Introduction
Gastric cancer is one of the most common malignant
carcinoma in the world. Currently, the approaches for gastric
cancer therapy mainly included surgical therapies, radiotherapies
and chemotherapies. However, the development of multidrug
resistance (MDR) greatly decreased the efficacy of chemotherapies.
Several studies suggested that attenuating the development of MDR
could restore the sensibility of cancer cells to chemotherapies
(1,2). Specially, the expression of MDR-related
proteins (MDR1, MRP1) played crucial roles in the development of
MDR (3). MDR1 and MRP1 belong to the
ATP-binding cassette (ABC) superfamily, which mediated the efflux
of drugs out of cells, reducing drug efficacy.
Kanglaite (KLT) is one of Chinese herb injection
reported to show antitumor activity (4). KLT is a coix seed oily substance
extracted from Coix lacryma-jobi (family Cramineae). The main
active ingredient of coix seed is a compound of triglycerides
containing four types of fatty acid (5). The coix seed oil is well known for its
antitumor and immunomodulatory effects among traditional Chinese
medicine (6). In 1997, KLT injection
was officially approved by the Ministry of Public Health in China
for the treatment of liver cancer, lung cancer and gastric cancer
(6,7). Moreover, KLT has showed good efficacy
in the USA and its clinical trial smoothly completed in Russia,
where it has been registered as a new drug and can be marketed
legally (8). In later research, KLT
was found to ameliorate MDR of cancers when combined with
radiotherapy and chemotherapy in clinical use (9). For example, Zhan et al reported
that patients with gastric cancer treated with KLT injection
combined with chemotherapy showed lower gastrointestinal reactions
and bone marrow suppression than that in the patients with
chemotherapy alone, which indicated that KLT injection enhanced
efficacy and reduced the side effects of chemotherapy (10). However, the mechanism of KLT working
against chemotherapy resistance in gastric cancer cells involving
the regulation of MDR-related proteins was poorly understood.
PVT1 is a long non-coding RNA located in human
chromosome 8q24 (11). To date,
overexpression of PVT1 has been frequently observed in several
malignant diseases, such as breast cancer, colorectal cancer,
ovarian cancer and gastric cancer, and is associated with
increasing cell proliferation and inhibiting cell apoptosis
(12–14). Recently, mounting evidence indicated
that PVT1 participated in the drug resistance of cancer cells by
regulating different pathways (15,16). In
our previous study, we also found that overexpression of PVT1
promoted the development of MDR in gastric cancer cells (16). Based on these data, in the present
study, we further investigated the role of PVT1 in the effect of
KLT on drug resistance in gastric cancer cells, which might shed
light on elucidating the potential mechanism of KLT in ameliorating
MDR of cancer cells.
Materials and methods
Cell lines and culture
The cisplatin-resistant BGC823/DDP cells and
SGC7901/DDP cells were obtained as the previous study (16). Briefly, human gastric cancer cell
lines BGC823 and SGC7901 obtaining from the Type Culture Collection
of the Chinese Academy of Sciences (Shanghai, China) were exposed
to cisplatin with gradually increasing concentration for about 12
months. The cisplatin concentration was from 0.05 mg/ml until the
cells acquired resistance to 1 mg/ml. Finally, the
cisplatin-resistant BGC823/DDP cells and SGC7901/DDP cells were
developed. Cells were cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% of
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100
U/ml of penicillin and 100 mg/ml of streptomycin (Gibco; Thermo
Fisher Scientific, Inc.) at 37°C in a humidified incubator with 5%
CO2.
Plasmids and cell transfection
PVT1-overexpression vector (Ad-PVT1) was constructed
and synthetized by Ribobio Co., Ltd. (Guangzhou, China). BGC823/DDP
and SGC7901/DDP cells were transfected Ad-PVT1 by using
Lipofectamine 2000 transfection reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer's
instructions.
Cell Counting Kit-8 (CCK-8) assay
CCK-8 assay was performed to detect the effect of
KLT on the cell viability of BGC823/DDP cells and SGC7901/DDP
cells. KLT® (Coix Seed Oil) injection (10 g/100 ml) and blank
emulsion (as vehicle) were obtained from the Zhejiang Kanglaite
Pharmaceutical Co., Ltd. (Hangzhou, China). The cells
(5×103 cells/ml) were cultured on a 96-well plate in a
RPMI-1640 medium supplemented with different concentrations of KLT
(1, 2.5 and 5 µl/ml) for 24, 36 and 48 h. After the incubation,
CCK-8 was added into each well, followed by incubation for 1 h in
humid atmosphere containing 5% CO2. Absorbance was
determined at 450 nm by microplate reader.
Apoptosis analysis by flow
cytometry
An Annexin V-FITC Apoptosis Detection kit I (BD
Pharmingen, San Diego, CA, USA) was used to examine the cell
apoptosis according to the manufacturer's instructions. Cells were
cultured on a 96-well plate in a RPMI-1640 medium supplemented with
different concentrations of KLT (1, 2.5, 5 µl/ml) for 48 h. Then
cells were washed with PBS and resuspended in 1X binding buffer at
a concentration of 1×106 cells/ml. Cell suspension (100
µl) was incubated with 5 ml of FITC Annexin-V and 5 µl of propidium
iodide (PI) for 15 min in the dark. Following the incubation, 400
µl 1X binding buffer was added. Flow cytometry was used to analyze
the cell apoptosis with the EPICS XL-MCL FACScan (BD Biosciences,
Mountain View, CA, USA). Annexin V-FITC-positive and PI-negative
cells indicated the cells undergoing apoptosis.
Western blot analysis
RIPA buffer was used to extract the total protein
from cell lysates and the protein concentration was measured by BCA
Protein Assay Kit (Pierce Chemical Co., Ltd., Rockford, IL, USA).
Then proteins (20 µg) were separated by 12% SDS-polyacrylamide gel
electrophoresis and transferred onto PVDF membranes (Millipore
Corp., Bedford, MA, USA). After blocking with 10% dry milk in TBST
for 1 h at room temperature, membranes were incubated with
antibodies against MDR1 (1:3,000) and MRP1 (1:1,500), or anti-GAPDH
(1:5,000) as a control overnight at 4°C. Blots were incubated with
a peroxidase-linked secondary antibody (1:1,000) for 1 h. The
enhanced chemiluminescence (ECL) detection system (Amersham
Biosciences, Uppsala, Sweden) was used to detect the antibody
binding.
Quantitative PCR
Quantitative PCR was performed to detect the level
of PVT1 and the mRNA level of MDR1 and MRP1. Briefly, total RNA
from cultured cells was extracted by using TRIzol reagent
(Invitrogen Life Technologies) according to the manufacturer's
instructions. Then the total RNA was used as the templet to convert
into cDNA using the RevertAidHMinus First Strand cDNA synthesis kit
(Fermentas; Thermo Fisher Scientific, Inc., Waltham, MA, USA). A
SYBR-Green PCR Mastermix was used to perform the quantitative PCR
on an Mx3000P Real-Time PCR system (Stratagene; Agilent
Technologies, Inc., Santa Clara, CA, USA). PCRs were performed with
the following primer sets: PVT1 forward, 5′-CAGCACTCTGGACGGAC-3′
and reverse primers, 5′-CAACAGGAGAAGCAAACA-3′; MDR1 forward,
5′-ACCAAGCGGCTCCGATACA-3′ and reverse primers,
5′-TCATTGGCGAGCCTGGTAGTC-3′; MRP1 forward,
5′-GGACCTGGACTTCGTTCTCA-3′ and reverse primers,
5′-CGTCCAGACTTCATCCG-3′; GAPDH forward, 5′-ACCACAGTCCATGCCATCAC-3′
and reverse primers, 5′-TCACCACCCTGTTGCTGTA-3′. RNA levels were
corrected with the GAPDH signal. All mRNA levels were calculated
using the 2−ΔΔCq method.
Statistical analysis
The values were expressed as the mean ± standard
deviation (SD). SPSS 17.0 software was used to analyze the data.
The Student's t-test was performed to detect differences between
the KLT and vehicle groups. P<0.05 was considered
significant.
Results
KLT inhibited the cell viability and
promoted the cell apoptosis of drug-resistant gastric cancer
cells
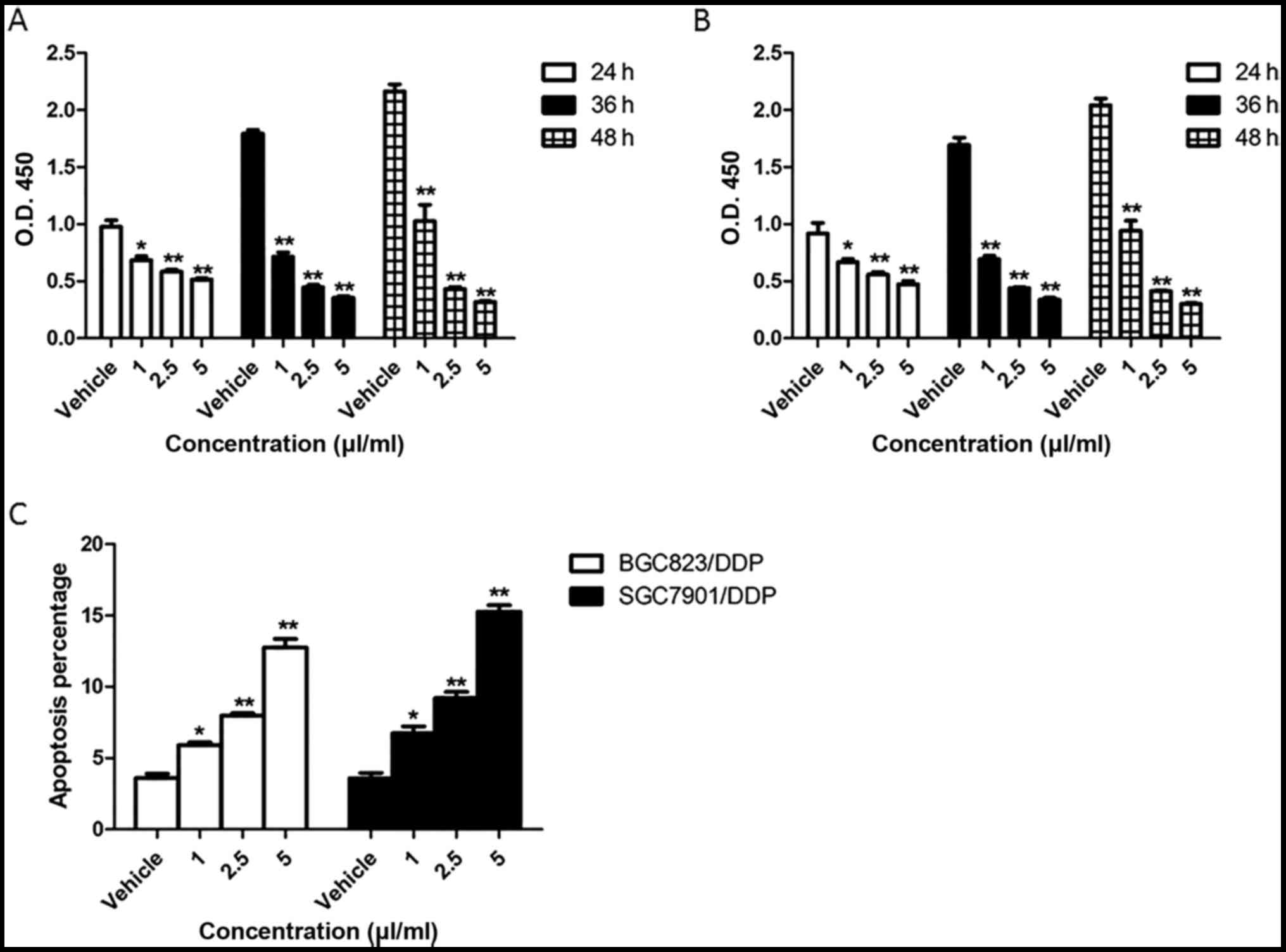
BGC823/DPP cells and SGC7901/DDP cells were treated
with 1, 2.5 and 5 µl/ml of KLT for 24, 36 and 48 h. The results of
CCK8 assay showed that the cell viability of BGC823/DPP cells and
SGC7901/DDP cells was significantly decreased by KLT in a
concentration-dependent manner (Fig. 1A
and B). To detect the effect of KLT on cell apoptosis of
drug-resistant gastric cancer cells, BGC823/DPP cells and
SGC7901/DDP cells were treated with 1, 2.5 and 5 µl/ml of KLT for
48 h and detected by Annexin V-PI assay. As shown in Fig. 1C, KLT highly promoted the cell
apoptosis of BGC823/DPP cells and SGC7901/DDP cells which was also
dose-dependent. Particularly, BGC823/DPP cells or SGC7901/DDP cells
treated with 5 µl/ml of KLT showed 3.5-fold increase or 4.2-fold
increase of apoptotic percentage compared to cells treated with
vehicle. These results indicated that KLT inhibited the cell
viability and promoted the cell apoptosis of drug-resistant gastric
cancer cells.
KLT suppressed the expression of drug
resistance-related proteins
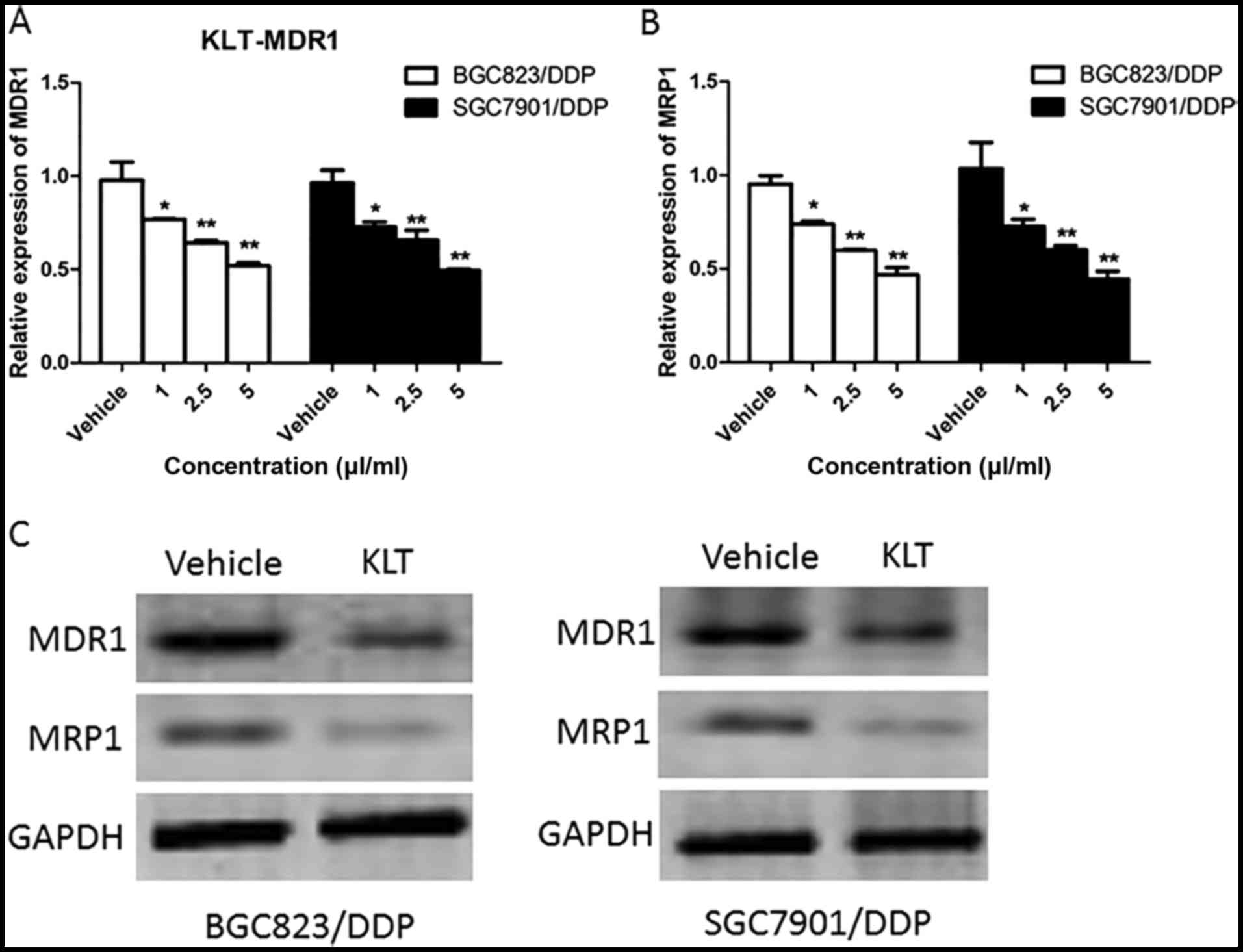
As drug resistance-related proteins might contribute
to MDR, we detected the mRNA levels of MDR1 and MRP1 by means of
Real Time-PCR in BGC823/DPP and SGC7901/DDP cells treated with
different concentration (1, 2.5, 5 µl/ml) of KLT for 24 h. As shown
in Fig. 2A and B, the expression of
MDR1 and MRP1 were both reduced by the pretreatment of KLT in a
concentration-dependent manner in BGC823/DPP and SGC7901/DDP cells.
Compared to the control (vehicle) the expression of MDR1 and MRP1
was 51 and 49% in BGC823/DPP cells treated with 5 µl/ml of KLT,
respectively. Then western blot was performed to examine the
protein level of MDR1 and MRP1 in BGC823/DPP and SGC7901/DDP cells
treated with 2.5 µl/ml of KLT for 48 h. KLT also greatly decreased
the protein level of MDR1 and MRP1 (Fig.
2C). These data indicated that KLT suppressed the expression of
drug resistance-related proteins.
KLT inhibited the expression of PVT1
in drug-resistant gastric cancer cells
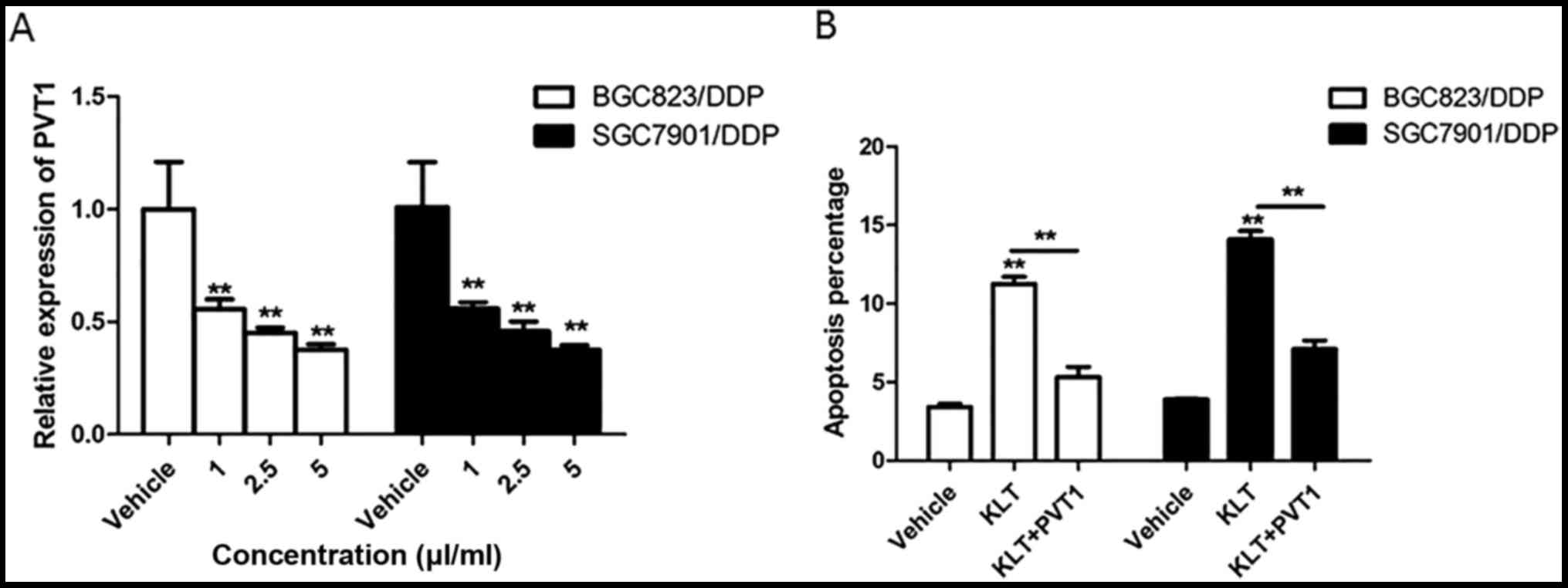
To investigate the effect of KLT on the expression
of PVT1 in gastric cancer cells, the level of PVT1 was detected in
BGC823/DPP and SGC7901/DDP cells treated with 1, 2.5 and 5 µl/ml of
KLT for 24 h. As shown in Fig. 3A,
significant downregulation of PVT1 was observed in all
Kanglaite-treated groups vs. the vehicle groups. Then BGC823/DPP
and SGC7901/DDP cells were transfected Ad-PVT1 followed by the
treatment of 2.5 µl/ml of KLT for 48 h. The result of cell
apoptosis showed that compared to BGC823/DPP and SGC7901/DDP cells
treated with KLT alone, the apoptotic percentage was 46 and 50% in
cells treated with KLT and Ad-PVT1, which indicated that PVT1
overexpression reversed the increased percentage of apoptotic cells
induced by KLT (Fig. 3B).
KLT inhibited the expression of drug
resistance-related proteins via suppressing the expression of
PVT1
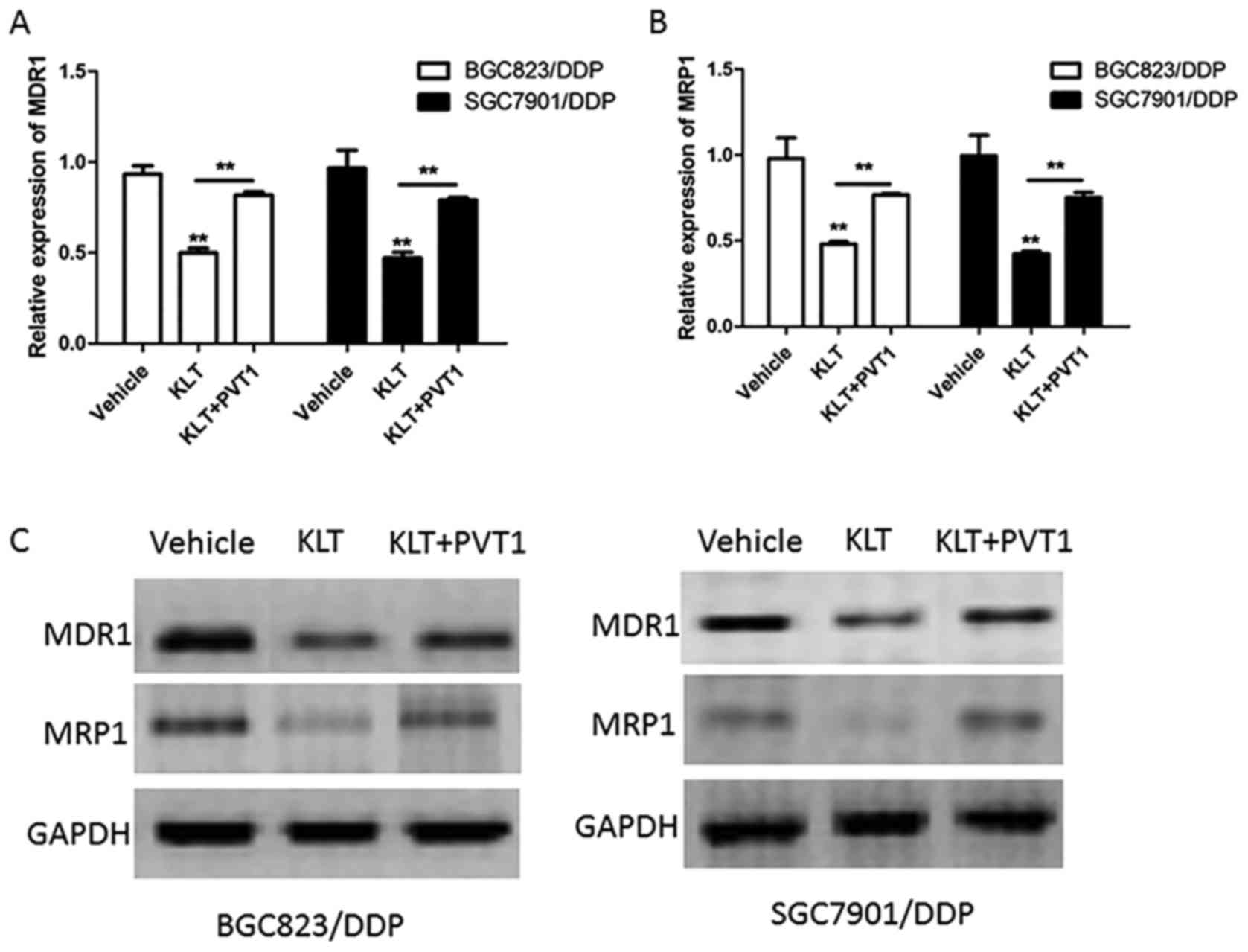
BGC823/DPP and SGC7901/DDP cells were transfected
Ad-PVT1 and then treated with 2.5 µl/ml of KLT for 24 h. The mRNA
level of MDR1 and MRP1 were detected by Real Time-PCR and the
results showed that PVT1 overexpression increased the mRNA level of
MDR1 and MRP1 which was inhibited by KLT (Fig. 4A and B). For example, BGC823/DPP
cells treated with KLT and Ad-PVT1 showed 1.66-fold increase of
MDR1 expression and 1.57-fold increase of MRP1 expression compared
to cells treated with KLT alone. In addition, PVT1 overexpression
also abrogated the effect of KLT on the protein level of MDR1 and
MRP1 in BGC823/DPP and SGC7901/DDP cells (Fig. 4C). These data suggested that KLT
inhibited the expression of drug resistance-related proteins via
suppressing the expression of PVT1.
Discussion
Traditional Chinese medicine has been known to show
significant advantages in improving the clinical symptom and the
therapeutic efficacy of chemotherapy, as well as in alleviating the
side effects of chemotherapy (17).
KLT is an antitumor drug widely used clinically in China. It has
been found to improve quality of life of cancer patients,
strengthen immune function and reduce cancer cachexia. Several
researches have aimed to clarify the antitumor mechanism of KLT and
demonstrated that KLT primarily blocked the G2/M phase of the cell
cycle, inducing cell apoptosis through activating proapoptotic
factors, such as p53, Fas and caspase-3 (4).
Nowadays, MDR has been the major obstacle for the
treatment of cancers, including the gastric cancer therapy, which
mainly leads to the failure of chemotherapies. KLT also ameliorated
the MDR of cancers in clinical use. KLT was reported to be
effective in reversing MDR of human lung adenocarcinoma cells and
increasing the sensitivity of cancer cells to chemotherapeutic
agents (18). A network
meta-analysis showed that compared with FOLFOX alone, combinations
with KLT injection could reduce nausea and vomiting and the
incidence of leukopenia (9).
Although the efficacy of KLT on MDR has been recognized, the
molecular mechanism was not fully understood. In this study, we
showed that KLT inhibited the cell viability and promoted the cell
apoptosis of drug-resistant gastric cancer cells. Furthermore, KLT
suppressed the expression of MDR1 and MRP1 in BGC823/DPP and
SGC7901/DDP cells, which suggested that KLT might attenuate MDR1-
and MRP1-regulated MDR in gastric cancer cells.
The MDR1 gene encoded P-glycoprotein (P-gp) which
was an efflux pump belonging to the ATP binding cassette
transporter superfamily. P-gp efflux pump was able to transport the
chemotherapeutic agents out of the cells causing the decreased drug
accumulation, then leading to the MDR (19). The mechanism of action of MRP1 for
MDR was similar to MDR1 which also belonged to the ATP binding
cassette transporter superfamily (20). These data indicated that KLT
inhibited anticancer drug resistance by targeting ATP binding
cassette transporter family members in gastric cancer.
To further investigate the mechanism through which
KLT regulated the expression of MDR1 and MRP1, we explored the
effect of KLT on the expression of PVT1, which was observed to
promote MDR in gastric cancer cells in our previous study. In
several cancers, PVT1 is found to be upregulated in cancer tissues
than in adjacent normal tissues, and acts to induce cell
proliferation and suppress apoptosis (21–23). In
addition, overexpression of PVT1 is involved in cancer resistance
to chemotherapy. Similar to the results of our previous study, PVT1
was overexpressed in SGC7901 paclitaxel-resistant cells than in
untreated SGC7901 cells (14). In
the malignant pleural mesothelioma cell, PVT1 knockdown reduced
cell proliferation and increased sensitivity to cisplatin (24). PVT1 was also shown to regulate
gemcitabine sensitivity in human pancreatic cancer cells ASPC-1
(25). We showed that KLT inhibited
the expression of PVT1 in drug-resistant gastric cancer cells in a
time-dependent manner. In addition, PVT1 overexpression reversed
the increased percentage of apoptotic cells and the inhibition of
the expression of MDR1 and MRP1 induced by KLT. These data
indicated that KLT inhibited the expression of MDR1 and MRP1 via
suppressing the expression of PVT1.
In conclusion, the results in the present study
indicated that KLT inhibited the cell viability and promoted the
cell apoptosis of drug-resistant gastric cancer cells. In addition,
KLT might attenuate the MDR of gastric cancer cells associating
with inhibiting MDR1 and MRP1. Furthermore, KLT inhibited the
expression of MDR1 and MRP1 via suppressing the expression of PVT1.
The present findings reveal a novel mechanism of KLT function on
the MDR of gastric cancer cells, which can potentially be exploited
to prevent the MDR of gastric cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China under grant no. 81673736.
References
|
1
|
Xin Y, Yin F, Qi S, Shen L, Xu Y, Luo L,
Lan L and Yin Z: Parthenolide reverses doxorubicin resistance in
human lung carcinoma A549 cells by attenuating NF-κB activation and
HSP70 up-regulation. Toxicol Lett. 221:73–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen SY, Zheng XW, Cai JX, Zhang WP, You
HS, Xing JF and Dong YL: Histone deacetylase inhibitor reverses
multidrug resistance by attenuating the nucleophosmin level through
PI3K/Akt pathway in breast cancer. Int J Oncol. 49:294–304.
2016.PubMed/NCBI
|
|
3
|
Gillet JP and Gottesman MM: Mechanisms of
multidrug resistance in cancer. Methods Mol Biol. 596:47–76. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu Y, Li CS and Dong Q: Chinese herb
related molecules of cancer-cell-apoptosis: A minireview of
progress between Kanglaite injection and related genes. J Exp Clin
Cancer Res. 27:312008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu F, Gao J, Zeng Y and Liu CX: Inhibition
of Coix seed extract on fatty acid synthase, a novel target for
anticancer activity. J Ethnopharmacol. 119:252–258. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qi F, Li A, Inagaki Y, Gao J, Li J, Kokudo
N, Li XK and Tang W: Chinese herbal medicines as adjuvant treatment
during chemo- or radio-therapy for cancer. Biosci Trends.
4:297–307. 2010.PubMed/NCBI
|
|
7
|
Zhu L, Yang Z, Wang S and Tang Y:
Kanglaite for treating advanced non-small-cell lung cancer: A
systematic review. Zhongguo Fei Ai Za Zhi. 12:208–215. 2009.(In
Chinese). PubMed/NCBI
|
|
8
|
Li DP: General survey and progress in
clinical trials abroad over Kanglaite injection. Chin J Integr Med.
10:233–235. 2004. View Article : Google Scholar
|
|
9
|
Wang JC, Tian JH, Ge L, Gan YH and Yang
KH: Which is the best Chinese herb injection based on the FOLFOX
regimen for gastric cancer? A network meta- analysis of randomized
controlled trials. Asian Pac J Cancer Prev. 15:4795–4800. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhan YP, Huang XE, Cao J, Lu YY, Wu XY,
Liu J, Xu X, Xiang J and Ye LH: Clinical safety and efficacy of
Kanglaite® (Coix Seed Oil) injection combined with chemotherapy in
treating patients with gastric cancer. Asian Pac J Cancer Prev.
13:5319–5321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beck-Engeser GB, Lum AM, Huppi K, Caplen
NJ, Wang BB and Wabl M: Pvt1-encoded microRNAs in oncogenesis.
Retrovirology. 5:42008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guan Y, Kuo WL, Stilwell JL, Takano H,
Lapuk AV, Fridlyand J, Mao JH, Yu M, Miller MA, Santos JL, et al:
Amplification of PVT1 contributes to the pathophysiology of ovarian
and breast cancer. Clin Cancer Res. 13:5745–5755. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takahashi Y, Sawada G, Kurashige J, Uchi
R, Matsumura T, Ueo H, Takano Y, Eguchi H, Sudo T, Sugimachi K, et
al: Amplification of PVT-1 is involved in poor prognosis via
apoptosis inhibition in colorectal cancers. Br J Cancer.
110:164–171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ding J, Li D, Gong M, Wang J, Huang X, Wu
T and Wang C: Expression and clinical significance of long
non-coding RNA-PVT1 in human gastric cancer. Onco Targets Ther.
7:1625–1630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu E, Liu Z, Zhou Y, Mi R and Wang D:
Overexpression of long non-coding RNA PVT1 in ovarian cancer cells
promotes cisplatin resistance by regulating apoptotic pathways. Int
J Clin Exp Med. 8:20565–20572. 2015.PubMed/NCBI
|
|
16
|
Zhang XW, Bu P, Liu L, Zhang XZ and Li J:
Overexpression of long non-coding RNA PVT1 in gastric cancer cells
promotes the development of multidrug resistance. Biochem Biophys
Res Commun. 462:227–232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qi F, Zhao L, Zhou A, Zhang B, Li A, Wang
Z and Han J: The advantages of using traditional Chinese medicine
as an adjunctive therapy in the whole course of cancer treatment
instead of only terminal stage of cancer. Biosci Trends. 9:16–34.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pin-Tian L, Kun Z, Ya-zhen W and Bin L:
Effect of Kanglaite Injection associated with Cisplatin in lung
adno carcinoma cells A549. Chin Traditional Patent Med. 33:393–396.
2011.
|
|
19
|
Minchinton AI and Tannock IF: Drug
penetration in solid tumours. Nat Rev Cancer. 6:583–592. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cole SP: Targeting multidrug resistance
protein 1 (MRP1, ABCC1): Past, present, and future. Annu Rev
Pharmacol Toxicol. 54:95–117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cui D, Yu CH, Liu M, Xia QQ, Zhang YF and
Jiang WL: Long non-coding RNA PVT1 as a novel biomarker for
diagnosis and prognosis of non-small cell lung cancer. Tumor Biol.
37:4127–4134. 2016. View Article : Google Scholar
|
|
22
|
Ding C, Yang Z, Lv ZDUC, Xiao H, Peng C,
Cheng S, Xie H, Zhou L, Wu J and Zheng S: Long non-coding RNA PVT1
is associated with tumor progression and predicts recurrence in
hepatocellular carcinoma patients. Oncol Lett. 9:955–963.
2015.PubMed/NCBI
|
|
23
|
Zhou Q, Chen J, Feng J and Wang J: Long
noncoding RNA PVT1 modulates thyroid cancer cell proliferation by
recruiting EZH2 and regulating thyroid-stimulating hormone receptor
(TSHR). Tumour Biol. 37:3105–3113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Riquelme E, Suraokar MB, Rodriguez J, Mino
B, Lin HY, Rice DC, Tsao A and Wistuba II: Frequent coamplification
and cooperation between C-MYC and PVT1 oncogenes promote malignant
pleural mesothelioma. J Thorac Oncol. 9:998–1007. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
You L, Chang D, Du HZ and Zhao YP:
Genome-wide screen identifies PVT1 as a regulator of Gemcitabine
sensitivity in human pancreatic cancer cells. Biochem Biophys Res
Commun. 407:1–6. 2011. View Article : Google Scholar : PubMed/NCBI
|