Introduction
Prostate cancer is the second most commonly
diagnosed cancer and the sixth leading cause of mortality in men
worldwide (1). The highest incidence
rates of prostate cancer are observed in the United States, Canada
and northwestern Europe, whereas this malignancy is less common
across Asia and South America (1,2).
Although the lowest incidence and mortality rates of prostate
cancer worldwide are observed in Asian men, these rates have
increased over the past 20–30 years (3). The advent of prostate-specific antigen
(PSA) screening in the 1980s has improved early-stage diagnosis of
prostate cancer and increased rates of diagnosis at potentially
treatable stages (4). However, many
prostate cancer patients that undergo definitive treatment that
aims to permanently eradicate the malignancy, including radical
prostatectomy and radiation therapy, suffer from biochemical
recurrence and eventually progress to metastatic disease (4). However, therapeutic options currently
available for advanced prostate cancer have short-lasting effects
and a minimal effect on patient survival rates, thus the
identification of novel treatment strategies is required (4).
Prostate stem cell antigen (PSCA) is a
glycosylphosphatidylinositol-anchored cell membrane protein that
belongs to the Thy-1/Ly-6 family of cell surface antigens, and
consists of 123 amino acids (5).
Expression of PSCA in normal tissues is predominantly
prostate-specific. However, low-level expression of PSCA has also
been detected in other tissues, including the placenta, stomach and
kidney (2). Elevated levels of PSCA
have been documented in >80% of prostate cancer tissues and in
all cases of bone metastatic prostate cancer in patients (6). In preclinical trials, administration of
murine anti-PSCA monoclonal antibodies to mice bearing human
prostate cancers led to the inhibition of tumor growth and
metastasis, and prolonged survival of mice (7,8). This
was observed for xenografts derived from both bone metastasis and
lymph node malignancies, and for non-castration and
castration-resistant tumors (7).
Furthermore, silencing of PSCA using small interfering RNA has been
demonstrated to inhibit the proliferation and reduce the migration
and invasion of human prostate cancer PC-3M cells (9).
The present study investigated the effect of
plant-derived flavonoid compounds, namely genistein, luteolin and
quercetin, on the expression of PSCA and inhibition of prostate
cancer cells in vitro.
Materials and methods
Materials
Genistein (≥98% purity), quercetin (≥97% purity) and
luteolin (≥98% purity) were purchased from Aladdin Industrial Corp.
(Shanghai, China). An Annexin V-FITC/PI apoptosis detection kit
(40302ES20) and Hoechst 33342 nucleic acid stain were purchased
from Shanghai Qcbio Science and Technologies Co., Ltd. (Shanghai,
China). The following reagents were from Beijing Solarbio Science
and Technology Co., Ltd. (Beijing, China): Propidium iodide (PI),
ethidium bromide (EB), dimethyl sulfoxide (DMSO), RNase and
materials for western blot analysis, including SDS gel,
nitrocellulose membranes, blot filter paper, bovine serum albumin
and Ponceau S dye. Human anti-PSCA (ab56338) and anti-β-actin
(ab3280) antibodies were obtained from Abcam (Cambridge, MA, USA).
Horseradish peroxidase-conjugated goat anti-mouse antibody
(TA130003) was purchased from Origene Technologies, Inc. (Beijing,
China).
Cell culture
The prostate cancer cell line DU145, which expresses
PSCA (10), was obtained from the
National Infrastructure of Cell Line Resource (Beijing, China).
DU145 cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 2 mM
L-glutamine, 10% heat-inactivated fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin G and 100 mg/ml
streptomycin (all Beijing Solarbio Science and Technology Co.,
Ltd.) at 37°C in a humidified atmosphere of 5% CO2 in
air.
Cell viability assay
DU145 cells (1×104 cells/well) were
plated into 96-well plates and incubated at 37°C for 24 h in
RPMI-1640 medium supplemented with 10% heat-inactivated fetal
bovine serum to allow the cells to attach prior to treatment with
flavonoids (genistein, luteolin and quercetin). Each flavonoid
compound was dissolved in 0.1% DMSO and made up with RPMI-1640
medium to a final flavonoid concentration of 20, 40, 80 and 100 µM.
DU145 cells were individually treated with 20, 40, 80 and 100 µM of
each flavonoid compound at 37°C for 24 h in RPMI-1640 medium
supplemented with 10% heat-inactivated fetal bovine serum. Cells
treated with 0.1% DMSO served as a negative control. Cell viability
was measured by counting the cells with a Neubauer chamber (BRAND
Trading Co., Ltd., Shanghai), according to the manufacturer's
protocol. Cell viability was expressed relative to control cells.
Half maximal inhibitory concentration (IC50) values were
subsequently determined as the concentration of compound that
produced a 50% reduction in cell number, and were determined
graphically from the concentration response curve.
Morphological observation
Morphological changes characteristic of cell death
were observed using a normal inverted light microscope. Cells were
individually treated with different concentrations of the three
flavonoids for 24 h. Morphological changes of cells were observed
at 24 h post-treatment.
Cell cycle analysis
DU145 cells (70% confluence in 6-well plates) were
starved in 1% FBS-supplemented RPMI-1640 media for 24 h to arrest
cells in the G0 phase of the cell cycle, after which they were
individually treated with each flavonoid compound (80 µM) for 24 h
in 10% FBS-supplemented RPMI-1640 medium. The cells in the control
group were treated with 0.1% DMSO under the same conditions.
Following flavonoid treatment, cells were trypsinized, washed twice
with cold phosphate-buffered saline (PBS) and centrifuged at 110 ×
g for 5 min at room temperature. The pellet was resuspended in 75%
ethanol for 24 h at −20°C. Cells were subsequently centrifuged at
110 × g for 5 min at room temperature and the supernatant
containing dead and apoptotic cells was discarded, whereas the
pellet was washed twice with cold PBS, suspended in 500 µl PBS and
incubated with 5 µl RNase (final concentration, 20 µg/ml) at 37°C
for 30 min. Cells were incubated in the dark with PI (final
concentration, 50 µg/ml) for 1 h at room temperature and analyzed
by flow cytometry using a BD FACSAria III Cell Sorter (BD
Biosciences, San Jose, CA, USA) and WinMDI (version 2.8) software
(Scripps Institute, La Jolla, CA, USA).
Observation of apoptosis by Hoechst
33342 staining
DU145 cells were seeded into 6-well plates
(5×105 cells/well) and individually incubated with each
of the three flavonoid compounds (80 µM) at 37°C for 24 h. Cells in
the control group were treated with 0.1% DMSO under the same
conditions. The cells were incubated with Hoechst 33342 (30 µM in
PBS) in the dark for 30 min. Cells were then washed twice with PBS
and cell fluorescence was observed using a FluoView FV1000 confocal
microscope (Olympus Corp., Tokyo, Japan).
Detection of apoptosis by flow
cytometry
Levels of cell apoptosis were measured using an
Annexin V-FITC apoptosis detection kit, according to the
manufacturer's protocol. Briefly, cells were individually treated
with each of the three flavonoids (80 µM) or DMSO control (0.1%)
for 24 h at 37°C. Cells were trypsinized and washed with PBS, then
resuspended in Annexin V binding buffer containing 10 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid/NaOH (pH 7.4),
140 mM NaCl and 2.5 mM CaCl2. Cells were subsequently
stained with FITC-conjugated Annexin V and PI at room temperature
for 15 min in the dark prior to the addition of binding buffer. The
number of apoptotic cells was measured by flow cytometry, as
described above. Cells were sorted into intact (Annexin
V−/PI−), early apoptotic (Annexin
V+/PI−), late apoptotic (Annexin
V+/PI+) and necrotic (Annexin
V−/PI+) cell populations.
Isolation of RNA and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from DU145 cells individually
treated with each of the three flavonoids (80 µM) or DMSO control
(0.1%) for 24 h using the TRIzol reagent-phenol chloroform
procedure (Gibco; Thermo Fisher Scientific, Inc.). DNase I (2 U,
D7076; Beyotime Institute of Biotechnology, Haimen, China) was
added to the RNA and incubated in a 37°C water bath for 20 min.
DNAse Inactivation reagent (2 µl, AM1906; Ambion; Thermo Fisher
Scientific, Inc.) was added to the RNA and incubated for 2 min at
room temperature. The tube was flicked once more during the
incubation to redisperse the DNase Inactivation reagent and
centrifuged at 10,000 × g for 1 min at 4°C. A total of 2 µg total
RNA from each sample, quantified with a NanoDrop 2000c
spectrophotometer (Thermo Fisher Scientific, Inc.) was subjected to
RT using a SYBR PrimeScript RT-PCR kit (Takara Biotechnology Co.,
Ltd., Dalian, China), according to the manufacturer's protocol. For
each sample, qPCR was carried out in triplicate in a total reaction
mixture of 20 µl [2 µl cDNA, 10 µl SYBR Premix Ex Taq, 0.4
µl ROX Reference Dye II, 0.5 µl of each forward and reverse primer
(both 10 µM) and 6.6 µl H2O] on an ABI PRISM 7500
real-time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Primers (Sangon Biotech Co., Ltd., Shanghai, China) used
were as follows: PSCA forward, 5′-ATCAGGAGGGCCCAGTAAAG-3′ and
reverse, 5′-TCCCAGGAACTCACGTCAAC-3′; and β-actin forward,
5′-AACACCCCAGCCATGTACG-3′ and reverse,
5′-ATGTCACGCACGATTTCCC-3′.
qPCR thermal cycling was initiated at 95°C for 10
sec, followed by 40 thermal cycles, each at 95°C for 5 sec and 60°C
for 34 sec. Levels of PCSA mRNA expression were measured using the
2−ΔΔCq method (11) and
were normalized to the expression of β-actin in each sample.
Melting curves for each qPCR analysis were generated to verify the
purity of the amplification product.
Western blot analysis
DU145 cells (70% confluent) were individually
treated with each of the three flavonoid compounds (80 µM) or DMSO
control (0.1%) at 37°C for 24 h. Following treatment, the media was
aspirated and cells were washed with cold PBS and subsequently
incubated with ice-cold lysis buffer (50 mM Tris-HCl, 150 mM NaCl,
1 mM EGTA, 1 mM EDTA, 20 mM NaF, 100 mM
Na3VO4, 0.5% NP-40, 1% Triton X-100, 1 mM
phenylmethylsulfonyl fluoride; pH 7.4) over ice for 30 min. Cells
were then scraped and the lysate was collected in a microfuge tube.
The lysate was cleared by centrifugation at 14,000 × g for 15 min
at 4°C and the supernatant (total cell lysate) was immediately used
or stored at −80°C. Protein concentration of the supernatant was
determined using a Bradford Protein assay (Bio-Rad Laboratories,
Inc., Hercules, CA, USA), according to the manufacturer's protocol.
Proteins (50 µg) from each sample were separated by 10–12% SDS-PAGE
and transferred to a nitrocellulose membrane. The blot was blocked
in blocking buffer (5% non-fat dry milk and 0.05% Tween-20 in 20 mM
TBS; pH 7.6) for 1 h at room temperature. Blots were then incubated
with anti-PSCA (1:1,000) overnight at 4°C and anti-β-actin
antibodies (1:1,000) for 2 h at room temperature, followed by
incubation with goat anti-mouse secondary antibody conjugated to
horseradish peroxidase (1:10,000) for 1 h at room temperature.
Protein bands were visualized using an enhanced chemiluminescence
detection system (Beyotime Institute of Biotechnology). β-actin was
used as an internal control.
Statistical analyses
All data are expressed as the mean ± standard error
of the mean of at least three independent experiments. Differences
between the mean values of multiple groups were analyzed using
one-way analysis of variance. Statistical analysis was performed
with SPSS 19.0 software (IBM SPSS, Armonk, NY, USA) and P<0.05
was considered to indicate a statistically significant
difference.
Results
Cytotoxic effects of flavonoids in
DU145 cells
To determine the effect of flavonoids on the
inhibition of cell viability, the cytotoxicity of various
concentrations (20–100 µM) of genistein, luteolin and quercetin
were evaluated by counting cells in a Neubauer chamber at 24 h
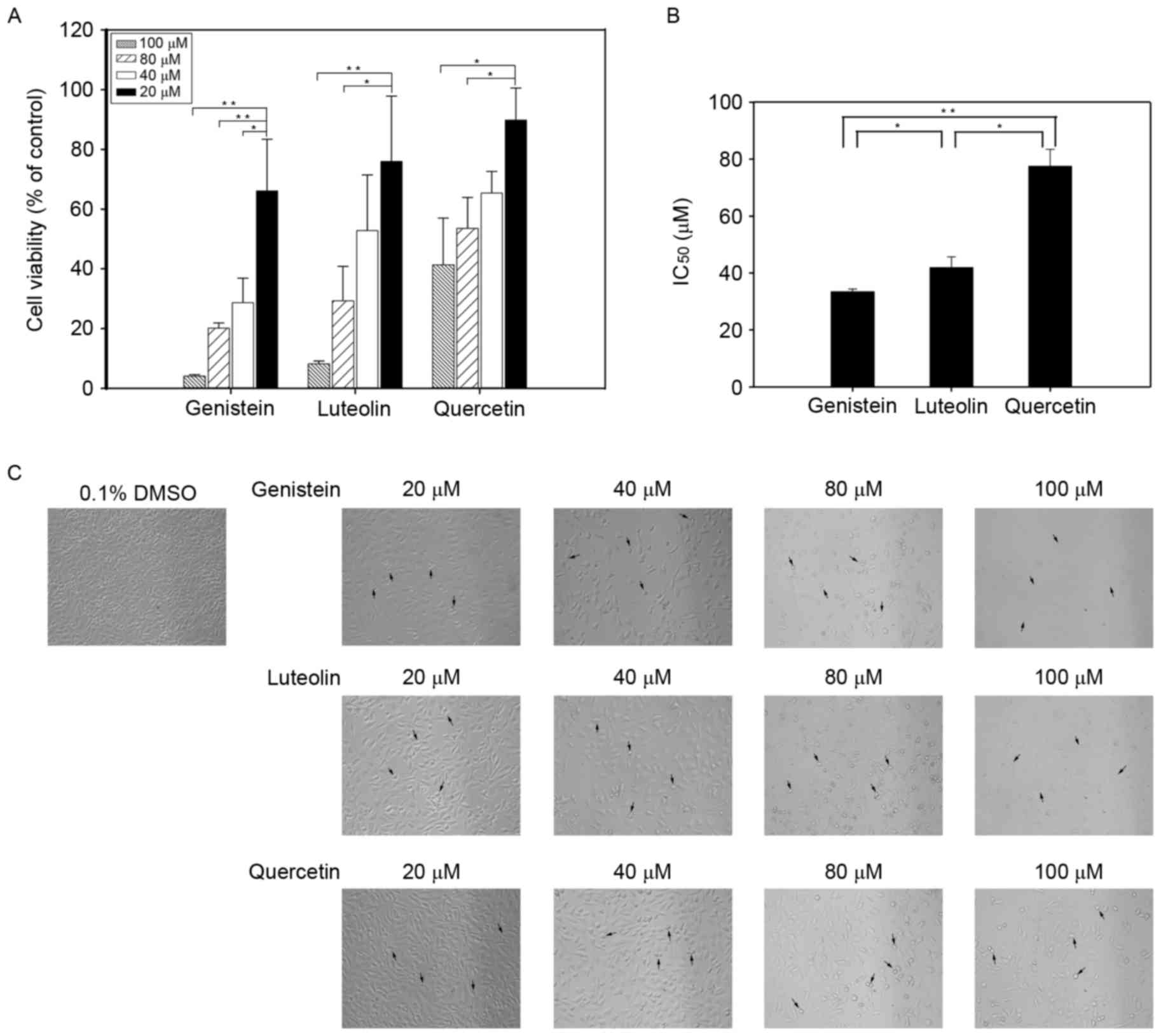
post-treatment. As depicted in Fig.
1A, flavonoids inhibited cell viability in a dose-dependent
manner (P<0.05). Genistein exhibited the greatest inhibitory
effect on cell cycle inhibition with an estimated IC50
value of 33 µM, which was significantly lower compared with that of
luteolin (42 µM; P<0.05) and quercetin (78 µM; P<0.01;
Fig. 1B). Observations by light
microscope also indicated that, following flavonoid treatment,
numbers of DU145 cells decreased in a dose-dependent manner, and
cell morphology was altered relative to that of control cells.
Notably, fewer DU145 cells were observed in cell cultures treated
with increasing concentrations of each flavonoid, while swelling
and damage of the plasma membrane was observed in the remaining
cells (Fig. 1C).
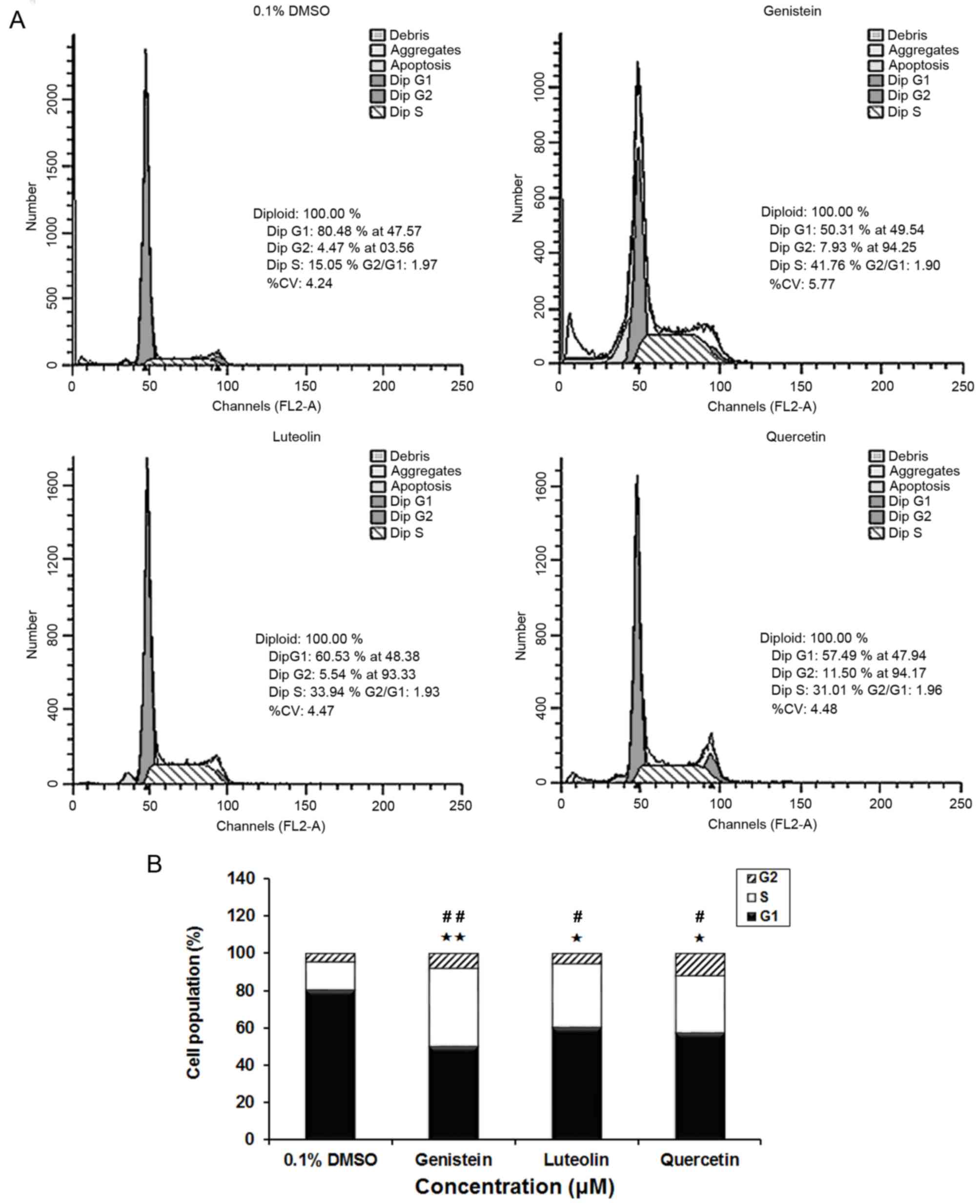
Flavonoids induce S phase cell cycle
arrest in DU145 cells
To determine whether the inhibitory effects of
flavonoids on cell growth were mediated by alterations in cell
cycle progression, synchronized cells were incubated with each
flavonoid (80 µM) for 24 h, and the effect of the flavonoids on
cell cycle phase distribution was determined. Representative
histograms are depicted in Fig. 2.
Relative to control cells, cell groups treated with genistein,
luteolin and quercetin exhibited a significantly increased
population of S phase cells, and a corresponding significant
decrease in G1 phase cells (both P<0.01 for genistein; both
P<0.05 for luteolin and quercitin). This was consistent with the
aforementioned growth inhibitory effects of genistein, luteolin and
quercetin.
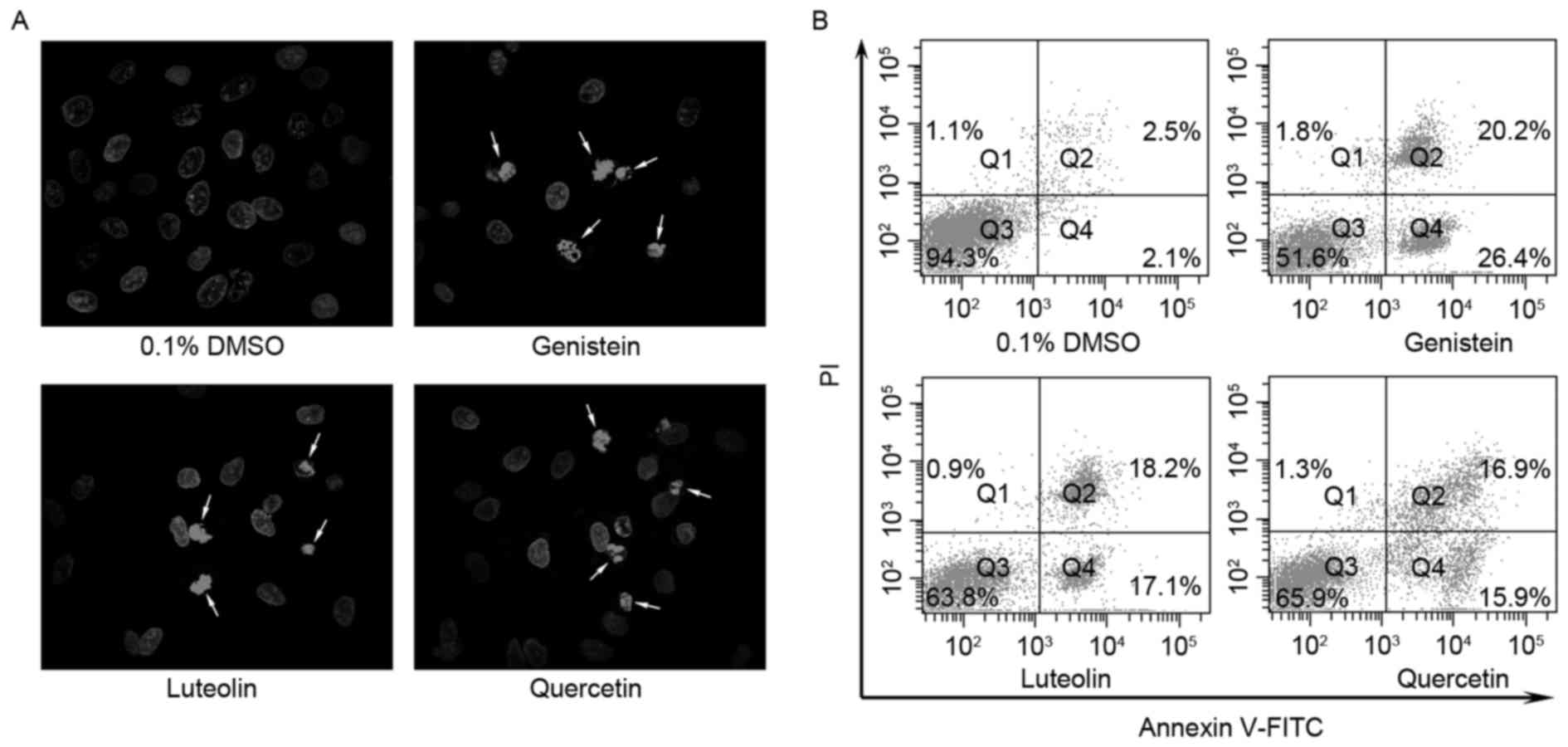
Flavonoids induce apoptosis in DU145
cells
Flavonoids may have inhibited the viability of DU145
cells through the induction of apoptosis, and thus cell
morphologies were assessed to determine the extent of apoptosis.
Hoechst 33342 staining demonstrated that treatment with genistein,
luteolin and quercetin induced chromatin condensation and
fragmentation of the nuclei into oligonucleosomes (Fig. 3A). Apoptotic cells were subsequently
quantified using a flow cytometry assay. Individual treatment with
each of the three flavonoid compounds (80 µM) for 24 h decreased
the proportion of intact cells (Fig.
3B) and increased the proportion of apoptotic cells. Notably,
treatment with genistein, luteolin and quercetin decreased the
proportions of intact cells from 94.3%, as observed for control
cells, to 51.6, 63.8 and 65.9%, respectively. By contrast,
genistein, luteolin and quercetin increased the proportions of
early apoptotic cells from 2.1% (control) to 26.4, 17.1 and 15.9%,
respectively. These data indicate that flavonoids may induce
apoptosis in DU145 cells.
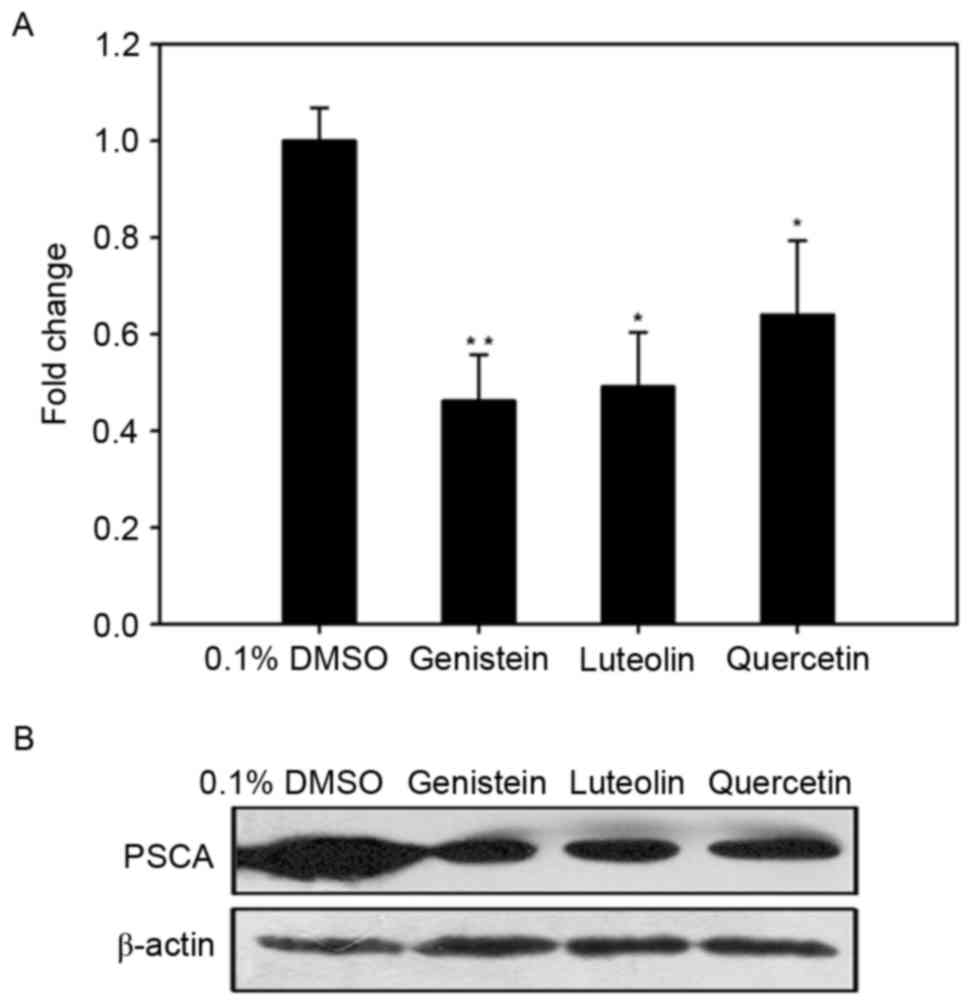
Flavonoids inhibit the expression of
PSCA in DU145 cells
To determine whether the inhibitory effects of
genistein, luteolin and quercetin were associated with a
downregulation in PSCA, RT-qPCR was used to measure the levels of
PSCA mRNA expression in DU145 cells following treatment with each
of the three flavonoids (80 µM) for 24 h. As depicted in Fig. 4A, levels of PSCA mRNA in DU145 cells
treated with genistein, luteolin and quercetin were decreased by
0.46-fold (P<0.01), 0.49-fold (P<0.05) and 0.64-fold
(P<0.05), respectively, when compared with controls. Thus,
genistein, luteolin, and quercetin may inhibit the expression of
PSCA at the mRNA level.
In addition, western blot analysis was conducted to
verify whether alterations in the expression of PSCA mRNA led to
alterations at the protein level. As depicted in Fig. 4B, genistein, luteolin and quercetin
markedly decreased the expression of PSCA at the protein level
after 24-h treatment.
Discussion
Flavonoids are polyphenolic compounds that are
considered to have potent preventative and chemotherapeutic
properties in the treatment of cancer (12). Flavonoids are principally categorized
into flavones, flavonols, flavanols, flavanones, flavanonols and
isoflavones (13,14). Genistein (isoflavone), luteolin
(flavone) and quercetin (flavonol) are typically present in
numerous fruits and vegetables, and have been shown to inhibit cell
proliferation with variable efficacy in human prostate cancer
(15–17). Consistent with these findings, the
present study observed that genistein, luteolin and quercetin
inhibited the proliferation of prostate cancer cells in a
dose-dependent manner and in the following order of potency:
Genistein>luteolin>quercetin. Based on the chemical
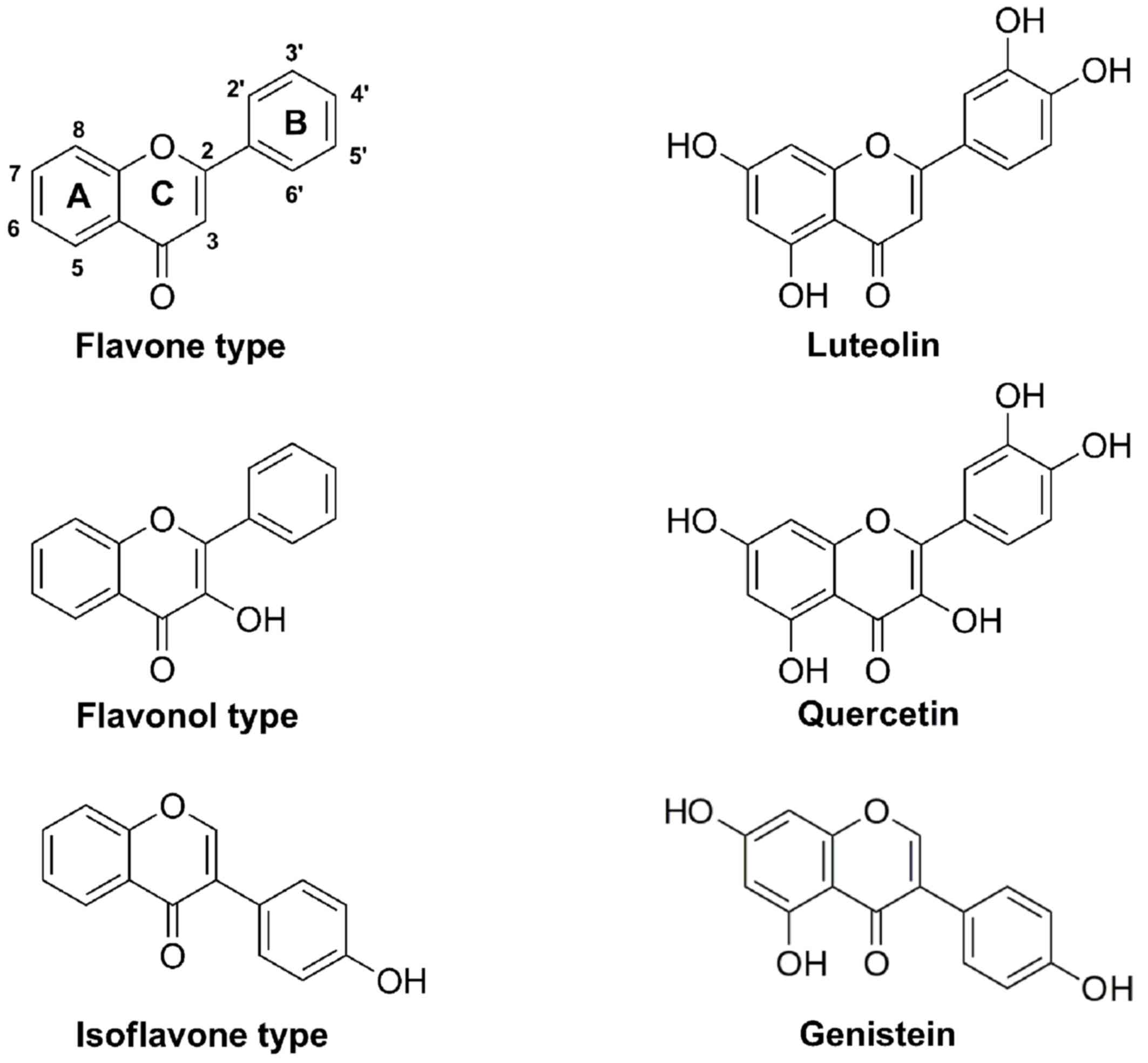
characteristics of genistein, luteolin and quercetin (Fig. 5), the inhibitory effect of these
flavonoids on cell proliferation may have been dependent on the
number of hydroxyl groups in the molecules.
Cell cycle progression mediates the growth and
proliferation of mammalian cells, and cell cycle inhibition is a
potential therapeutic strategy in the management of cancer
(18). The present study observed
that genistein, luteolin, and quercetin exerted inhibitory effects
on the growth of DU145 prostate cancer cells by arresting cells in
S phase of the cell cycle. Similarly, previous studies have
demonstrated that flavonoids inhibit cell growth by inducing S
phase cell cycle arrest in prostate cancer cells (19–21).
Flavonoids have also been documented to arrest cell-cycle
progression at G0/G1 and G2/M in prostate cancer cells (22–24).
These results indicate that flavonoids may block cell growth at
multiple stages of the cell cycle (25).
Previous studies have focused on cell cycle-mediated
apoptosis as a potential strategy for cancer cell elimination
(26,27). Analogous to previous studies in human
prostate cancer cells (24,28–30), the
present study demonstrated that genistein, luteolin and quercetin
induced apoptosis in DU145 cells. Thus, genistein, luteolin and
quercetin may inhibit the growth of prostate cancer cells through
the induction of cell cycle arrest and apoptosis.
Biomarkers are considered to be critical in the
development of anticancer therapies (31,32). Due
to improved insight into the molecular basis of cancer, anticancer
strategies now focus on targeting specific molecular alterations
that occur in cancer cells (33,34). In
prostate cancer, numerous biomarkers have been identified,
including PSA, prostate-specific membrane antigen, PSCA, early
prostate cancer antigen, B7-H3, chromogranin A, α-methylacyl
coenzyme A racemase, glutathione S-transferase P1 (GSTP1),
sarcosine, caveolin-1, TMPRSS2-ERG, Ki-67, prostate cancer antigen
3 (PCA3) and disabled homolog 2-interacting protein (35). Previous studies have demonstrated
that a number of these biomarkers, including PSA, GSTP1, Ki-67 and
PCA3, were the targets by which flavonoids exerted anticancer
effects in prostate cancer (36–39).
Using human xenografts grown in mice with severe combined
immunodeficiency, it has also been demonstrated that anti-PSCA
monoclonal antibodies inhibited the growth and metastasis of tumors
(40), thus indicating that PSCA may
be an immunotherapeutic target in the treatment of prostate cancer
(40,41).
In conclusion, the current study was the first to
demonstrate that the anticancer effects of genistein, luteolin and
quercetin in DU145 prostate cancer cells were associated with a
downregulation in PSCA at the mRNA and protein levels. The present
findings indicate that flavonoids may be potential therapeutic
agents in the treatment and prevention of prostate cancer.
Acknowledgements
The present study was supported by the Youth
Academic Backbone Supporting Plan Project of the General Colleges
and Universities of Heilongjiang Province, China (grant no.
1254G057).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Quinn M and Babb P: Patterns and trends in
prostate cancer incidence, survival, prevalence and mortality. Part
I: International comparisons. BJU Int. 90:162–173. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pu YS, Chiang HS, Lin CC, Huang CY, Huang
KH and Chen J: Changing trends of prostate cancer in Asia. Aging
Male. 7:120–132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gu Z, Yamashiro J, Kono E and Reiter RE:
Anti-prostate stem cell antigen monoclonal antibody 1G8 induces
cell death in vitro and inhibits tumor growth in vivo via a
Fc-independent mechanism. Cancer Res. 65:9495–9500. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barve A, Jin W and Cheng K: Prostate
cancer relevant antigens and enzymes for targeted drug delivery. J
Control Release. 187:118–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nejatollahi F, Abdi S and Asgharpour M:
Antiproliferative and apoptotic effects of a specific antiprostate
stem cell single chain antibody on human prostate cancer cells. J
Oncol. 2013:8398312013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Antonarakis ES, Carducci MA, Eisenberger
MA, Denmeade SR, Slovin SF, Jelaca-Maxwell K, Vincent ME, Scher HI
and Morris MJ: Phase I rapid dose-escalation study of AGS-1C4D4, a
human anti-PSCA (prostate stem cell antigen) monoclonal antibody,
in patients with castration-resistant prostate cancer: A PCCTC
trial. Cancer Chemother Pharmacol. 69:763–771. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saffran DC, Raitano AB, Hubert RS, Witte
ON, Reiter RE and Jakobovits A: Anti-PSCA mAbs inhibit tumor growth
and metastasis formation and prolong the survival of mice bearing
human prostate cancer xenografts. Proc Natl Acad Sci USA. 98:pp.
2658–2663. 2001; View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang R, Zhao S, Liu L, Li F, Li E, Luo L,
Xu L, Wan S and Zhao Z: Knockdown of PSCA induces EMT and decreases
metastatic potentials of the human prostate cancer DU145 cells.
Cancer Cell Int. 16:202016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao Z, Ma W, Zeng G, Qi D, Ou L and Liang
Y: Small interference RNA-mediated silencing of prostate stem cell
antigen attenuates growth, reduces migration and invasion of human
prostate cancer PC-3M cells. Urol Oncol. 31:343–351. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ravishankar D, Rajora AK, Greco F and
Osborn HM: Flavonoids as prospective compounds for anti-cancer
therapy. Int J Biochem Cell Biol. 45:2821–2831. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harborne JB and Williams CA: Advances in
flavonoid research since 1992. Phytochemistry. 55:481–504. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ren W, Qiao Z, Wang H, Zhu L and Zhang L:
Flavonoids: Promising anticancer agents. Med Res Rev. 23:519–534.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chiyomaru T, Yamamura S, Fukuhara S,
Yoshino H, Kinoshita T, Majid S, Saini S, Chang I, Tanaka Y,
Enokida H, et al: Genistein inhibits prostate cancer cell growth by
targeting miR-34a and oncogenic HOTAIR. PLoS One. 8:e703722013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsui KH, Chung LC, Feng TH, Chang PL and
Juang HH: Upregulation of prostate-derived Ets factor by luteolin
causes inhibition of cell proliferation and cell invasion in
prostate carcinoma cells. Int J Cancer. 130:2812–2823. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsai PH, Cheng CH, Lin CY, Huang YT, Lee
LT, Kandaswami CC, Lin YC, Lee KP, Hung CC, Hwang JJ, et al:
Dietary flavonoids luteolin and quercetin suppressed cancer stem
cell properties and metastatic potential of isolated prostate
cancer cells. Anticancer Res. 36:6367–6380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schwartz GK and Shah MA: Targeting the
cell cycle: A new approach to cancer therapy. J Clin Oncol.
23:9408–9421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee HZ, Leung HW, Lai MY and Wu CH:
Baicalein induced cell cycle arrest and apoptosis in human lung
squamous carcinoma CH27 cells. Anticancer Res. 25:959–964.
2005.PubMed/NCBI
|
|
20
|
Shenouda NS, Zhou C, Browning JD, Ansell
PJ, Sakla MS, Lubahn DB and Macdonald RS: Phytoestrogens in common
herbs regulate prostate cancer cell growth in vitro. Nutr Cancer.
49:200–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Knowles LM, Zigrossi DA, Tauber RA,
Hightower C and Milner JA: Flavonoids suppress androgen-independent
human prostate tumor proliferation. Nutr Cancer. 38:116–122. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Z, Wang CZ, Du GJ, Qi LW, Calway T,
He TC, Du W and Yuan CS: Genistein induces G2/M cell cycle arrest
and apoptosis via ATM/p53-dependent pathway in human colon cancer
cells. Int J Oncol. 43:289–296. 2013.PubMed/NCBI
|
|
23
|
Markaverich BM, Vijjeswarapu M, Shoulars K
and Rodriguez M: Luteolin and gefitinib regulation of EGF signaling
pathway and cell cycle pathway genes in PC-3 human prostate cancer
cells. J Steroid Biochem Mol Biol. 122:219–231. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu KC, Yen CY, Wu RS, Yang JS, Lu HF, Lu
KW, Lo C, Chen HY, Tang NY, Wu CC and Chung JG: The roles of
endoplasmic reticulum stress and mitochondrial apoptotic signaling
pathway in quercetin-mediated cell death of human prostate cancer
PC-3 cells. Environ Toxicol. 29:428–439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Neves MP, Cidade H, Pinto M, Silva AM,
Gales L, Damas AM, Lima RT, Vasconcelos MH and de São José
Nascimento M: Prenylated derivatives of baicalein and
3,7-dihydroxyflavone: Synthesis and study of their effects on tumor
cell lines growth, cell cycle and apoptosis. Eur J Med Chem.
46:2562–2574. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ahmad N, Adhami VM, Afaq F, Feyes DK and
Mukhtar H: Resveratrol causes WAF-1/p21-mediated G(1)-phase arrest
of cell cycle and induction of apoptosis in human epidermoid
carcinoma A431 cells. Clin Cancer Res. 7:1466–1473. 2001.PubMed/NCBI
|
|
27
|
Calcabrini A, García-Martínez JM, González
L, Tendero MJ, Ortuño MT, Crateri P, Lopez-Rivas A, Arancia G,
González-Porqué P and Martín-Pérez J: Inhibition of proliferation
and induction of apoptosis in human breast cancer cells by lauryl
gallate. Carcinogenesis. 27:1699–1712. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang G, Song L, Wang H and Xing N:
Quercetin synergizes with 2-methoxyestradiol inhibiting cell growth
and inducing apoptosis in human prostate cancer cells. Oncol Rep.
30:357–363. 2013.PubMed/NCBI
|
|
29
|
Zhu QY, Hu R, Liu L, Yuan L, Huang WZ, Ma
L and Gu XJ: Quercetin induces the apoptosis of human PC-3 cells.
Zhonghua Nan Ke Xue. 17:790–793. 2011.(In Chinese). PubMed/NCBI
|
|
30
|
Senthilkumar K, Elumalai P, Arunkumar R,
Banudevi S, Gunadharini ND, Sharmila G, Selvakumar K and Arunakaran
J: Quercetin regulates insulin like growth factor signaling and
induces intrinsic and extrinsic pathway mediated apoptosis in
androgen independent prostate cancer cells (PC-3). Mol Cell
Biochem. 344:173–184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Verma M and Srivastava S: New cancer
biomarkers deriving from NCI early detection research. Recent
Results Cancer Res. 163:72–84, 264-266. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kelloff GJ, Bast RC Jr, Coffey DS, D'Amico
AV, Kerbel RS, Park JW, Ruddon RW, Rustin GJ, Schilsky RL, Sigman
CC and Woude GF Vande: Biomarkers, surrogate end points and the
acceleration of drug development for cancer prevention and
treatment: An update prologue. Clin Cancer Res. 10:3881–3884. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Collins I and Workman P: New approaches to
molecular cancer therapeutics. Nat Chem Biol. 2:689–700. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kummar S, Gutierrez M, Doroshow JH and
Murgo AJ: Drug development in oncology: Classical cytotoxics and
molecularly targeted agents. Br J Clin Pharmacol. 62:15–26. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Madu CO and Lu Y: Novel diagnostic
biomarkers for prostate cancer. J Cancer. 1:150–177. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
White RW deVere, Tsodikov A, Stapp EC,
Soares SE, Fujii H and Hackman RM: Effects of a high dose,
aglycone-rich soy extract on prostate-specific antigen and serum
isoflavone concentrations in men with localized prostate cancer.
Nutr Cancer. 62:1036–1043. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Adjakly M, Bosviel R, Rabiau N, Boiteux
JP, Bignon YJ, Guy L and Bernard-Gallon D: DNA methylation and soy
phytoestrogens: Quantitative study in DU-145 and PC-3 human
prostate cancer cell lines. Epigenomics. 3:795–803. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Khan N, Bharali DJ, Adhami VM, Siddiqui
IA, Cui H, Shabana SM, Mousa SA and Mukhtar H: Oral administration
of naturally occurring chitosan-based nanoformulated green tea
polyphenol EGCG effectively inhibits prostate cancer cell growth in
a xenograft model. Carcinogenesis. 35:415–423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stettner M, Kaulfuss S, Burfeind P,
Schweyer S, Strauss A, Ringert RH and Thelen P: The relevance of
estrogen receptor-beta expression to the antiproliferative effects
observed with histone deacetylase inhibitors and phytoestrogens in
prostate cancer treatment. Mol Cancer Ther. 6:2626–2633. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ross S, Spencer SD, Holcomb I, Tan C,
Hongo J, Devaux B, Rangell L, Keller GA, Schow P, Steeves RM, et
al: Prostate stem cell antigen as therapy target: Tissue expression
and in vivo efficacy of an immunoconjugate. Cancer Res.
62:2546–2553. 2002.PubMed/NCBI
|
|
41
|
Dannull J, Diener PA, Prikler L,
Fürstenberger G, Cerny T, Schmid U, Ackermann DK and Groettrup M:
Prostate stem cell antigen is a promising candidate for
immunotherapy of advanced prostate cancer. Cancer Res.
60:5522–5528. 2000.PubMed/NCBI
|