express CXCR5 continuously and stably
(24). CXCR5 is a specific receptor
of CXCR13, which is a follicular homing chemokine that induces TFH
cell migration from the T zone in peripheral lymphoid organs to the
center of lymphoid follicles (25).
Finally, TFH cells localize to and interact with B cells.
Therefore, CXCR5 is known as an important transport molecule in the
process of migration and positioning of TFH cells (26). The results of the present study
indicate that the mRNA and protein expression levels of CXCR5 were
increased in the asthma group, as compared with the control
group.
ICOS, as an induced coordinated stimulus molecule,
induces the process of TH cell activation and differentiation
(23). A previous study indicated
that ICOS interacts with ICOSL to deliver coordination and
stimulate positive signals (27). It
has an important role not only for the generation and survival of
TFH cells but also for the formation of GC and memory B cells
(28). Choi et al (29) revealed that ICOS provides a critical
early signal to induce differentiation and maturation of TFH cells.
The present study also revealed that the expression levels of ICOS
and ICOSL mRNA and protein were increased in the bronchial asthma
group, as compared with the control group.
Flow cytometric analysis demonstrated that, compared
with the control group, the ratio of
CD4+CXCR5+/CD4+ and
CD4+CXCR5+ICOS+/CD4+CXCR5+
in the spleen was higher in the asthma group. These results suggest
that the increasing ratio of TFH cell subsets may be an important
determinant in the pathogenesis of asthma.
Zinc finger protein, BCL-6, is a transcription
receptor that was initially determined to act on B cells (30). Its expression has an important role
in the formation of GC. Expression of BCL-6 was indicated at the
beginning of TFH cell proliferation and the activation of T cells
(28,31). The present study revealed that the
expression of BCL-6 at a molecular and protein level in the asthma
group was higher than in the control group. The current study
demonstrated that an abundance of TFH cells proliferated in the
process of asthma pathogenesis.
IL-21 is primarily released from TFH cells in the
body. IL-21 combines with its corresponding receptor on the surface
of TFH or B cells, thus the expression of IL-21 increases by
autocrine function. IL-21 is involved in TFH cell proliferation,
development and effectiveness (32).
The present study revealed that the expression of IL-21, at the
mRNA and protein level, was increased in asthmatic lung tissue.
In conclusion, the results of the current study
demonstrated that TFH cells and associated markers have a role in
the pathogenesis of asthma. These results suggest that regulation
of the abnormal TFH cells in vivo may be a novel strategy
for treating asthma, in the future.
Acknowledgements
The present study was funded by Funds for National
Science Foundation of Autonomous Region of Xinjiang (grant no.
2015211C012). The authors would like to thank Dr Yuyuan Guo
(Xinjiang Medical University) and Mr. Umer Farooq Dost (Xinjiang
Medical University) for improving the essay.
References
|
1
|
Lemanske RF Jr and Busse WW: Asthma:
Clinical expression and molecular mechanisms. J Allergy Clin
Immunol. 125 2 Suppl 2:1–102. 2010. View Article : Google Scholar
|
|
2
|
Herrick CA and Bottomly K: To respond or
not to respond: T cells in allergic asthma. Nat Rev Immunol.
3:405–412. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wei M, Chu X, Guan M, Yang X, Xie X, Liu
F, Chen C and Deng X: Protocatechuic acid suppresses
ovalbumin-induced airway inflammation in a mouse allergic asthma
model. Int Immunopharmacol. 15:780–788. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Balaha MF, Tanaka H, Yamashita H, Rahman
MN Abdel and Inagaki N: Oral Nigella sativa oil ameliorates
ovalbumin-induced bronchial asthma in mice. Int Immunopharmacol.
14:224–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park HJ, Lee CM, Jung ID, Lee JS, Jeong
YI, Chang JH, Chun SH, Kim MJ, Choi IW, Ahn SC, et al: Quercetin
regulates Th1/Th2 balance in a murine model of asthma. Int
Immunopharmacol. 9:261–267. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi YH, Shi GC, Wan HY, Jiang LH, Ai XY,
Zhu HX, Tang W, Ma JY, Jin XY and Zhang BY: Coexistence of Th1/Th2
and Th17/Treg imbalances in patients with allergic asthma. Chin Med
J (Engl). 124:1951–1956. 2011.PubMed/NCBI
|
|
7
|
Zhao Y, Yang J and Gao YD: Altered
expressions of helper T cell (Th)1, Th2, and Th17 cytokines in
CD8(+) and γδ T cells in patients with allergic asthma. J Asthma.
48:429–436. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi YS, Yang JA and Crotty S: Dynamic
regulation of Bcl6 in follicular helper CD4 T (TFH) cells. Curr
Opin Immunol. 25:366–372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Linterman MA and Vinuesa CG: T follicular
helper cells during immunity and tolerance. Prog Mol Biol Transl
Sci. 92:207–248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
King C: A fine romance: T
follicularhelpercells and B cells. Immunity. 34:827–829. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dong W, Zhu P, Wang Y and Wang Z:
Follicular helper T cells in systemic lupus eiythematosus: A
potential therapeutic target. Autoimmun Rev. 10:299–304. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma J, Zhu C, Ma B, Tian J, Baidoo SE, Mao
C, Wu W, Chen J, Tong J, Yang M, et al: Increased frequency of
circulating follicular helper T cells in patients with rheumatoid
arthritis. J Immunol Res. 2012:8274802012.
|
|
13
|
Zhu C, Ma J, Liu Y, Tong J, Tian J, Chen
J, Tang X, Xu H, Lu L and Wang S: Increased frequency of follicular
helper T cells in patients with autoimmune thyroid disease. J Clin
Endocrinol Metab. 927:943–950. 2012. View Article : Google Scholar
|
|
14
|
Du Q, Zhang Q, Shen L, Cai JK, Huang M and
Yin KS: Effects of Astragaloside IV on airway remodeling in a
murine model of chronic asthma. Chin Pharmacol Bulletin. 1–1434.
2011.
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fazilleau N, Mark L, McHeyzer-Williams LJ
and McHeyzer-Williams MG: Follicular helper T cells: Lineage and
location. Immunity. 30:324–335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Afshar R, Strassner JP, Seung E, Causton
B, Cho JL, Harris RS, Hamilos DL, Medoff BD and Luster AD:
Compartmentalized chemokine-dependent regulatory T-cell inhibition
of allergic pulmonary inflammation. J Allergy Clin Immunol.
131:1644–1652. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maazi H, Shirinbak S, Willart M, Hammad
HM, Cabanski M, Boon L, Ganesh V, Baru AM, Hansen G, Lambrecht BN,
et al: Contribution of regulatory T cells to alleviation of
experimental allergic asthma after specific immunotherapy. Clin Exp
Allergy. 42:1519–1528. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma C, Ma Z, Fu Q and Ma S: Curcumin
attenuates allergic airway inflammation by regulation of CD4+CD25+
regulatory T cells (Tregs)/Th17 balance in ovalbumin-sensitized
mice. Fitoterapia. 87:57–64. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song L, Guo Y, Deng Q and Li J: TH17
functional study in severe asthma using agent based model. J Theor
Biol. 309:29–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang X, Ing S, Fraser A, Chen M, Khan O,
Zakem J, Davis W and Quinet R: Follicular helper T cells: New
insights into mechanisms of autoimmune diseases. Ochsner J.
13:131–139. 2013.PubMed/NCBI
|
|
22
|
Feng J, Lu L, Hua C, Qin L, Zhao P, Wang
J, Wang Y, Li W, Shi X and Jiang Y: High frequency of CD4+ CXCR5+
TFH cells in patients with immune-active chronic hepatitis B. PLoS
One. 6:e216982011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nurieva RI and Chung Y: Understanding the
development and function of T follicular helper cells. Cell Mol
Immunol. 7:190–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu D, Batten M, Mackay CR and King C:
Lineage specification and heterogeneity of T follicular helper
cells. Curr Opin Immunol. 21:619–625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu X, Nurieva RI and Dong C:
Transcriptional regulation of follicular T-helper (TFH) cells.
Immunol Rev. 252:139–145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Crotty S: Follicular helper CD4 T cells
(TFH). Annu Rev Immunol. 29:621–663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kuo TC, Shaffer AL, Haddad J Jr, Choi YS,
Staudt LM and Calame K: Repression of BCL-6 is required for the
formation of human memory B cells in vitro. J Exp Med. 204:819–830.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Simpson TR, Quezada SA and Allison JP:
Regulation of CD4 T cell activation and effector function by
inducible costimulator (ICOS). Curr Opin Immunol. 22:326–332. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choi YS, Kageyama R, Eto D, Escobar TC,
Johnston RJ, Monticelli L, Lao C and Crotty S: ICOS receptor
instructs T follicular helper cell versus effector cell
differentiation via induction of the transcriptional repressor
Bcl6. Immunity. 34:932–946. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu R, Wu Q, Su D, Che N, Chen H, Geng L,
Chen J, Chen W, Li X and Sun L: A regulatory effect of IL-21 on T
follicular helper-like cell and B cell in rheumatoid arthritis.
Arthritis Res Ther. 14:R2552012. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Linterman MA, Beaton L, Yu D, Ramiscal RR,
Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ and Vinuesa
CG: IL-21 acts directly on B cells to regulate Bcl-6 expression and
germinal center responses. J Exp Med. 207:353–363. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tiriveedhi V, Fleming TP, Goedegebuure PS,
Naughton M, Ma C, Lockhart C, Gao F, Gillanders WE and Mohanakumar
T: Mammaglobin-A cDNA vaccination of breast cancer patients induces
antigen-specific cytotoxic CD4+ICOShi T cells. Breast Cancer Res
Treat. 138:109–118. 2013. View Article : Google Scholar : PubMed/NCBI
|