Introduction
Myocardial infarction (MI) adversely affects major
killers of human health. Acute MI is caused by acute persistent
ischemia and hypoxia (1). The
clinical manifestations are severe and persistent retrosternal
pain. Rest and nitrate cannot completely achieve remission,
accompanied by serum myocardial enzyme spectrum changes. Changes in
electrocardiogram, arrhythmia, shock or heart failure risk
increase, and become life-threatening (2,3).
Approximately 1.5 million individuals in the United States have MI
each year (4,5). In China, with the development of
economy and the improvement of living standards, the annual new
onset is ≥0.5 to 2 million individuals. Although early MI can
achieve good therapeutic effect through intervention and drug
treatment with the improvement of the level of medical technology,
MI early warning to prevent the occurrence of MI remains, however,
the current research hotspot (6–8).
Estrogen receptor β (ERβ) genes, encoding ERβ plays
an important role in the regulation of normal physiology, aging and
many diseases (9). The most
classical function of ER is described as a ligand activated
transcription factor, which can mediate gene transcription in
tissues and organs regulated by hormones (10). ERβ is usually highly expressed in
breast cancer, prostate cancer and other tumors, and may change
with the menstrual cycle. Clinical studies have indicated that the
level of expression of ERα is closely related to the degree of
differentiation of breast cancer cells and their degree of
malignancy (11–14). In MI, ERβ increase cardiac function
in patients with acute MI by modulating the PI3K/Akt signaling
pathway (15). In addition, ERβ can
improve myocardial fibrosis after MI (16).
On the basis of previous research, we investigated
the role of ERβ in left ventricular remodeling in mice after MI in
order to understand the mechanism and provide theoretical basis for
MI and fibrosis therapy, as well as the targets for drug
development.
Materials and methods
Experimental animals
The use of experimental animals in this study was
approved by the ethics committee of our university. The general
characteristics of Tg-ERβ have been reported in previous literature
(8). Microinjection DNA box sequence
to the C57BL/6 mouse fertilized oocytes were implanted into
pseudopregnant mice. The injection of sequence contained the cDNA
ORF region of ERΒ gene in the mice, and was regulated by α-myosin
heavy chain promoter.
Non-transgenic littermate control (NLC) mice were
C57BL/6 mice that did not receive the microinjection (for the
microinjection of the mouse brothers and sisters) in order to
ensure the genetic background to be similar to Tg-ERβ mice.
Grouping
NLC mice were divided into the transgenic group and
the control group. Each group had 12 mice, the average body weight
was 23.9±3.6 g, and the average age was 3.1±0.3 weeks. Coronary
artery ligation (CAL) was used to construct a mouse model of MI in
mice randomly selected from each group (n=6). Cardiac structure and
function changes were observed in the mice 1, 3 and 7 days after
surgery, respectively. RT-PCR method was used to detect the
expression of collagen I, α-SMA, TGF-β mRNA in the mouse heart, and
Masson staining was used to detect cardiac fibrosis.
Instrument
Biological safety cabinet (Esco Micro Pte., Ltd.,
Singapore, Republic of Singapore), PCR amplification instrument
(Eppendorf AG, Hamburg, Germany), gel imaging instrument (Syngene,
Frederick, MD, USA), electrophoresis apparatus (Beijing 61
Instrument Factory, Beijing, China), centrifuge (Eppendorf AG),
micropipet (Eppendorf AG), Haier ice machine, western blot
electrophoresis apparatus trophoresis (Bio-Rad Laboratories,
Hercules, CA, USA), −80°C refrigerator (Thermo Fisher Scientific,
Waltham, MA, USA), 10 ml syringe, 5 ml syringe (Tianjin Hanaco
Medical Co., Ltd., Tianjin, China), experimental animal surgical
instruments (Beijing Medical Equipment Factory, Beijing, China),
NanoDrop2000 photometric analyzer (Thermo Fisher Scientific), EP
tube (Eppendorf AG), water bath (Beijing Medical Equipment
Factory), and pathological section machine (Leica, Mannheim,
Germany) were used in the present study.
Reagent
Taq Master Mix (SinoBio, Shanghai, China), agarose
(Biowest, Nuaille, France), sterile double distilled water, sterile
double distilled water, monoclonal rabbit β-actin antibody
(dilution, 1:5,000; cat. no. MA5-15739; Invitrogen Life
Technologies, Carlsbad, CA, USA), polyclonal rabbit ERβ antibody
(dilution, 1:500; cat. no. BD-PT1637; BioLegend, Inc., San Diego,
CA, USA), monoclonal rabbit phosphorylation ERβ antibody (dilution,
1:1,000; cat. no. orb10614; Cell Signaling Technology, Inc.,
Danvers, MA, USA), 0.9% stroke-physiological saline solution
(Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan), pentobarbital
sodium (Guangzhou Chemical Reagent Factory, Guangzhou, China),
TRIzol (Invitrogen Life Technologies), AngII (RayBiotech,
Norcross, GA, USA), and acetic acid (Guangzhou Chemical Reagent
Factory) constituted the reagents used in this study. Phosphate
buffer was purchased from Gibco Life Technologies (Carlsbad, CA,
USA).
Method
Ligation of coronary artery
The procedure followed for ligation of coronary
artery (8) was: i) Preoperative
preparation: The artery was fixed by phenobarbital anesthesia,
tracheal incision intubation was undertaken after successful
intubation under the use of ventilation. ii) The skin was cut;
muscles were separated in the 4th intercostal level to get into the
chest. After good exposure of the heart, the left coronary artery
was separated. The 6–0 non-destructive suture ligation was used.
iii) After the ligation, the heart was returned to the chest, and
the incision skin was sutured.
Small animal ultrasound
The small animal ultrasound (9) was used to evaluate the diastolic
(PWTD), end systolic (PWTS), left ventricular systolic diameter
(LVESD) and left ventricular diastolic diameter (LVEDD). Mice were
anesthetized by isoflurane and then tested before ultrasound
examination.
Detection of levels of collagen I, α-SMA, and
TGF-β
RT-PCR assay was used to detect the levels of
collagen I, α-SMA, and TGF-β (10–12). The
tissue samples obtained in the first step were used to extract the
RNA in the tissue by TRIzol method (Invitrogen Life Technologies).
After the extraction, NanoDrop2000 photometric analyzer (Thermo
Fisher Scientific) was used to measure the concentration 260/280.
Under normal circumstances, OD260/280 of RNA was in the range of
1.7–2.0, if <1.7, it suggested that there might be protein or
phenol pollution, if >2.0, it suggested that there was acid
residue, that needed further extraction for the detection of
purity.
Immunohistochemistry
Prior to immuno-histochemistrystaining, the paraffin
section of rat brain was first prepared, including fixation,
dehydration, transparency, embedding, slice, patch and so on. After
the paraffin section was prepared, the immunofluorescent staining
was performed as follows: At 20°C, the section was placed still for
60 min, and then was immersed by xylene for 25 min. The section was
immersed by anhydrous alcohol for 2 min, and by 95, 80 and 70%
alcohol, each for 2 min, then was washed by PBS 2–3 times for 5
min. The section was incubated with 3% H2O2
deionized water for 10 min and washed by PBS for 2–3 times for 5
min. The section was boiled in citrate buffer (pH 6.0) at 95°C for
15–20 min and cooled to room temperature with cold water, and then
was washed by PBS for 2–3 times for 5 min. The section was
incubated with normal goat serum blocking solution at room
temperature for 20 min, and excess solution was discarded. The
section was incubated with primary polyclonal rabbit ERβ antibody
(dilution, 1:500; cat. no. BD-PT1637; BioLegend, Inc.) at room
temperature for 1 h and then washed with PBS for 2–3 times for 5
min. The section was incubated with secondary goat anti-rabbit
(HRP) IgG antibody (dilution, 1:2,000; cat.no. ab6721) at room
temperature for 1 h and then washed with PBS for 2–3 times for 5
min. The section was incubated with streptavidin peroxidase at room
temperature for 30 min and then washed with PBS for 2–3 times for 5
min. DAB visualization was performed for 5–10 min. Under a
microscope, those cells with brown cytoplasm were judged as
positive cells. Tap water washing was performed for 10 min to stop
the reaction. Hematoxylin staining was performed for 2 min, and
hydrochloric alcohol differentiation was performed; The section was
washed by tap water for 10 min and then was dehydrated, cleared and
mounted. Neutral latex was added, and the coverslip was covered
before microscopic examination. Immunohistochemical staining
results were analyzed using Image-Pro Plus 6.0 (version X; Media
Cybernetics, Silver Springs, MD, USA).
Masson staining
Paraffin sections were dewaxed in water prior to
washing by tap water and distilled water in turn. We used Regaud
hematoxylin stain or Weigert staining sperm nuclear for 5–10 min.
If necessary, hydrochloric acid alcohol differentiation was washed
by distilled water. The Masson acid complex red blood staining of
myocardial tissue was for 5–10 min as previously described
(14). We used 2% acetic acid
aqueous solution soaking for 10 min and 1% phosphomolybdic acid
aqueous solution of differentiation for 3–5 min. We directly used
aniline blue or light green liquid dye for 5 min. We used 0.2%
acetic acid aqueous solution soaking and sealed with 95% alcohol,
anhydrous alcohol and xylene transparent neutral gum. Collagen
fibers, mucus, and cartilage were blue, cytoplasm, muscle,
cellulose and glial were red, and the nucleus was black blue.
Statistical analysis
Statistical analysis was performed with SPSS 19.0
(SPSS, Inc., Chicago, IL, USA). Analysis of variance (ANOVA) and
χ2 test were used to analyze the normal distribution
data. Fisher's exact probability method was used for the data of
the four cases that did not satisfy the condition. The comparison
of skewed distribution data was tested by paired t-test or
χ2. P<0.05 was considered to indicate a statistically
significant difference.
Results
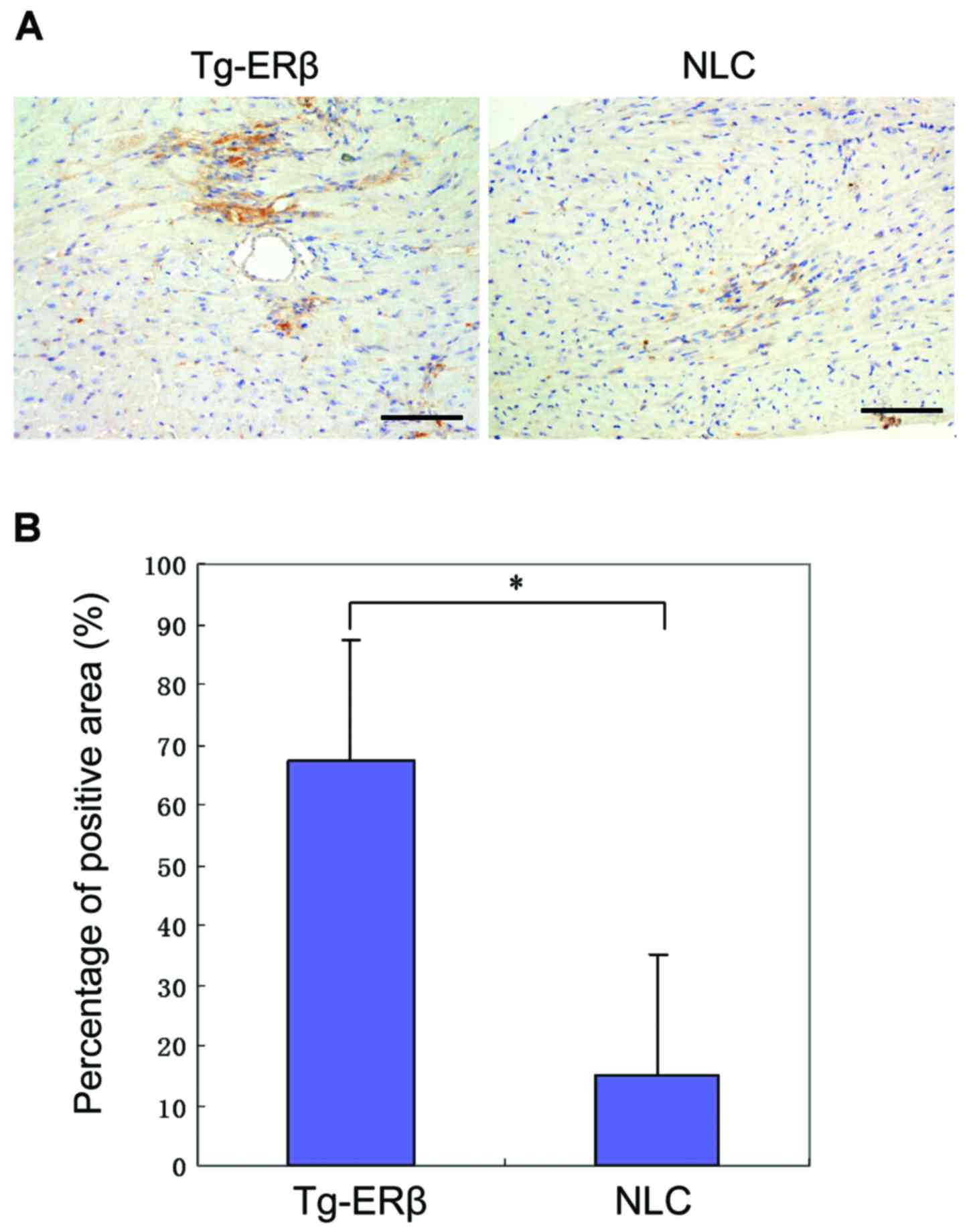
Expression of ERβ before and after
CAL
In order to detect the expression of ERβ in the
groups before and after CAL, one randomly selected mouse was
sacrificed it in the transgenic and the control group,
respectively, and then cardiac ERβ was stained. There was slight
expression of ERβ in the myocardium of NLC mice, and the difference
was statistically significant compared with that of the transgenic
mice (P>0.05). The expression of ERβ in the Tg-ERβ group was
significantly higher than that in NLC mice (P<0.05). After
AngII reperfusion, there was a trend of increase of ERβ
expression in Tg-ERβ (P>0.05; Fig.
1).
Mouse cardiac ultrasound index after
construction of MI model
In order to observe the cardiac function of the two
groups of mice after MI, we performed echocardiography. As compared
with the NLC mice, end echocardiographic diastolic PWTD and end
systolic PWTS of Tg-ERβ mice were significantly reduced
(P<0.05). By contrast, end systolic LVESD and end
echocardiographic diastolic LVEDD were also significantly increased
in Tg-ERβ mice (P<0.05). Ejection fraction (EF%) and short axis
shortening rate (FS%) decreased significantly in Tg-ERβ mice
(P<0.05). In addition, 7 days after MI, end echocardiographic
diastolic PWTD and end systolic PWTS were significantly increased
in Tg-ERβ mice (P<0.05). Furthermore, end systolic LVESD and end
echocardiographic diastolic LVEDD were significantly higher in NLC
mice than those of Tg-ERβ mice (P<0.05). However, compared with
the NLC mice, end echocardiographic diastolic PWTD and end systolic
PWTS of Tg-ERβ mice were significantly reduced (P<0.05), whereas
the end systolic LVESD and end echocardiographic diastolic LVEDD
were also significantly increased (P<0.05) in Tg-ERβ mice,
suggesting the protecting ventricular remodeling effect of Tg-ERβ
after MI (Table I).
 | Table I.Statistics of echocardiography (mean ±
standard deviation). |
Table I.
Statistics of echocardiography (mean ±
standard deviation).
| Characteristics | NLC | NLC + CAL | Tg-ERβ | Tg-ERβ + CAL |
|---|
| No. | 5 | 6 | 5 | 6 |
| BW (g) | 22.7±2.2 | 24.2±1.2 | 24.8±1.2 | 23.8±1.3 |
| HR (bmp) | 412.3±27.7 | 431.4±11.9 | 466.8±28.7 | 428.4±19.6 |
| PWTD
(mm)a | 1.17±0.48 | 0.71±0.21 | 0.83±1.24 | 0.67±0.21 |
| PWTS
(mm)a | 1.61±0.14 | 1.24±0.21 | 1.42±0.32 | 1.20±0.45 |
| AWTD
(mm)a | 0.85±0.12 | 0.72±0.02 | 0.87±0.03 | 0.70±0.11 |
| AWTS
(mm)a,b | 1.71±0.13 | 1.31±0.12 | 1.48±0.17 | 1.28±0.21 |
| LVEDD
(mm)a | 3.03±0.44 | 3.98±0.24 | 3.52±0.26 | 3.88±0.31 |
| LVESD
(mm)a,b | 1.59±0.32 | 2.49±0.31 | 2.16±0.47 | 2.41±0.13 |
| CO
(ml/min)a,b | 15.93±5.85 | 18.19±2.43 | 17.25±4.38 | 18.20±3.24 |
| FS (%)a,b | 58.24±2.43 | 37.24±2.19 | 37.10±1.28 | 42.36±1.25 |
| EF (%)a,b | 81.95±2.16 | 67.81±1.33 | 67.42±1.05 | 73.22±1.48 |
| LV mass (AW)
correcteda,b | 83.29±11.47 | 82.44±10.96 | 84.34±10.52 | 86.93±13.82 |
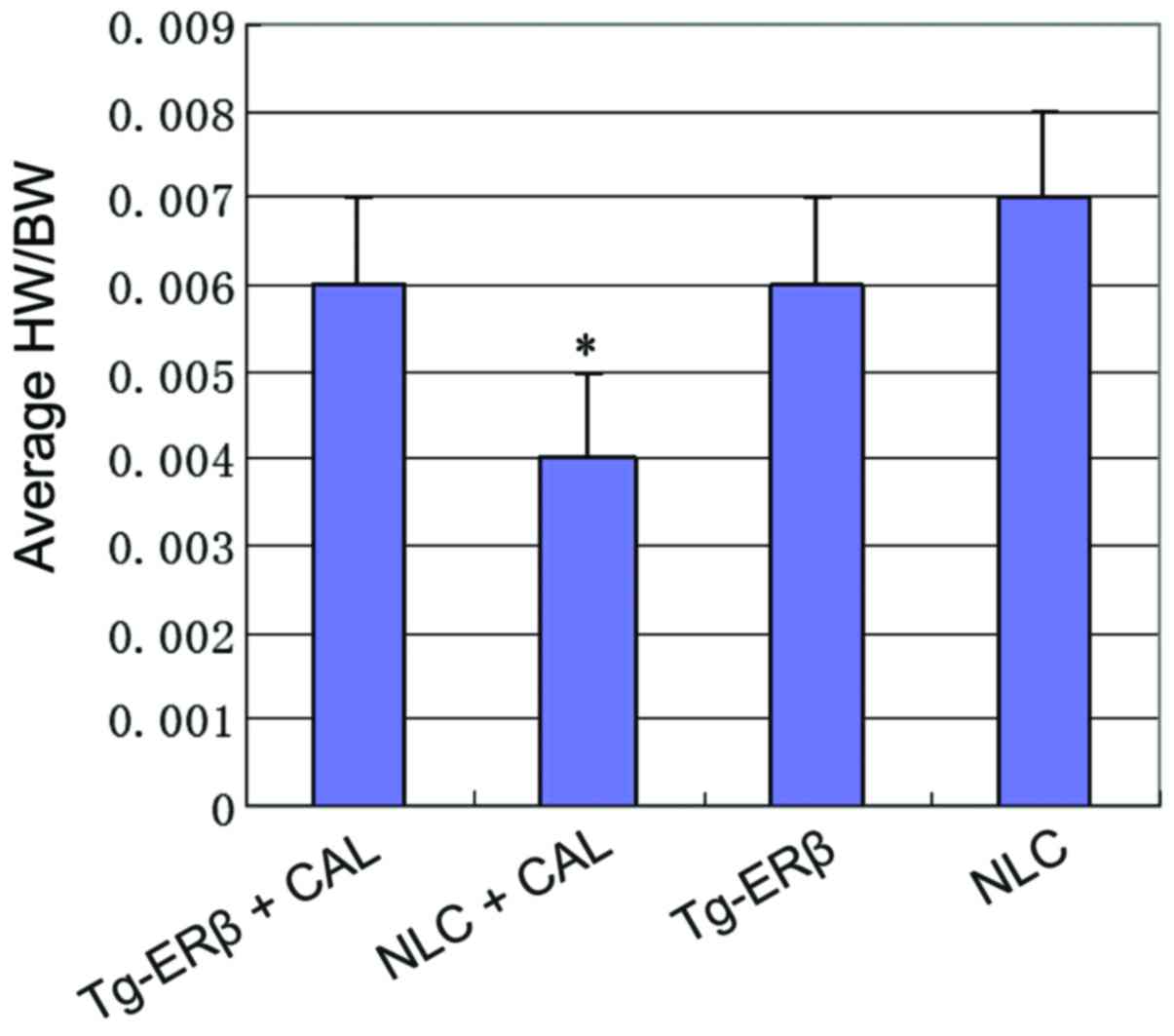
Heart weight ratio test
To study the absolute changes in the weight of the
heart of the mouse, we measured the heart weight and body weight of
each group, and calculated the ratio of heart weight to body weight
(HW/BW). We found that the average HW/BW of Tg-ERβ + AngII
group was significantly increased (P<0.05) compared with the NLC
group (Fig. 2).
Masson staining was used to compare
the collagen deposition in the heart of mice
After Masson staining, the mouse myocardial
interstitial and perivascular appeared as blue collagen fiber,
compared with Tg-ERβ + CAL group, heart coronary artery and
myocardial fibrosis area of NLC + CAL mice significantly increased
(P<0.05) (Fig. 3).
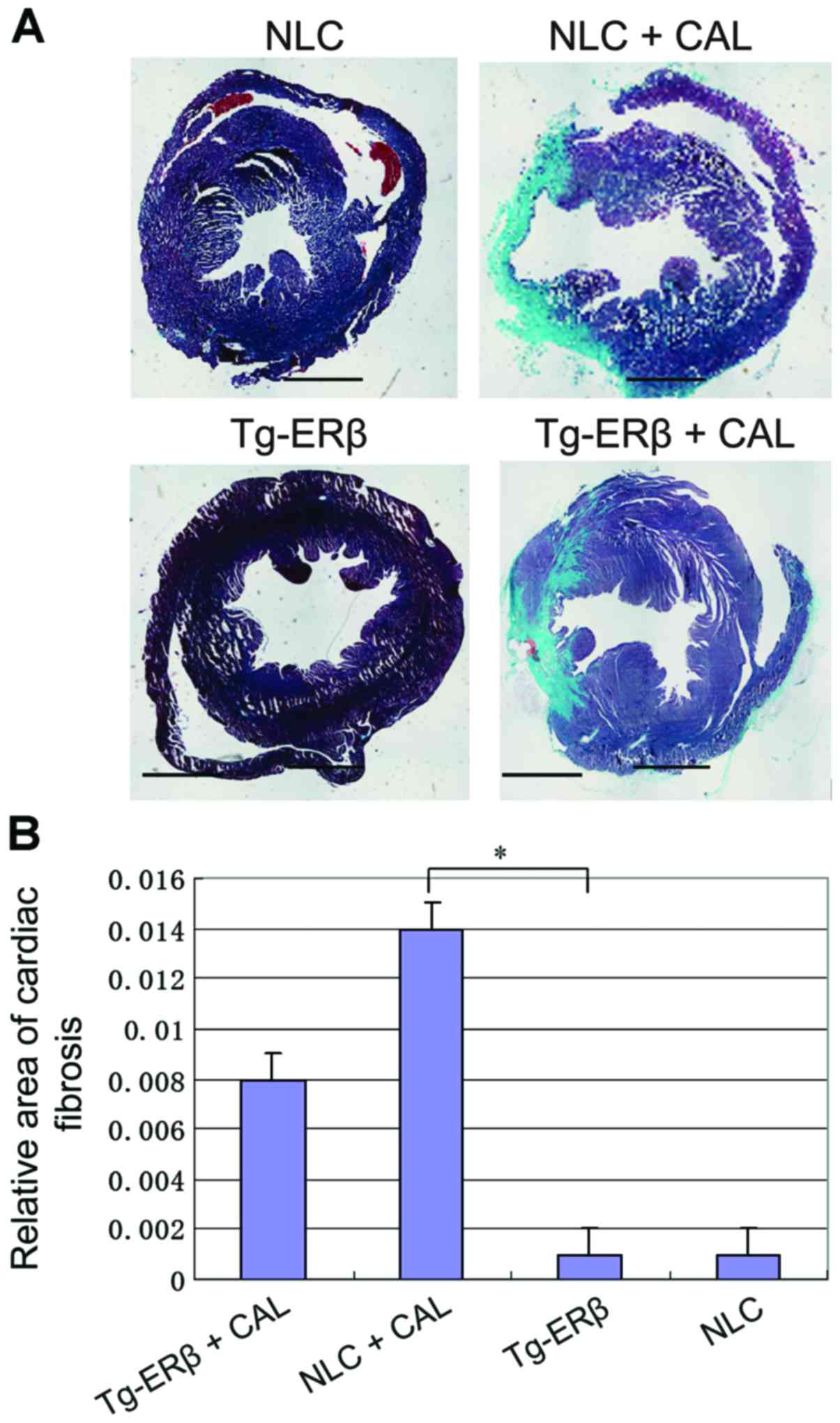 | Figure 3.Masson staining of paraffin sections
was performed on NLC, NLC + CAL, Tg-ERβ, Tg-ERβ + CAL, respectively
(myocardial interstitium ×100 magnification, perivascular ×200
magnification). Image-Pro6.0 software was used to analyze the
cardiac fibrosis area quantitatively and statistical results were
obtained. Compared with the NLC + CAL group, the area of coronary
artery and myocardial interstitial fibrosis was significantly
reduced in the Tg-ERβ + CAL group, and the difference was
statistically significant, *P<0.05. ERβ, estrogen receptor β;
NLC, non-transgenic littermate control; CAL, coronary artery
ligation. |
Collagen I, α-SMA, and TGF-β
expression level of cardiac tissue
RT-PCR was employed to assess the expression level
of collagen I, α-SMA, TGF-β in the hearts of mice. We found that,
after ligation of coronary artery, the levels of expression of
collagen I, α-SMA and TGF-β in NLC and Tg-ERβ mice were
significantly increased (P<0.05). However, the level of
increased of collagen I, α-SMA, TGF-β in NLC mice was significantly
higher than in Tg-ERβ mice (P<0.05) (Fig. 4).
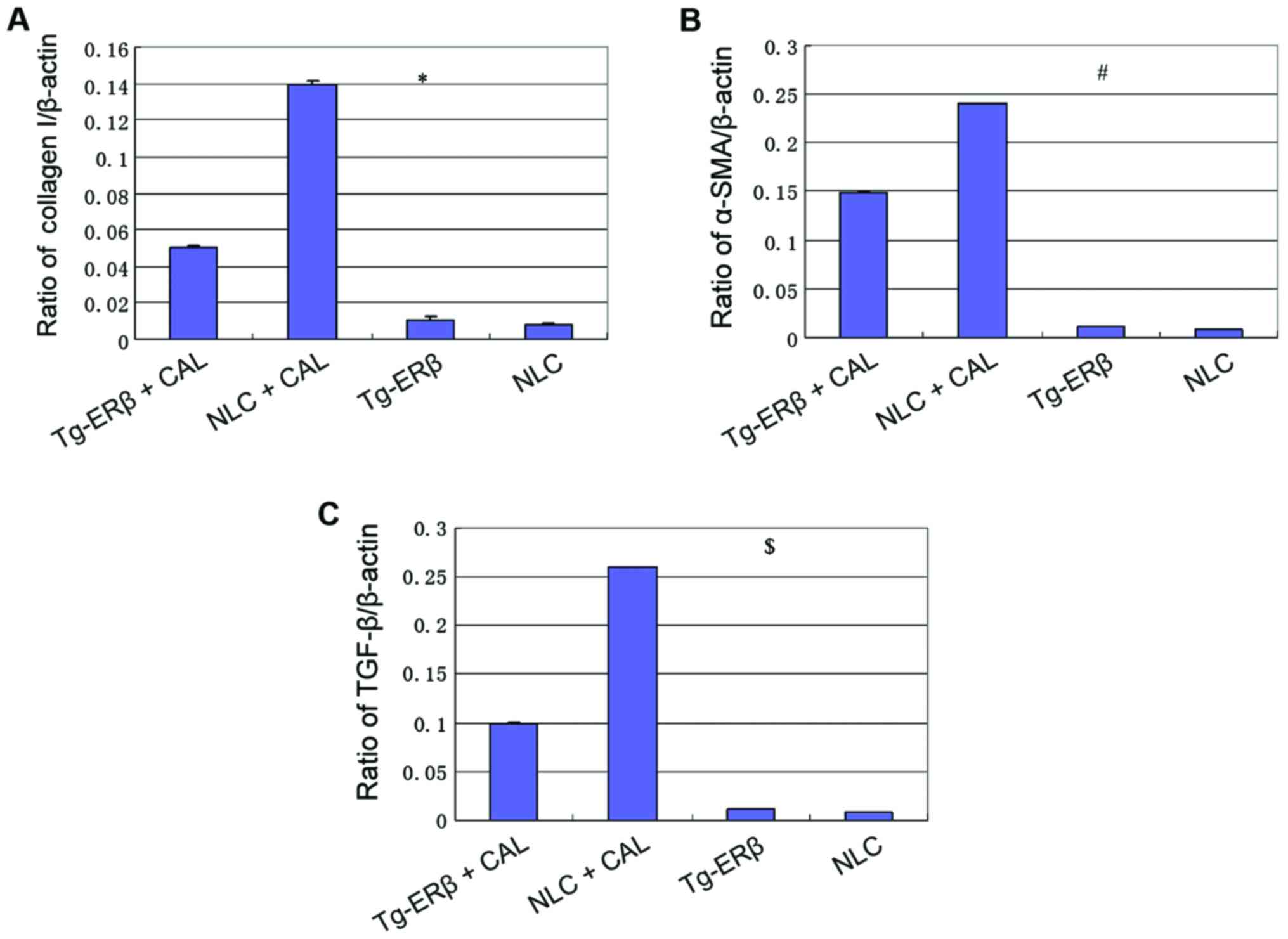 | Figure 4.RT-PCR detection of the expression of
collagen I, TGF-β, α-SMA. (A) At 7 days after cardiac infarction,
the expression levels of collagen I were significantly increased,
but the increased level of NLC mice was significantly higher than
that of Tg-ERβ mice, and the difference was statistically
significant, *P<0.05. (B) At 7 days after cardiac infarction,
the expression levels of α-SMA in heart tissue were significantly
increased, but the increased level of NLC mice was significantly
higher than that of Tg-ERβ mice, and the difference was
statistically significant, #P<0.05. (C) At 7 days
after cardiac infarction, the expression levels of TGF-β in heart
tissue were significantly increased, but the increased level of NLC
mice was significantly higher than that of Tg-ERβ mice, and the
difference was statistically significant, $P<0.05.
ERβ, estrogen receptor β; NLC, non-transgenic littermate control;
CAL, coronary artery ligation. |
Discussion
MI is a common cardiovascular disease. The target
organ damage caused by MI is one of the main causes of morbidity
and mortality in patients with cardiovascular and cerebrovascular
events (1). MI induced heart
remodeling occurs after MI, cardiac or vascular anatomical
structure and morphology changes adaptively caused by hemodynamic,
neurohumoral, endocrine and metabolic abnormalities. In the heart,
the ventricular wall becomes thin, the heart cavity increases,
inflammatory cells and infiltration of inflammatory factors appear
in the myocardial tissue of the infarct area, gradually replaced by
scar tissue (2–4).
In the extracellular matrix of the heart, ~80% is
type I collagen, collagen is mainly composed of cardiac fibroblasts
synthesis and secretion. Its function is regulated by a variety of
factors (17,18). TGF-β is one of the most important
inflammatory factors that adjust fibroblast function. We found that
after CAL, TGF-β mRNA expression increased significantly,
suggesting that TGF-β was involved in the fibrosis process after
MI. This was consistent with other findings (19). In addition, the ability of collagen
synthesis of fibroblasts was also significantly increased (20–22). In
the present study, we constructed a mouse model of MI by CAL. At 7
days after MI, echocardiography indicated that compared with the
NLC + CAL group, the degree of reduction of end echocardiographic
diastolic PWTD and end systolic PWTS was significantly eased in the
Tg-ERβ group. The degree of increase of end systolic LVESD and end
echocardiographic diastolic LVEDD was significantly decreased
(P<0.05). In addition, Masson staining showed that after MI,
collagen synthesis in NLC mice was significantly increased. By
contrast, heart tissue collagen of Tg-ERβ mice was relatively low
(P<0.05), indicating that ERβ is helpful to inhibit the process
of myocardial fibrosis and remodeling after MI, improving the
ability of cardiac anti-fibrosis.
Some studies suggest that the increase in acute MI
in postmenopausal women may be related to the decrease of estrogen
in the body and decrease in the expression of ERβ (23). In the present study, after Tg-ERβ
mice + AngII perfusion, the expression levels of collagen I,
α-SMA, TGF-β mRNA were significantly lower in the wild-type mice
(P<0.05). ER can be cloned and proliferated in human vascular
endothelial cells after the liquid shear stress is induced
(24). The shear stress on the
target is a strong inducer. Expression of nitric oxide (NO)
synthase can be promoted (25). This
suggests that estrogen may play a role through the L-arginine/NO
pathway (26). Many laboratories
have demonstrated that non-endothelium-dependent vascular
relaxation ER mediates will not occur after L-NAME treatment with
endothelial or NO synthase inhibitors (26). Some recent studies have further
gained understanding of the function of vascular endothelial cells
mediated by GPER (27,28). In cerebral vascular disease and acute
kidney injury, both in male and female, the protective effect of
estrogen is regulated by GPER (29,30) and
plays a protective function. Collectively, cardiac remodeling and
cardiac fibrosis after MI is a complex regulation of various
inflammatory factors. In the present study, in vivo
experiments confirmed Tg-ERβ has a protective effect on cardiac
remodeling after MI. However, the specific mechanisms of myocardial
protection of ERβ need to be further explored.
References
|
1
|
Lee KH, Jeong MH, Ahn Y, Cho MC, Kim CJ
and Kim YJ: New horizons of acute myocardial infarction: From the
Korea Acute Myocardial Infarction Registry. J Korean Med Sci.
28:173–180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gerber Y, Weston SA, Jiang R and Roger VL:
The changing epidemiology of myocardial infarction in Olmsted
County, Minnesota, 1995–2012. Am J Med. 128:144–151. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kirchberger I, Wolf K, Heier M, Kuch B,
von Scheidt W, Peters A and Meisinger C: Are daylight saving time
transitions associated with changes in myocardial infarction
incidence? Results from the German MONICA/KORA Myocardial
Infarction Registry. BMC Public Health. 15:7782015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shah AS, Griffiths M, Lee KK, McAllister
DA, Hunter AL, Ferry AV, Cruikshank A, Reid A, Stoddart M, Strachan
F, et al: High sensitivity cardiac troponin and the under-diagnosis
of myocardial infarction in women: Prospective cohort study. BMJ.
350:g78732015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kirchberger I, Meisinger C, Golüke H,
Heier M, Kuch B, Peters A, Quinones PA, von Scheidt W and Mielck A:
Long-term survival among older patients with myocardial infarction
differs by educational level: Results from the MONICA/KORA
myocardial infarction registry. Int J Equity Health. 13:192014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stillman AE, Oudkerk M, Bluemke D,
Bremerich J, Esteves FP, Garcia EV, Gutberlet M, Hundley WG,
Jerosch-Herold M, Kuijpers D, et al: North American Society of
Cardiovascular Imaging; European Society of Cardiac Radiology:
Assessment of acute myocardial infarction: Current status and
recommendations from the North American society for Cardiovascular
Imaging and the European Society of Cardiac Radiology. Int J
Cardiovasc Imaging. 27:7–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sim DS, Jeong MH, Ahn Y, Kim YJ, Chae SC,
Hong TJ, Seong IW, Chae JK, Kim CJ, Cho MC, et al: Korea Acute
Myocardial Infarction Registry (KAMIR) Investigators: Effectiveness
of drug-eluting stents versus bare-metal stents in large coronary
arteries in patients with acute myocardial infarction. J Korean Med
Sci. 26:521–527. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cho JS, Youn HJ, Her SH, Park MW, Kim CJ,
Park GM, Jeong MH, Cho JY, Ahn Y, Kim KH, et al: Korea Acute
Myocardial Infarction Registry Investigators: The prognostic value
of the left ventricular ejection fraction is dependent upon the
severity of mitral regurgitation in patients with acute myocardial
infarction. J Korean Med Sci. 30:903–910. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Omoto Y and Iwase H: Clinical significance
of estrogen receptor β in breast and prostate cancer from
biological aspects. Cancer Sci. 106:337–343. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pastore MB, Jobe SO, Ramadoss J and
Magness RR: Estrogen receptor-α and estrogen receptor-β in the
uterine vascular endothelium during pregnancy: Functional
implications for regulating uterine blood flow. Semin Reprod Med.
30:46–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mufudza C, Sorofa W and Chiyaka ET:
Assessing the effects of estrogen on the dynamics of breast cancer.
Comput Math Methods Med. 2012:4735722012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Crooke PS, Justenhoven C, Brauch H,
Dawling S, Roodi N, Higginbotham KS, Plummer WD, Schuyler PA,
Sanders ME, Page DL, et al: GENICA Consortium: Estrogen metabolism
and exposure in a genotypic-phenotypic model for breast cancer risk
prediction. Cancer Epidemiol Biomarkers Prev. 20:1502–1515. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cardaci S and Ciriolo MR: TCA cycle
defects and cancer: When metabolism tunes redox state. Int J Cell
Biol. 2012:1618372012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Colditz GA: Relationship between estrogen
levels, use of hormone replacement therapy, and breast cancer. J
Natl Cancer Inst. 90:814–823. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang M, Wang Y, Weil B, Abarbanell A,
Herrmann J, Tan J, Kelly M and Meldrum DR: Estrogen receptor beta
mediates increased activation of PI3K/Akt signaling and improved
myocardial function in female hearts following acute ischemia. Am J
Physiol Regul Integr Comp Physiol. 296:R972–R978. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pedram A, Razandi M, O'Mahony F, Lubahn D
and Levin ER: Estrogen receptor-beta prevents cardiac fibrosis. Mol
Endocrinol. 24:2152–2165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vornehm ND, Wang M, Abarbanell A, Herrmann
J, Weil B, Tan J, Wang Y, Kelly M and Meldrum DR: Acute
postischemic treatment with estrogen receptor-alpha agonist or
estrogen receptor-beta agonist improves myocardial recovery.
Surgery. 146:145–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bashey RI, Donnelly M, Insinga F and
Jimenez SA: Growth properties and biochemical characterization of
collagens synthesized by adult rat heart fibroblasts in culture. J
Mol Cell Cardiol. 24:691–700. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carver W, Nagpal ML, Nachtigal M, Borg TK
and Terracio L: Collagen expression in mechanically stimulated
cardiac fibroblasts. Circ Res. 69:116–122. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang W, Chancey AL, Tzeng HP, Zhou Z,
Lavine KJ, Gao F, Sivasubramanian N, Barger PM and Mann DL: The
development of myocardial fibrosis in transgenic mice with targeted
overexpression of tumor necrosis factor requires mast
cell-fibroblast interactions. Circulation. 124:2106–2116. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Duerrschmid C, Crawford JR, Reineke E,
Taffet GE, Trial J, Entman ML and Haudek SB: TNF receptor 1
signaling is critically involved in mediating
angiotensin-II-induced cardiac fibrosis. J Mol Cell Cardiol.
57:59–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weber KT, Sun Y, Bhattacharya SK, Ahokas
RA and Gerling IC: Myofibroblast-mediated mechanisms of
pathological remodelling of the heart. Nat Rev Cardiol. 10:15–26.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Burns KA, Li Y, Arao Y, Petrovich RM and
Korach KS: Selective mutations in estrogen receptor alpha D-domain
alters nuclear translocation and non-estrogen response element gene
regulatory mechanisms. J Biol Chem. 286:12640–12649. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Prossnitz ER and Barton M: The
G-protein-coupled estrogen receptor GPER in health and disease. Nat
Rev Endocrinol. 7:715–726. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fleming I: Molecular mechanisms underlying
the activation of eNOS. Pflugers Arch. 459:793–806. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meyer MR, Prossnitz ER and Barton M: The G
protein-coupled estrogen receptor GPER/GPR30 as a regulator of
cardiovascular function. Vascul Pharmacol. 55:17–25. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gros R, Ding Q, Liu B, Chorazyczewski J
and Feldman RD: Aldosterone mediates its rapid effects in vascular
endothelial cells through GPER activation. Am J Physiol Cell
Physiol. 304:C532–C540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ding Q, Hussain Y, Chorazyczewski J, Gros
R and Feldman RD: GPER-Independent effects of estrogen in rat
aortic vascular endothelial cells. Mol Cell Endocrinol. 399:60–68.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hutchens MP, Fujiyoshi T, Komers R, Herson
PS and Anderson S: Estrogen protects renal endothelial barrier
function from ischemia-reperfusion in vitro and in vivo. Am J
Physiol Renal Physiol. 303:F377–F385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Murata T, Dietrich HH, Xiang C and Dacey
RG Jr: G protein-coupled estrogen receptor agonist improves
cerebral microvascular function after hypoxia/reoxygenation injury
in male and female rats. Stroke. 44:779–785. 2013. View Article : Google Scholar : PubMed/NCBI
|