Introduction
Intervertebral disc degeneration (IDD) is a common
spinal disorder and is characterized by clinical signs ranging from
low back pain (LBP) to neurological deficits. In addition, LBP is
one of the most common musculoskeletal complaints in the world, and
it has become a severe socioeconomic and health issue influencing
of our society. Previous studies have exhibited that the mechanism
of IDD includes a complicated biochemical process. One of the most
important characteristics of IDD is the deficiency of proteoglycan
(PG) content in intervertebral discs (IVDs). This process is
closely related to the gene expression of ADAMTSs (activity of
disintegrins and metalloproteinases with thrombospondin motifs),
MMPs (matrix metalloproteinases) and TIMPs (tissue inhibitors of
metalloproteinases) (1–7). As a strong inflammatory stimulation
factor, previous researches have revealed that lipopolysaccharide
(LPS) can lead to gene upregulation and the secretion of diverse
proinflammatory cytokines and matrix-degrading enzymes, including
ADAMTSs and MMPs in NP cells, thereout causing a decrease in PG
volume and IDD (8,9). Moreover, proinflammatory cytokines such
as tissue necrosis factor-α (TNF-α) also play important parts in
IDD (10,11). Cell factors do not affect the IVD
like ADAMTSs or MMPs do directly; instead of that, they expedite
IDD by inducing the production of inflammatory factors by the
intervertebral disc cells. As a TLR ligand, LPS can initiate TLR
signaling in intervertebral disc cells, resulting in the
up-regulated expression of proinflammatory cytokines and MMPs.
Apart from MMPs and ADAMTSs, cytokines can also promote the
chemokine ligand (CCL) expression in intervertebral disc cells. In
terms of previous studies, CCLs can induce macrophage migration
into the IVD, deteriorating the inflammatory stage and causing pain
(12–17).
Traditional Chinese medicines (TCMs) are
experience-based remedies derived from hundreds or thousands of
years of clinical use in China. Most TCMs are extracted from one or
more medicinal herbs. The existence of multiple bioactive
ingredients makes many TCMs potential novel resources for the
discovery of new anti-inflammatory and anti-matrix degradation
drugs (18,19). Thus far, many naturally occurring
phytochemicals were reported to possess anti-inflammatory and
anti-matrix degradation effect and got considerable research
attention.
Carthamins yellow (CY), which is the flavonoid
compounds derived from safflower, has been extensively used as a
natural food color additive in China. Safflower is known as the
herb used in traditional medicines that promotes blood circulation
to remove blood stasis and alleviate pain (20,21).
Safflower injection has been used clinically for cerebrovascular
disease, coronary heart disease, and angiitis in China (22,23).
However, the effects and potential mechanisms of CY in inflammation
and matrix degradation in the intervertebral disc desease has not
yet been illuminated. Therefore, the purpose of our study was to
evaluate the anti-inflammatory and anti-matrix degradation effect
of CY in the IDD and investigate its potential mechanisms.
Materials and methods
Main reagents
CY and LPS was purchased from Sigma-Aldrich (St.
Louis, MO, USA). CY was dissolved in DMSO at concentrations of 1
mol/l. And it was stored at −20°C. The additional proportion of
DMSO in the culture medium was less than 0.05%. LPS was dissolved
in PBS at concentrations of 1 mg/ml and was stored at 4°C. The
P-ERK, ERK, P-JNK, JNK, P-P38, P38 and GAPDH antibody and secondary
antibody were purchased from Cell Signaling Technology, Inc.
(Beverly, MA, USA). Collagen II and aggrecan were purchased from
Abcam (Cambridge, UK). The IHC secondary antibody was MaxVision TM
HRP-Polymer kit from Maixin Bio (Fuzhou, China).
NP cell isolation and culture
The present study was conducted in strict accordance
with the recommendations in the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health. NP cells
were isolated from the lumbar spines of Sprague Dawley rats (6–8
weeks old, mixed male and female), using standard enzymatic
digestion and culture in complete media (high-glucose DMEM with 10%
FBS, 100 U/ml penicillin and 100 mg/ml streptomycin) up to passage
2–3 at 37°C in a humidifed atmosphere with 5% CO2.
Cell cytotoxicity assay
A cell counting kit-8 (CCK8) (Dojindo, Kumamoto,
Japan) was used to test the viability of NP cells after CY
treatment for 1 day according to previously reported methods
(24). Approximately
5×103 NP cells were seeded on each film and transferred
to 96-well plates. incubated with various concentrations of CY for
24 h. The conditioned culture medium was removed before CCK8
examination. Subsequently, 100 µl of DMEM and 10 µl of CCK8
solution were added to each well, followed by CCK8 incubation at
37°C for 2.5 h. The optical density (OD) at 450 nm was determined
using a microplate reader (BioTek, Winooski, VT, USA). Cell
viability was calculated as follows: Cell viability to control
(%)=(ODdrug-treated
group-ODblank)/(ODcontrol
group-ODblank).
Apoptosis assay
Cell apoptosis was measured by flow cytometry using
Annexin V/propidium iodide (PI) double-immunofluorescent staining
according to previously reported methods (25). NP cells were cultured with 200 µM CY
and/or 1 µg/ml LPS for 24 h. Then the cells were washed with cold
PBS and resuspended with 1X Annexin-binding buffer. After that, all
cells were stained with Annexin V-FITC and Propidium iodide
according to the manufacturer's protocols. The apoptosis rate was
measured by flow cytometry (FCM). Apoptotic events were indicated
as a combination of fluorescein isothiocyanate (FITC)+/PI- (early
apoptotic) and FITC+/PI+ (late apoptotic or dead) events. The final
results are expressed as the percentage of early, late and total
apoptotis.
Gene expression
NP cells were incubated with different
concentrations of CY and 1 µg/ml LPS for 24 h. LPS could induce
inflammation and matrix degradation in IVD. Total RNA was isolated
by the AxyPrep™ Multisource Total RNA Miniprep kit (Axygen
Biosciences, Union City, CA, USA). Then 1 µg RNA was converted into
complementary DNA (cDNA) with PrimeScript™ RT reagent kit (Takara
Bio, Inc., Otsu, Japan). Quantitative PCR was performed using an
ABI 7500 Sequencing Detection System and SYBR® Premix Ex Taq™
(Takara Bio, Inc.). Cycling conditions were as follows: 40 cycles
at 95°C for 5 sec and 60°C for 34 sec. The primers were used to
amplify target genes are listed in Table
I. The primers were designed and selected using blast in
pubmed, and GAPDH was used as the internal control.
 | Table I.Sequences of primers used in
quantitative PCR. |
Table I.
Sequences of primers used in
quantitative PCR.
| Gene | Primer sequences
(5′-3′) |
|---|
| TNF-α | Forward:
GGCTTTCGGAACTCACTGGA |
|
| Reverse:
GGGAACAGTCTGGGAAGCTC |
| Collagen II | Forward:
GGCCAGGATGCCCGAAAATTA |
|
| Reverse:
ACCCCTCTCTCCCTTGTCAC |
| Aggrecan | Forward:
CAGATGGCACCCTCCGATAC |
|
| Reverse:
GACACACCTCGGAAGCAGAA |
| ADAMT-4 | Reverse:
ACCGATTACCAGCCTTTGGG |
|
| Forward:
CCGACTCCGGATCTCCATTG |
| ADAMT-5 | Forward:
CCGAACGAGTTTACGGGGAT |
|
| Reverse:
TGTGCGTCGCCTAGAACTAC |
| MMP3 | Forward:
TTTGGCCGTCTCTTCCATCC |
|
| Reverse:
GCATCGATCTTCTGGACGGT |
| GAPDH | Forward:
TGCCACTCAGAAGACTGTGG |
|
| Reverse:
TTCAGCTCTGGGATGACCTT |
Western blotting
For signaling pathway protein assay, the cells were
treated with various concentrations of CY and/or 1 µg/ml LPS for 24
h. For aggrecan and collagen II protein assay, the cells were
treated with various concentrations of CY and/or 1 µg/ml LPS for 5
or 8 days. Cell total proteins were extracted using NE-PER® Nuclear
and Cytoplasmic Extraction Reagents according to the manufacturer's
instructions. 20 µg protein (each sample) was loaded into gel, and
separated by 7.5–12.5% SDS-PAGE, then transferred to 0.22-µm PVDF
membranes (Millipore Corp., Billerica, MA, USA). The membranes were
blocked with 5% fat-free milk at room temperature for 1 h and
subsequently incubated with primary antibodies overnight at 4°C
(1:1,000 dilution, Cell Signaling Technology). After three washes
in TBST, the membranes were probed with the corresponding secondary
antibody for 1 h at room temperature. The membranes were washed
again in TBST, and the protein bands were visualized using an
Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE,
USA). Positive immunoreactive bands were densitometrically
quantified and normalized to GAPDH.
Immunohistochemistry staining
2×104/ml cells were seeded in 24-well
plates, and these NP cells were cultured with diverse CY
concentrations and/or 1 µg/ml LPS for 5 or 8 days. Cells were fixed
with 4% paraformaldehyde before making cells slides. After
fixation, NP cells were treated with 0.1% Triton X-100 for 15 min.
Then the cells were blocked with 2% bovine serum albumin
(Sigma-Aldrich) for 1 h. Then, cells slides were incubated with the
corresponding antibody, including anti-collagen II and
anti-aggrecan antibody (1:200 dilution; Abcam) overnight at 4°C.
For immunohistochemistry, the secondary antibody was used for 15
min at room temprature. The DAB (Maixin Bio) solution was used as
the chromogen. We used an inverted microscope microscopy (Olympus,
Tokyo, Japan) to acquire the images. The integral optical density
(IOD) of every photo was measured using the Image-Pro Plus 6.0
software (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
The statistical package for the social sciences
(SPSS) version 19.0 was used to analyze the data. The significance
of differences between experimental groups and controls were
assessed using the Student's t-test or one-way analysis of variance
(ANOVA) as appropriate. The data are expressed as the mean ± SD.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cell viability of NP cells after CY
treatment
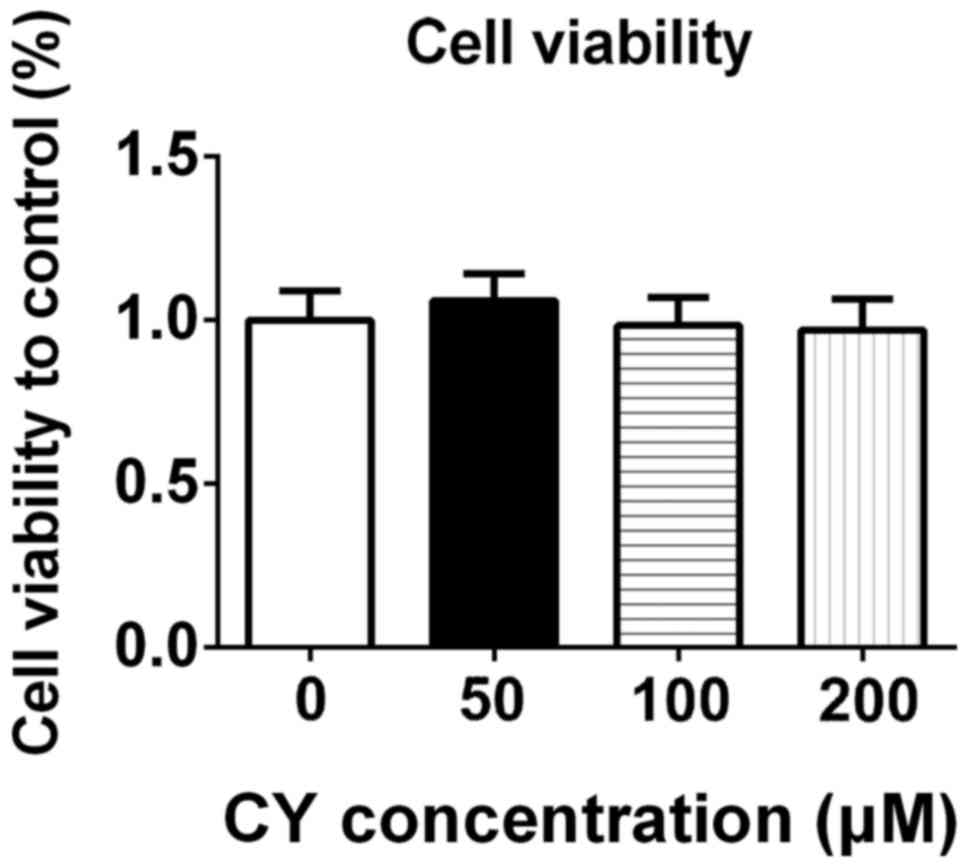
To study the potential cytotoxicity of CY, we
measured the cell viability of NP cells. After incubating with
different concentrations of CY for 24 h, we used CCK-8 to assay the
cell viability of NP cells. As shown in Fig. 1, CY did not inhibit proliferation of
NP cells at concentration of 50, 100 and 200 µM.
Apoptosis analysis of NP cells
following CY treatment
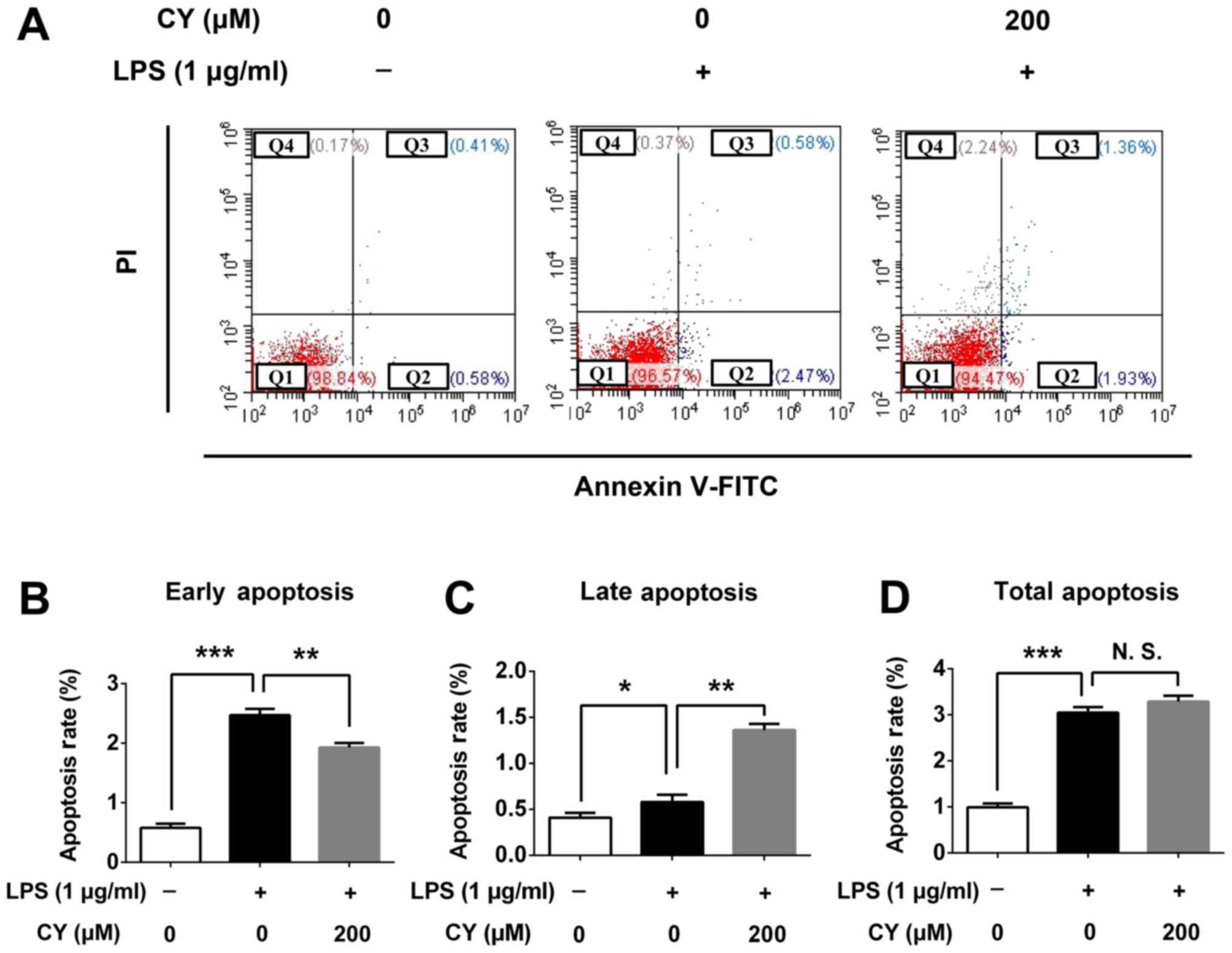
To verify the cytotoxicity of CY, we examined the
apoptosis rate of NP cells after treating with CY for 24 h. CY
could decrease the early apoptosis rate at 200 µM with 1 µg/ml LPS
(2.47% in 1 µg/ml LPS vs. 1.93% which were treated with CY of 200
µM and 1 µg/ml LPS, P<0.05), and CY could increase the late
apoptosis rate at 200 µM with 1 µg/ml LPS (0.58% in 1 µg/ml LPS vs.
1.36% which were treated with CY of 200 µM and 1 µg/ml LPS,
P<0.05), but the total apoptosis has no difference between two
groups (Fig. 2A). And quantitative
data of early, late and total apoptosis rate was consistant with
this phenomenon (Fig. 2B-D).
CY inhibited LPS induced
matrix-degradation in NP cells
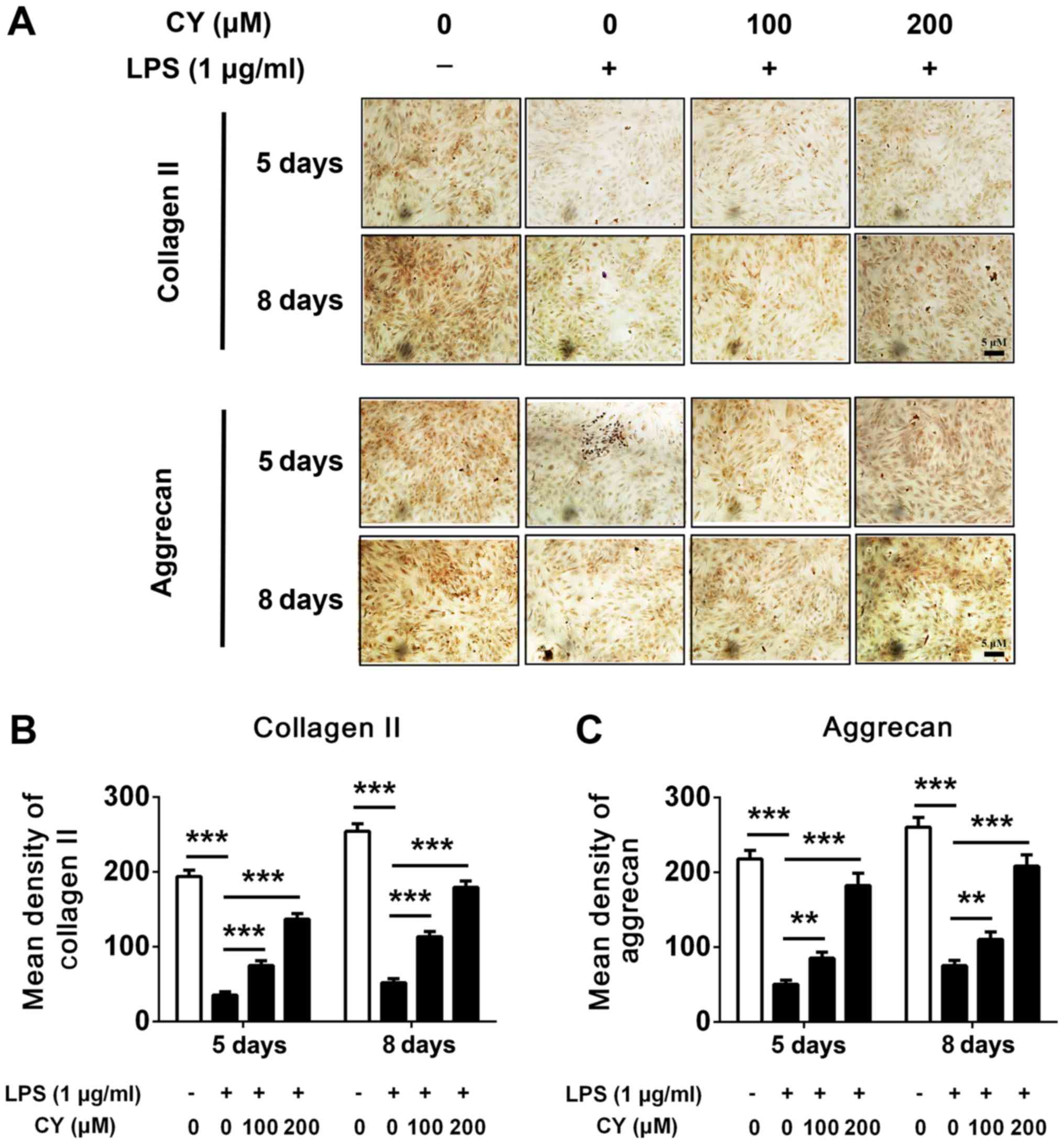
NP cells were incubated with 1 µg/ml LPS in the
various concentration of CY (100 or 200 µM) for 5 or 8 days. After
that, we detected extracellular matrix content by cell
immunohistochemistry. Our results indicated that the expression of
collagen II and aggrecan increased over time in NP cells, while LPS
strikingly decreased the aggrecan and collagen II amount (Fig. 3A). Immunohistochemistry staining
showed CY significantly increased the content of aggrecan and
collagen II compared to the LPS groups (Fig. 3A). The quantification of IOD also
indicated that the CY group gained more aggrecan and collagen II
staining (Fig. 3B and C).
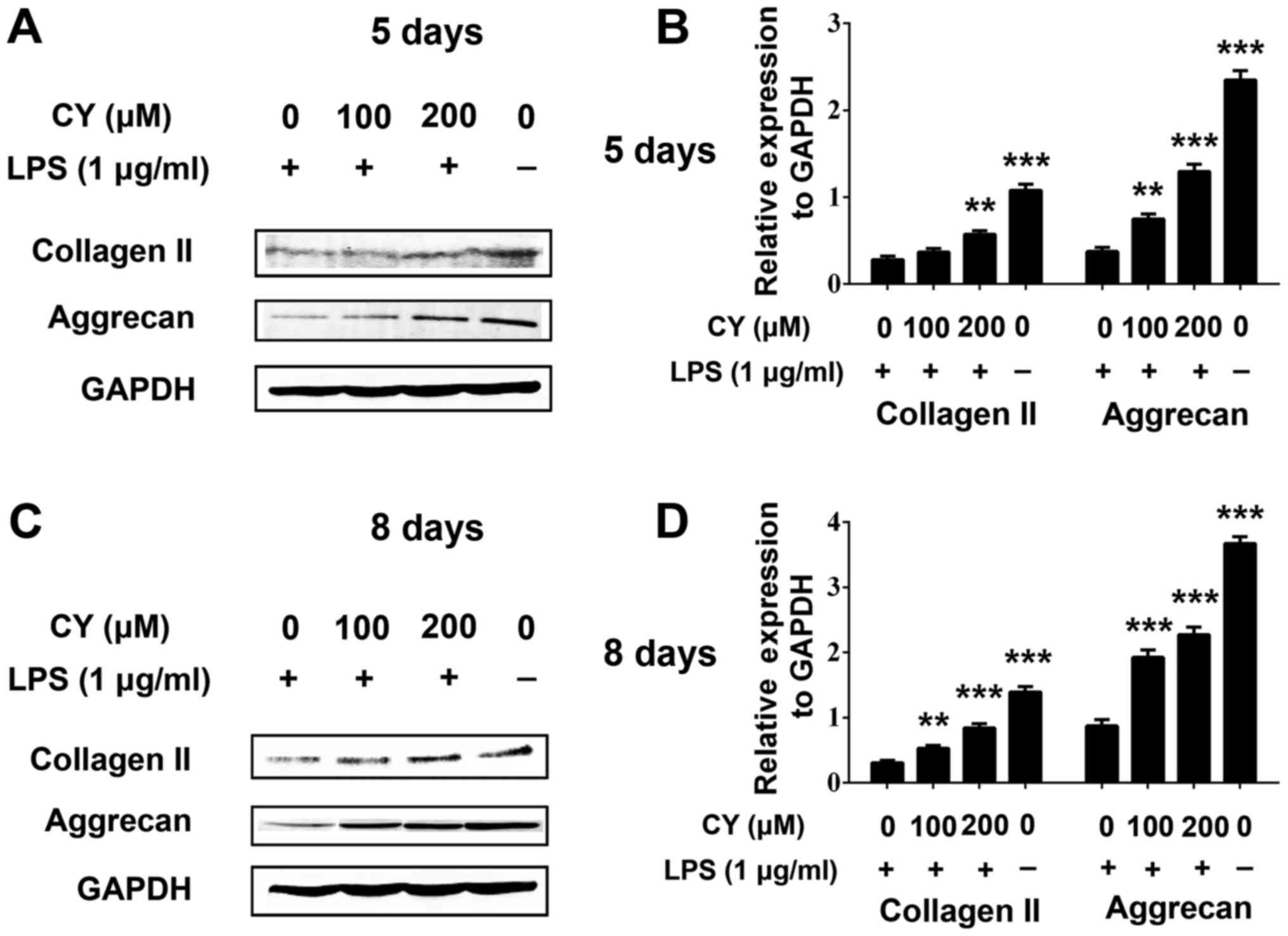
After incubated with LPS and various concentration
of CY for 5 or 8 days, we also used western blotting to measure the
collagen II and aggrecan protein content. The result was similar
with immunohistochemistry staining. As shown in Fig. 4, LPS induced collagen II and aggrecan
protein down-expression, and CY significantly attenuated the
collagen II and aggrecan loss at day 5 and 8. This effect was more
obvious at day 8 (Fig. 4).
CY reversed LPS induced gene
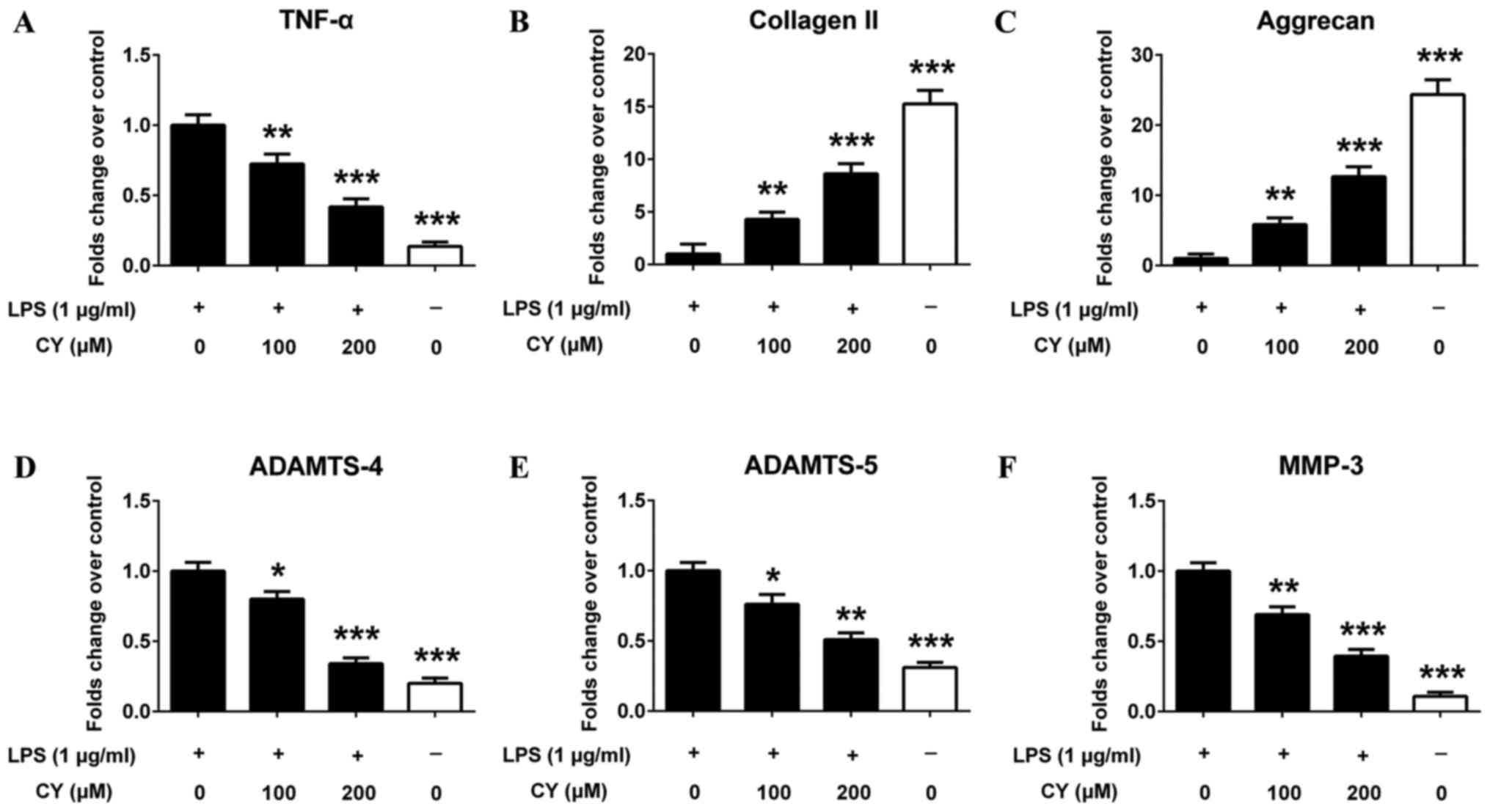
expression changes in NP cells
NP cells were stimulated with 1 µg/ml LPS and 0, 100
or 200 µM CY, followed by quantitative PCR assay. PCR results
showed that LPS significantly up-regulated the gene expression of
proinflammatory cytokines TNF-α (Fig.
5A). LPS also down-regulated the gene expression of collagen II
and aggrecan in NP cells, which was reversed by CY (Fig. 5B and C). And CY also inhibited
multiple matrix-degrading enzymes (MMP-3, ADAMTS-4 and ADAMTS-5)
gene overexpression induced by LPS in NP cells (Fig. 5D-F).
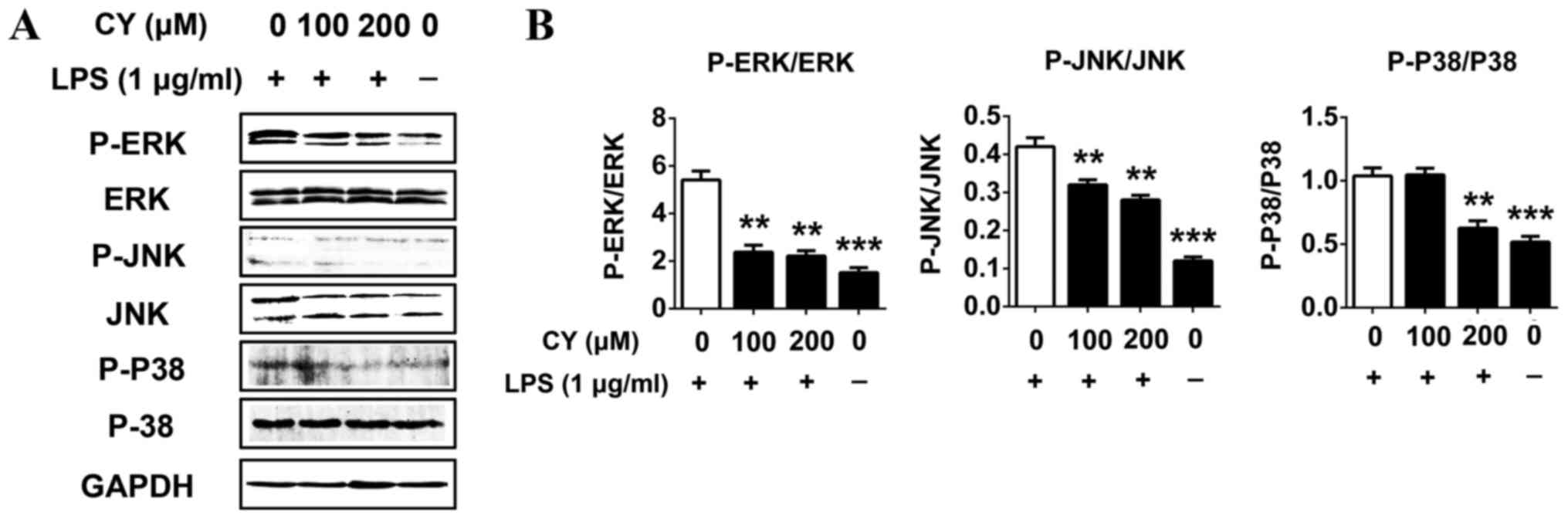
CY inhibited the LPS-induced
activation of the MAPK pathway in NP cells
We measured the activation of the MAPKs pathway
related proteins by western blotting to evaluate the potential
mechanisms of effects of CY on LPS induced NP cells. Cells were
pretreated with various concentrations of CY for 2 h, then treated
with or without 1 µg/ml LPS for 30 min. The results showed that 1
µg/ml LPS significantly activated the MAPKs pathways by promoting
JNK, ERK and P38 protein phosphorylation (Fig. 6). And CY could inhibit the
phosphorylation of JNK, ERK and P38 in a dose dependent manner
(Fig. 6). This result indicated the
MAPKs pathway was involved while CY exerted anti-inflammatory
effect in NP cells.
Discussion
At present, CY has already been widely used as a
bioactive natural products and food additive in China (26). However, there is no study focus on
the potential therapeutic effect of CY in intervertebral disc
degeneration. In our study, we tested the cytotoxicity of CY to NP
cells by CCK8 and apoptosis assay firstly. The results showed that
CY did not inhibit proliferation of NP cells at different
concentration, and there is a statistically significant difference
between different treatment in early and late apoptosis, but the
apoptosis rate could be ignored as the apoptosis rate is too little
in different treatment. As the total apoptosis rate has no
difference between LPS and LPS with CY group, there is no
cytotoxicity of CY to NP cells. Furthermore, our research showed
that CY produced pharmacological anti-inflammatory reaction and
anti-degeneration results in LPS-induced NP cells for the first
time. And our study also evidenced that CY could block the
LPS-induced activation of the MAPK pathway in NP cells.
The gene expression of ADAMTSs and MMPs has been a
wide range of researches in the stage of IVD degeneration (27). According to many researches, MMP-3
are not expressed in normal human intervertebral discs but have
up-regulated expression in degenerated human intervertebral discs.
Besides, the protein expression of MMP-3 has a positive correlation
with IVD histomorphological degenerative studies (28). The upregulated expression of ADAMTSs
has also been detected in degenerative intervertebral discs.
ADAMTS-4 and ADAMTS-5 have been revealed as the most important
aggrecanases because of their strong abilties in cleaving aggrecan
among the 20 various ADAMTSs (29).
Inhibitors of ADAMTSs and MMPs have a therapeutic action on IDD
in vitro and in vivo studies (30). Aggrecan and collagen II are the main
elements of nucleus pulposus, and the decrease of collagen II
content is highly correlated with IVD degeneration (31). Our research shows that CY can both
prevent LPS-induced collagen II and aggrecan loss and induce their
synthesis.
We also demonstrated that CY could inhibit
LPS-induced inflammation of NP cells. Previous studies suggested
that LPS markedly induced gene upregulation and the production of
various proinflammatory cytokines in NP cells (32). TNF-α secreted from inflammatory cells
and IVD cells triggers MMPs expression which ends up in vicious
cycle of cell apoptosis and matrix degradation (33). The result of the current study
demonstrated CY can down-regulate TNF-α expression. TNF-α blocker
is now widely used in the treatment of inflammatory arthritis and
in certain cases of IVD herniation (34). The MAPK pathways play important roles
in the regulation of the inflammatory response (35). The effect of inhibiting p38
expression in NP cells was tested in previous study, the result
indicated that inflammatory cytokines induced caveolin-1/β-catenin
signalling in rat nucleus pulposus cell apoptosis through the p38
MAPK pathway (36). The role of the
ERK pathway had also been investigated in human intervertebral
disc, and exogenous and autocrine growth factors could stimulate
human intervertebral disc cell proliferation via the ERK and Akt
pathways (37). And another research
showed that crocin exerts anti-inflammatory and anti-catabolic
effects on rat intervertebral discs by suppressing the activation
of JNK. And JNK is a critical MAPK pathway for intervertebral disc
degeneration (15). Though MAPK
pathway is related to NP cell death, CCK8 and apoptosis assay
showed that there was no impact on NP cell proliferation and total
apoptosis following CY treatment. That revealed that there may be
other signaling influencing NP cell death. And in our study, MAPK
pathway was activated after inflammatory stimulation, so MAPK
pathway is more related to inflammatory. Our western blotting
results showed that CY inhibited the activation of MAPK pathway in
NP cells, thus exerting anti-inflammation effect. So the inhibitory
effect of CY on LPS induced inflammation of NP cells was through
suppressing MAPK pathway.
As the above, we are honored to report the role of
CY in LPS-induced inflammatory and matrix degradation in the
intervertebral disc. We found that CY could indirectly or directly
affect the vitality of MAPK signaling and PG content of
intervertebral discs. However, more investigations should conducted
following this basal research. Firstly, the in-depth molecular
mechanisms controlling the CY-mediated transformations to
inflammatory- and PG-related signaling pathways should be better
stated. Secondly, animal experients should be constructed to verify
the therapeutic effect of CY in vivo. Finally, our results
should be verified by patient treatment.
Taken together, our study reveals that CY could
exhibit a strong anti-inflammatory and anti-degeneration effect by
suppressing LPS-induced MAPK activation in NP cells. CY may be a
potential new traditional Chinese medicine for curing IDD in the
future. However, more and further studies are required to confirm
this.
Acknowledgements
This study was supported by grants from Research
project of Shanghai municipal health and Family Planning Commission
(grant no. 201640304).
References
|
1
|
Li Y, Li K, Mao L, Han X, Zhang K, Zhao C
and Zhao J: Cordycepin inhibits LPS-induced inflammatory and matrix
degradation in the intervertebral disc. PeerJ. 4:e19922016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Y, Li K, Han X, Mao C, Zhang K, Zhao T
and Zhao J: The imbalance between TIMP3 and matrix-degrading
enzymes plays an important role in intervertebral disc
degeneration. Biochem Biophys Res Commun. 469:507–514. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Campbell RJ, Mobbs RJ and Phan K: Evidence
update-association between CILP and degeneration of the
intervertebral disc: A meta-analysis. J Spine Surg. 2:242–243.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ye S, Ju B, Wang H and Lee KB: Bone
morphogenetic protein-2 provokes interleukin-18-induced human
intervertebral disc degeneration. Bone Joint Res. 5:412–418. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feng Y, Egan B and Wang J: Genetic factors
in intervertebral disc degeneration. Genes Dis. 3:178–185. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Teichtahl AJ, Urquhart DM, Wang Y, Wluka
AE, O'Sullivan R, Jones G and Cicuttini FM: Lumbar disc
degeneration is associated with modic change and high paraspinal
fat content-a 3.0T magnetic resonance imaging study. BMC
Musculoskelet Disord. 17:4392016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hart LG, Deyo RA and Cherkin DC: Physician
office visits for low back pain. Frequency, clinical evaluation,
and treatment patterns from a U.S. national survey. Spine (Phila Pa
1976). 20:11–19. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu MC, Chen WH, Wu LC, Hsu WC, Lo WC, Yeh
SD, Wang MF, Zeng R and Deng WP: Establishment of a promising human
nucleus pulposus cell line for intervertebral disc tissue
engineering. Tissue Eng Part C Methods. 20:1–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim JS, Ellman MB, Yan D, An HS, Kc R, Li
X, Chen D, Xiao G, Cs-Szabo G, Hoskin DW, et al: Lactoferricin
mediates anti-inflammatory and anti-catabolic effects via
inhibition of IL-1 and LPS activity in the intervertebral disc. J
Cell Physiol. 228:1884–1896. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang B, Wang D, Yan T and Yuan H:
MiR-138-5p promotes TNF-α-induced apoptosis in human intervertebral
disc degeneration by targeting SIRT1 through PTEN/PI3K/Akt
signaling. Exp Cell Res. 345:199–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu XG, Hou HW and Liu YL: Expression
levels of IL-17 and TNF-α in degenerated lumbar intervertebral
discs and their correlation. Exp Ther Med. 11:2333–2340.
2016.PubMed/NCBI
|
|
12
|
Yang W, Yu XH, Wang C, He WS, Zhang SJ,
Yan YG, Zhang J, Xiang YX and Wang WJ: Interleukin-1β in
intervertebral disk degeneration. Clin Chim Acta. 450:262–272.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu B, Shi C, Xu C, Cao P, Tian Y, Zhang Y,
Deng L, Chen H and Yuan W: Heme oxygenase-1 attenuates IL-1β
induced alteration of anabolic and catabolic activities in
intervertebral disc degeneration. Sci Rep. 6:211902016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fang F and Jiang D: IL-1β/HMGB1 signalling
promotes the inflammatory cytokines release via TLR signalling in
human intervertebral disc cells. Biosci Rep. 36(pii): e003792016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li K, Li Y, Ma Z and Zhao J: Crocin exerts
anti-inflammatory and anti-catabolic effects on rat intervertebral
discs by suppressing the activation of JNK. Int J Mol Med.
36:1291–1299. 2015.PubMed/NCBI
|
|
16
|
Zhang Y, Liu L, Wang S, Zhao Y, Liu Y, Li
J, Nie L and Cheng L: Production of CCL20 on nucleus pulposus cells
recruits IL-17-producing cells to degenerated IVD tissues in rat
models. J Mol Histol. 47:81–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karli P, Martlé V, Bossens K, Summerfield
A, Doherr MG, Turner P, Vandevelde M, Forterre F and Henke D:
Dominance of chemokine ligand 2 and matrix metalloproteinase-2 and
−9 and suppression of pro-inflammatory cytokines in the epidural
compartment after intervertebral disc extrusion in a canine model.
Spine J. 14:2976–2984. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng CY and Lee YC: Anti-inflammatory
effects of traditional Chinese medicines against ischemic injury in
in vivo models of cerebral ischemia. Evid Based Complement Alternat
Med. 2016:57394342016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu F, Yin L, Ji L, Yang F, Zhang G, Shi L
and Xu L: Suppressive effect of Sanmiao formula on experimental
gouty arthritis by inhibiting cartilage matrix degradation: An in
vivo and in vitro study. Int Immunopharmacol. 30:36–42. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang CC, Choy CS, Liu YH, Cheah KP, Li JS,
Wang JT, Yu WY, Lin CW, Cheng HW and Hu CM: Protective effect of
dried safflower petal aqueous extract and its main constituent,
carthamus yellow, against lipopolysaccharide-induced inflammation
in RAW264.7 macrophages. J Sci Food Agric. 91:218–225. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kohno Y, Totsuka K, Ikoma S, Yoda K,
Shibata M, Matsushima R, Tomita Y, Maeda Y and Kobayashi K:
Photostability enhancement of anionic natural dye by intercalation
into hydrotalcite. J Colloid Interface Sci. 337:117–121. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan S, Lin N, Shan G, Zuo P and Cui L:
Safflower yellow for acute ischemic stroke: A systematic review of
randomized controlled trials. Complement Ther Med. 22:354–361.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou MX, Fu JH, Zhang Q and Wang JQ:
Effect of hydroxy safflower yellow A on myocardial apoptosis after
acute myocardial infarction in rats. Genet Mol Res. 14:3133–3141.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han XG, Li Y, Mo HM, Li K, Lin D, Zhao CQ,
Zhao J and Tang TT: TIMP3 regulates osteosarcoma cell migration,
invasion, and chemotherapeutic resistances. Tumour Biol.
37:8857–8867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han XG, Du L, Qiao H, Tu B, Wang YG, Qin
A, Dai KR, Fan QM and Tang TT: CXCR1 knockdown improves the
sensitivity of osteosarcoma to cisplatin. Cancer Lett. 369:405–415.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li HX, Han SY, Wang XW, Ma X, Zhang K,
Wang L, Ma ZZ and Tu PF: Effect of the carthamins yellow from
Carthamus tinctorius L. on hemorheological disorders of blood
stasis in rats. Food Chem Toxicol. 47:1797–1802. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vo NV, Hartman RA, Yurube T, Jacobs LJ,
Sowa GA and Kang JD: Expression and regulation of
metalloproteinases and their inhibitors in intervertebral disc
aging and degeneration. Spine J. 13:331–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weiler C, Nerlich AG, Zipperer J,
Bachmeier BE and Boos N: 2002 SSE award competition in basic
science: Expression of major matrix metalloproteinases is
associated with intervertebral disc degradation and resorption. Eur
Spine J. 11:308–320. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gendron C, Kashiwagi M, Lim NH, Enghild
JJ, Thøgersen IB, Hughes C, Caterson B and Nagase H: Proteolytic
activities of human ADAMTS-5: Comparative studies with ADAMTS-4. J
Biol Chem. 282:18294–18306. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leckie SK, Bechara BP, Hartman RA, Sowa
GA, Woods BI, Coelho JP, Witt WT, Dong QD, Bowman BW, Bell KM, et
al: Injection of AAV2-BMP2 and AAV2-TIMP1 into the nucleus pulposus
slows the course of intervertebral disc degeneration in an in vivo
rabbit model. Spine J. 12:7–20. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Y, Li K, Hu Y, Xu B and Zhao J:
Piperine mediates LPS induced inflammatory and catabolic effects in
rat intervertebral disc. Int J Clin Exp Pathol. 8:6203–6213.
2015.PubMed/NCBI
|
|
32
|
Iwata M, Ochi H, Asou Y, Haro H, Aikawa T,
Harada Y, Nezu Y, Yogo T, Tagawa M and Hara Y: Variations in gene
and protein expression in canine chondrodystrophic nucleus pulposus
cells following long-term three-dimensional culture. PloS One.
8:e631202013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kang YM, Hong SH, Yang JH, Oh JC, Park JO,
Lee BH, Lee SY, Kim HS, Lee HM and Moon SH: Pamidronate
down-regulates tumor necrosis factor-alpha induced matrix
metalloproteinases expression in human intervertebral disc cells. J
Bone Metab. 23:165–173. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Williams LM, Lali F, Willetts K, Balague
C, Godessart N, Brennan F, Feldmann M and Foxwell BM: Rac mediates
TNF-induced cytokine production via modulation of NF-kappaB. Mol
Immunol. 45:2446–2454. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Goldring MB, Otero M, Plumb DA, Dragomir
C, Favero M, El Hachem K, Hashimoto K, Roach HI, Olivotto E, Borzì
RM and Marcu KB: Roles of inflammatory and anabolic cytokines in
cartilage metabolism: Signals and multiple effectors converge upon
MMP-13 regulation in osteoarthritis. Eur Cell Mater. 21:202–220.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang J, Chen H, Cao P, Wu X, Zang F, Shi
L, Liang L and Yuan W: Inflammatory cytokines induce
caveolin-1/β-catenin signalling in rat nucleus pulposus cell
apoptosis through the p38 MAPK pathway. Cell Prolif. 49:362–372.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pratsinis H, Constantinou V, Pavlakis K,
Sapkas G and Kletsas D: Exogenous and autocrine growth factors
stimulate human intervertebral disc cell proliferation via the ERK
and Akt pathways. J Orthop Res. 30:958–964. 2012. View Article : Google Scholar : PubMed/NCBI
|