Introduction
Infants are very susceptible to infection with
external pathogens that can cause a series of diseases, which
results from the underdevelopment of teh infantile immune system so
that the resistance for the external adverse conditions is weak
(1). As a common disease in infants,
respiratory disease has high incidence and recurrence (2). Mild pediatric respiratory diseases
mainly manifest as acute upper respiratory infection, bronchitis,
asthma and pneumonia, while severe respiratory system disease in
children may gradually develop to severe pneumonia and even lung
failure. Therefore, it is extremely significant to enhance the
diagnosis and treatment for respiratory diseases in children
(3). As a common respiratory disease
in children, bronchitis is mainly caused by pulmonary capillary
bronchial lesions by virtue of the common cold or the flu. The
study indicated that approximately 80% of children with bronchitis
suffered from viral infection caused by respiratory syncytial
virus, and adenovirus infection followed (4). At present, the research for pediatric
bronchitis has been mainly concentrated on the level of bacteria,
viruses, and other exotic microbes, which are rarely reported in
the study of related genes (5).
Previous findings have shown that human c-Jun
N-terminal kinase (JNK) and stress-activated protein kinase (SAPK)
signaling pathways are involved in the production and deterioration
of various diseases of the respiratory system (6). In addition, it was previously found
that there was a correlation between the phosphorylation of JNK as
well as related proteins and diseases such as bronchial asthma
(7), suggesting that JNK1 may be one
of the important binding sites of anti-airway remodeling. It has
been proven that there was a certain correlation between
JNK1 gene and asthma in children. However, the correlation
of JNK1 and children bronchitis was not reported.
In the present study, we first investigated the
mutuality between JNK1 and bronchitis in children, and
identified that JNK1 promotes the production and
deterioration of bronchitis in children, which provides certain
theoretical and experimental basis for the diagnosis and treatment
of the pediatric bronchitis.
Materials and methods
General information
Between April 2013 and April 2015, 32 children with
an average age of 6.5±3.8 years were admitted to the Xuzhou
Children's Hospital (Jiangsu, China) for bronchitis were enrolled
in the present study, including 17 males with an average age of
6.2±4.5 years and 15 females with an average age of 6.7±3.2 years.
The patients in the observation group were consistent with a
previous study (8). Exclusion
criteria for the study included other respiratory diseases and
inflammation. Twenty-eight healthy children were selected as the
control group; average age of 6.3±4.1 years in the same period,
including 14 males with an average age of 5.8±3.7 years and 14
females with an average age of 5.4±4.2 years. Venous blood samples
(5 ml) were obtained from the subjects in the experimental and
control groups, respectively. The cells were collected by
centrifugation at 2,750 × g for 5 min. Frozen storage solution was
added and the cells were stored in the refrigerator at −80°C.
Approval of the study was provided by the Ethics
Committee of Xuzhou Children's Hospital. Written informed consent
was obtained from the children's parents or guardians.
Method
The protein extraction kit used in the present study
was purchased from Shanghai Biological Engineering Co., Ltd.,
Shanghai, China; rabbit anti-rat primary antibody used in western
blotting was purchased from Abcam Co. (Cambridge, UK); goat
anti-rabbit HRP secondary antibody was purchased from Suzhou Keqing
(Suzhou, Jiangsu, China); and the RNA extraction kit was purchased
from Takara Co. (Dalian, China). Other relevant molecular reagents
were purchased from Axygen Biotechnology Co., Ltd. (Silicon Valley,
CA, USA).
Quantitative polymerase chain reaction
(qPCR)
RNA extraction
Frozen samples (0.2 g) were defrosted in the
prepared ice box. RNA Plus (0.5 ml) was added, samples were crushed
in a precooling mortar, and then immediately moved into a 1.5 ml
RNase-free EP tube (Thermo Fisher Scientific, Beijing, China). RNA
Plus (0.25 ml) was used to rewash mortar and the washing solution
was placed into a centrifugal tube. Then, 200 µl chloroform was
added to the centrifugal tube and agitated for 15 sec, prior to
placing the tube on the ice for 15 min. This was followed by
centrifugation at 10,000 × g, at 4°C for 15 min. The supernatant
was transferred into an RNase-free EP tube and an equal amount of
isopropanol was added. After quickly reversing and mixing, the tube
was placed on ice for at least 10 min prior to centrifugation at
10,500 × g for 10 min at 4°C. The supernatant was discarded, 750 µl
75% alcohol was added and gently mixed, and centrifuged at 10,000 ×
g for 10 min at 4°C. Again the supernatant was discarded and
residual alcohol cleared. RNase-free water was added and the
extracted RNA measured. The remaining RNA was used for reverse
transcription (9).
qPCR
Referring to the Takara fluorescence qPCR
specification for operation, with a little improvement, the qPCR
primer was synthesized by Shanghai Biological Engineering
Technology Co., Ltd. The sequences are shown in Table I.
 | Table I.Fluorescence quantitative PCR
primer. |
Table I.
Fluorescence quantitative PCR
primer.
| Primer name | Sequence |
|---|
| JNK1-F |
ATGAGCAGAAGCAAGCCGTGAC |
| JNK1-R |
CTGGGCTTTAAGTCCCGATG |
| GAPDH-F |
GTCGATGGCTAGTCGTAGCATCGAT |
| GAPDH-R |
TGCTAGCTGGCATGCCCGATCGATC |
Enzyme-linked immunosorbent assay (ELISA)
The levels of JNK1 protein expression in different
samples were detected following the ELISA kit instructions, with
slight modifications (10). In the
present study, the ELISA standard protein sample was diluted at
1:50 in the assay buffer, and the ELISA standard curve was prepared
according to the procedures in the manual. Samples tested were
diluted at 1:100 with the phosphate-buffered saline (PBS) (pH 7.2).
Then, 100 µl of dilution was added into each well, and 50 µl of
detection solution was subsequently added. After 2-h incubation at
room temperature, the TMB chromogenic substrate was added.
Absorbance was measured at 495 nm, and the D44V6 content and
concentration of each tested sample were calculated by
interpolation from the standard curve.
Western blotting
Extracted total protein (150 mg) was extracted from
experimental samples stored at −80°C (11) and ground with a mortar and pestle in
liquid nitrogen. Powdered tissue was collected in a 1.5 ml EP tube.
Protein extraction solution (300 µl) and 10 µl protease inhibitor
were added, incubated in ice water for 30 min and centrifuged at
10,000 × g for 15 min, and then supernatant was collected for
analysis. Subsequently, the protein expression levels were detected
using western blotting. Approximately 10 µl supernatant was
extracted and mixed with sample buffer, followed by SDS-PAGE
electrophoresis and routine transmembrane were performed. The
samples were sealed at room temperature for 1 h, and incubated with
rabbit polyclonal DDR-1 antibody (dilution, 1:250; cat. no.
ab135643) and then with secondary goat anti-rabbit (HRP) IgG
antibody (dilution, 1:2,000; cat. no. ab6721) (both from Abcam,
Cambridge, MA, USA), respectively. After washing the membrane three
times, color was developed using diaminobenzidine (DAB). Images
were obtained using the Fluorchem 9900 imaging system (Tokyo,
Japan). Integral optical density (IOD) values of each chromogenic
protein band were measured and the relative protein content of DDR1
was calculated (12).
Statistical analysis
SPSS 20.0 software (IBM Corp., Armonk, NY, USA) was
used for statistical analysis. The experimental data were presented
as means ± standard deviation. One-way analysis of variance (ANOVA)
was applied for intra-group comparison. P<0.05 was considered
statistically significant.
Results
mRNA expression level of JNK1 in the
control and observation groups
Blood samples were obtained from normal children and
children with bronchitis, and the mRNA expression level of JNK1 in
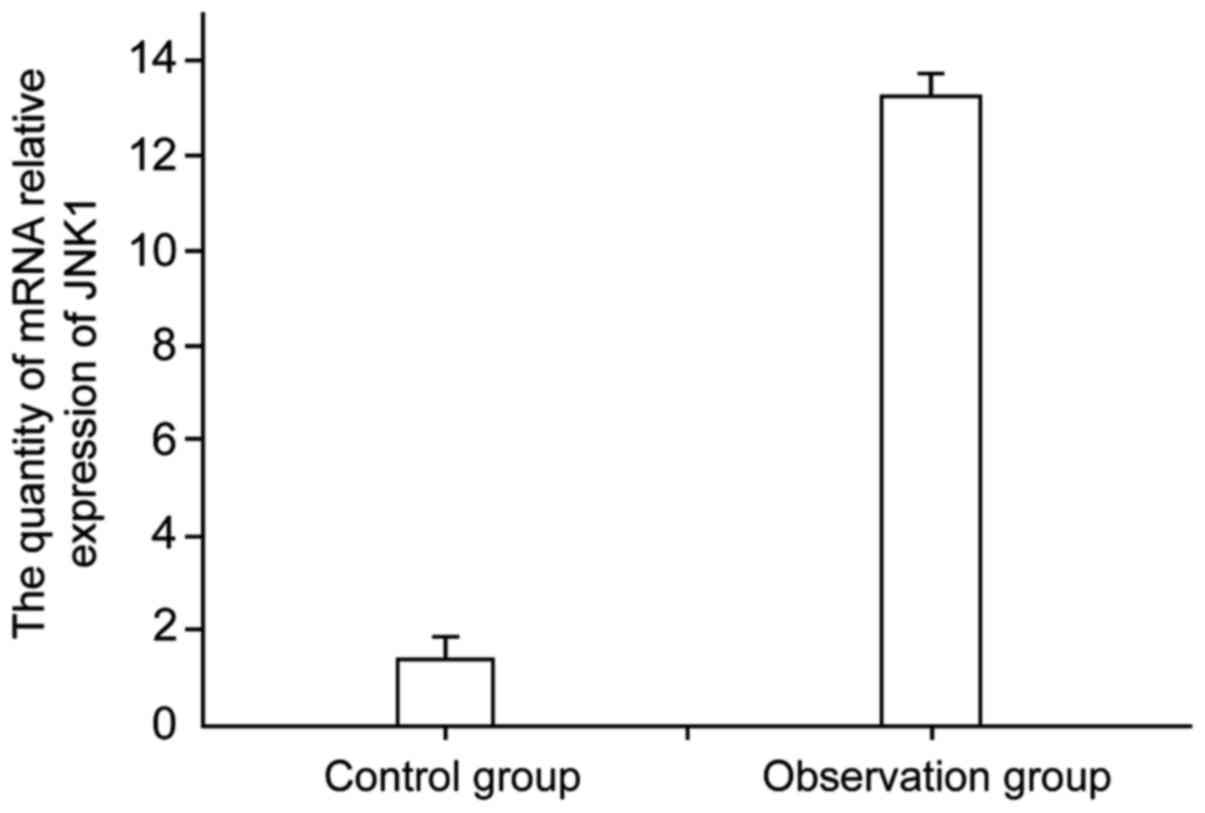
the different samples was detected by qPCR (Fig. 1). The results show that the JNK1
expression in children with bronchitis was higher than that of the
control group, with a significant difference between the two groups
(P<0.05). This indicated that there was a certain correlation
between the level of mRNA expression of JNK1 and pediatric
bronchitis attack, namely, that JNK1 expression levels increased in
patients with bronchitis.
Protein expression level of JNK1 in
the control and observation group was detected using ELISA
JNK1 protein in the blood of normal children and
children with bronchitis was detected using ELISA (Table II). From the results it can be
concluded that JNK1 protein expression in the blood of patients
with pediatric bronchitis was significantly increased (15.71±0.16
µg/l) compared to that of the normal children with (1.04±0.13
µg/l), showing a significant difference between the two groups,
which indicated that the content of JNK1 protein in the blood of
patients with pediatric bronchitis was higher than that of normal
children. As the result was consistent with qPCR, it showed a
certain positive correlation between content of JNK1 protein and
pediatric bronchitis.
 | Table II.Level of JNK1 protein expression in
the blood of normal children and children with bronchitis
(µg/l). |
Table II.
Level of JNK1 protein expression in
the blood of normal children and children with bronchitis
(µg/l).
| Groups | Content of JNK1
protein (mean ± SD) | t | P-value |
|---|
| Control |
1.04±0.13 | 0.739 | <0.01 |
| Observation | 15.71±0.16 |
|
|
Protein expression level of JNK1 in
the control and observation groups was detected by western
blotting
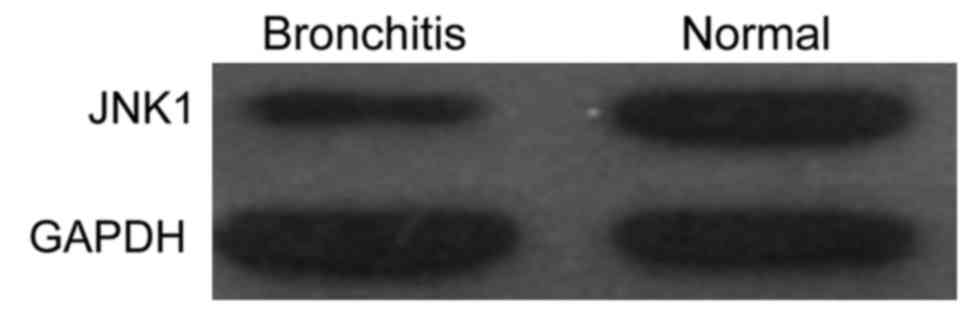
The level of JNK1 protein expression in the blood of
normal children and children with bronchitis was detected by
western blotting (Fig. 2). The
results showed that, following a comparison of the content of JNK1
protein in the control group, the content of JNK1 protein in the
blood of children with bronchitis was significantly higher than
that of normal children, which was consistent with the results of
qPCR and ELISA.
Level of JNK1 protein expression in
patients with different conditions of bronchitis
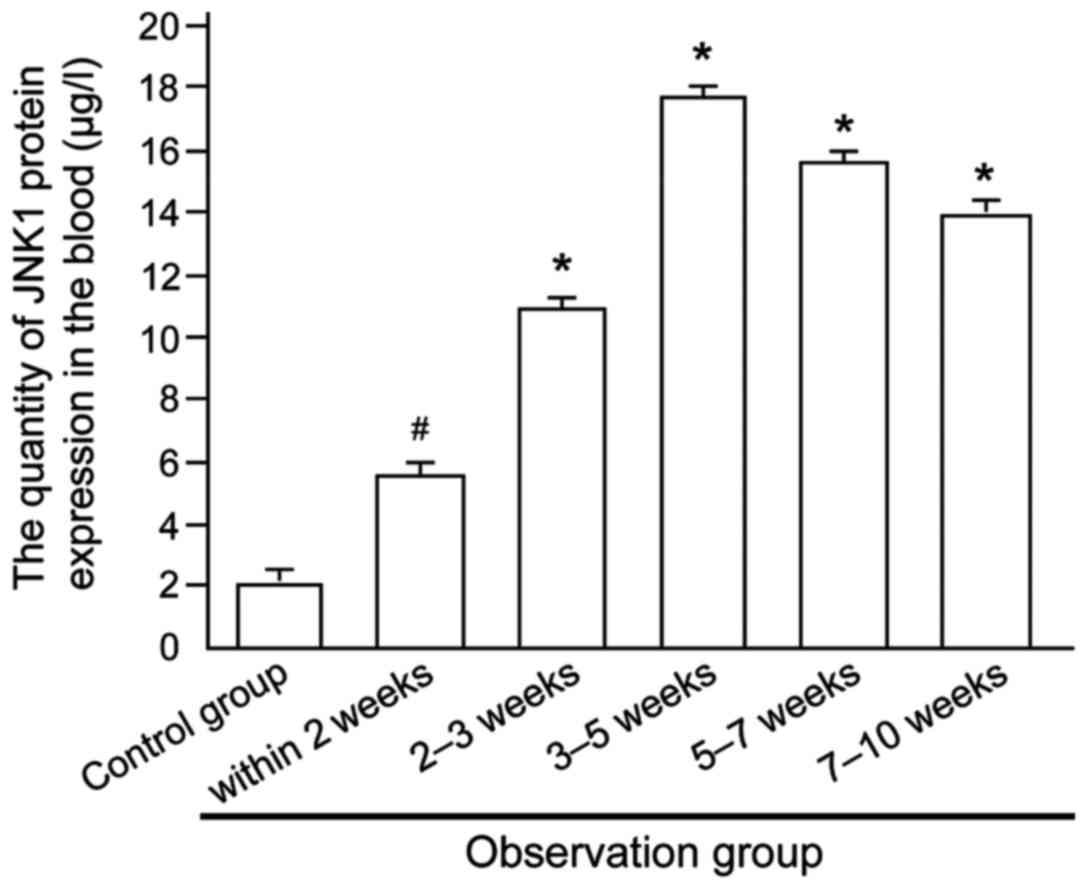
The level of JNK1 protein expression in patients
with different conditions of bronchitis in the observation group
was detected (Fig. 3), and the
result showed that JNK1 protein content in the blood of patients
increased first and then decreased with the prolonged course of
disease, indicating a positive correlation between JNK1 protein
content at certain levels and conditions of bronchitis in children,
but manifested as a negative correlation if beyond that certain
limit.
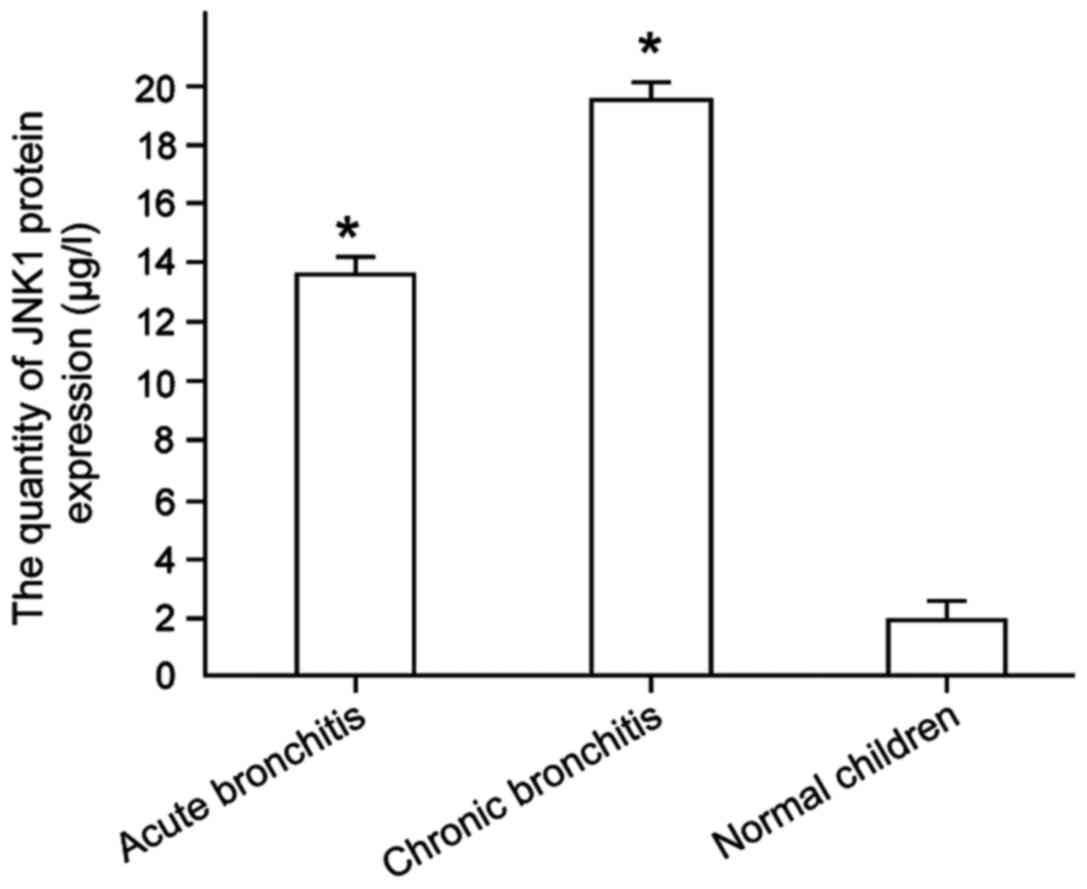
Level of JNK1 gene expression in
bronchitis patients with different condition
The level of JNK1 protein expression in the blood of
children with bronchitis (acute and chronic bronchitis) with
different conditions was detected (Fig.
4), and the result has shown the JNK1 protein content as
18.42±0.18 µg/l in the blood of patients with acute bronchitis was
lower than that of chronic bronchitis with 12.59±0.14 µg/l, showing
a significant difference between them (P<0.01).
Discussion
As one of multiple sicknesses in children,
respiratory system disease ordinarily is a common illness in
pediatric clinic, and the diagnosis and treatment for respiratory
diseases in children is extremely significant by virtue of its wide
distribution, high incidence, and triggering many acute diseases
(13,14). As a common illness in respiratory
system disease, the incidence of bronchitis in children accounts
for 32.6% of the total number of children with respiratory system
attack (15). Therefore, the study
on its pathogenesis has become a significant research direction in
the treatment of children with bronchitis.
Findings of a previous study indicated that as an
important cell signaling molecule in the human body, JNK1 can be
activated by three stages of phosphorylation, while the activated
JNK1 protein can involve the process of collective transcription
and regulation of many genes (16,17).
Through the detection for quantity of different genes in asthma, it
has been identified that human asthma and bronchial wall thickness
showed a positive correlation in comparison with levels of JNK1
gene expression of normal population (18–21). In
the present study, the JNK1 expression level in normal children and
children with bronchitis were detected, and the results showed
that, the JNK1 protein expression level in patients with pediatric
bronchitis significantly increased to 15.71±0.16 µg/l compared to
that of normal children at 1.04±0.13 µg/l. In addition, the
expression first increased and then decreased with the prolonged
course of disease, indicating a positive correlation between
conditions of patients and JNK1 expression at the initial period of
bronchitis attack in children, while other genes in the body may be
responsible for the decrease of JNK1 expression. The attack of
pediatric bronchitis, not only involves JNK1, but also occurs due
to the combined action of multiple genes, which provides certain
theoretical research direction for further study of children
bronchitis.
References
|
1
|
Shibata W, Maeda S, Hikiba Y, Yanai A,
Sakamoto K, Nakagawa H, Ogura K, Karin M and Omata M: c-Jun NH2-
terminal kinase 1 is a critical regulator for the development of
gastric cancer in mice. Cancer Res. 68:5031–5039. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fernandez-Valdivia R, Takeuchi H,
Samarghandi A, Lopez M, Leonardi J, Haltiwanger RS and Jafar-Nejad
H: Regulation of mammalian Notch signaling and embryonic
development by the protein O-glucosyltransferase Rumi. Development.
138:1925–1934. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu A, Wang L, Zhang X and Zhang M:
Combined treatment for child refractory Mycoplasma pneumoniae
pneumonia with ciprofloxacin and glucocorticoid. Pediatr Pulmonol.
46:1093–1097. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adékambi T and Drancourt M: Isolation of
Mycobacterium septicum from the sputum of a patient suffering from
hemoptoic pneumonia. Res Microbiol. 157:466–470. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kang CD, Ahn BK, Jeong CS, Kim KW, Lee HJ,
Yoo SD, Chung BS and Kim SH: Downregulation of JNK/SAPK activity is
associated with the cross-resistance to P-glycoprotein-unrelated
drugs in multidrug-resistant FM3A/M cells overexpressing
P-glycoprotein. Exp Cell Res. 256:300–307. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sabapathy K, Hochedlinger K, Nam SY, Bauer
A, Karin M and Wagner EF: Distinct roles for JNK1 and JNK2 in
regulating JNK activity and c-Jun-dependent cell proliferation. Mol
Cell. 15:713–725. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Graham BS: Biological challenges and
technological opportunities for respiratory syncytial virus vaccine
development. Immunol Rev. 239:149–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kan RX, Zhang CL, Zhen Q and Chen J:
Magnesium sulfate micro air pump suction for bronchiolitis
treatment in infants under two years old. Eur Rev Med Pharmacol
Sci. 20:1180–1184. 2016.PubMed/NCBI
|
|
9
|
Bressan S, Mion T, Andreola B, Bisogno G
and Da Dalt L: Severe Mycoplasma pneumoniae-associated mucositis
treated with immunoglobulins. Acta Paediatr. 100:e238–e240. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang YH, Wang SQ, Sun CR, Wang M, Wang B
and Tang JW: Inhibition of JNK1 expression decreases migration and
invasion of mouse hepatocellular carcinoma cell line in vitro. Med
Oncol. 28:966–972. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yalcin-Ozuysal O, Fiche M, Guitierrez M,
Wagner KU, Raffoul W and Brisken C: Antagonistic roles of Notch and
p63 in controlling mammary epithelial cell fates. Cell Death
Differ. 17:1600–1612. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lamb JA, Ventura JJ, Hess P, Flavell RA
and Davis RJ: JunD mediates survival signaling by the JNK signal
transduction pathway. Mol Cell. 11:1479–1489. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
But DY, Lai CL and Yuen MF: Natural
history of hepatitis-related hepatocellular carcinoma. World J
Gastroenterol. 14:1652–1656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang Z, Yan DP and Ge BX: JNK regulates
cell migration through promotion of tyrosine phosphorylation of
paxillin. Cell Signal. 20:2002–2012. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hui L, Zatloukal K, Scheuch H, Stepniak E
and Wagner EF: Proliferation of human HCC cells and chemically
induced mouse liver cancers requires JNK1-dependent p21
downregulation. J Clin Invest. 118:3943–3953. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seki E, Brenner DA and Karin M: A liver
full of JNK: signaling in regulation of cell function and disease
pathogenesis, and clinical approaches. Gastroenterology.
143:307–320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang SN, Lee KT, Tsai CJ, Chen YJ and Yeh
YT: Phosphorylated p38 and JNK MAPK proteins in hepatocellular
carcinoma. Eur J Clin Invest. 42:1295–1301. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu H, Zhu H, Xiao S, Sun H, Xie C and Ma
Y: Activation and crosstalk between the endoplasmic reticulum road
and JNK pathway in ischemia-reperfusion brain injury. Acta
Neurochir (Wien). 154:1197–1203. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mahfoudh-Boussaid A, Zaouali MA, Hadj-Ayed
K, Miled AH, Saidane-Mosbahi D, Rosello-Catafau J and Ben Abdennebi
H: Ischemic preconditioning reduces endoplasmic reticulum stress
and upregulates hypoxia inducible factor-1α in ischemic kidney: the
role of nitric oxide. J Biomed Sci. 19:72012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kunz M, Ibrahim S, Koczan D, Thiesen HJ,
Köhler HJ, Acker T, Plate KH, Ludwig S, Rapp UR, Bröcker EB, et al:
Activation of c-Jun NH2-terminal kinase/stress-activated protein
kinase (JNK/SAPK) is critical for hypoxia-induced apoptosis of
human malignant melanoma. Cell Growth Differ. 12:137–145.
2001.PubMed/NCBI
|
|
21
|
Libby P: Inflammation in atherosclerosis.
Nature. 420:868–874. 2002. View Article : Google Scholar : PubMed/NCBI
|