Introduction
Diabetic retinopathy (DR) is one of the primary
causes of blindness globally. More than 300 million people are
estimated to have diabetes worldwide, and 40% have retinopathy to
some extent, which includes ~8.2% with vision-threatening
retinopathy (1,2). Primary pathological hallmarks of DR are
retinal ischemia and proliferation, induced by microcirculatory
disturbance and microangiopathy. The process includes oxidative
stress, vascular endothelial growth factor (VEGF)-induced damage
and inflammatory changes (3).
Previous studies have indicated that somatostatin
(an endogenous peptide) or its analogue exhibit anti-inflammatory
effects and neuroprotection against DR or retinal
ischemia-reperfusion injury (4–6).
Somatostatin receptors (SSTR1-5) are widely distributed in the
retina (7,8). Activation of retinal SSTR2
may reduce VEGF release from damaged neurons and limit the VEGF
response (9). It has been
demonstrated in an oxygen-induced retinopathy model that activation
of SSTR2 may downregulate VEGF and exert an
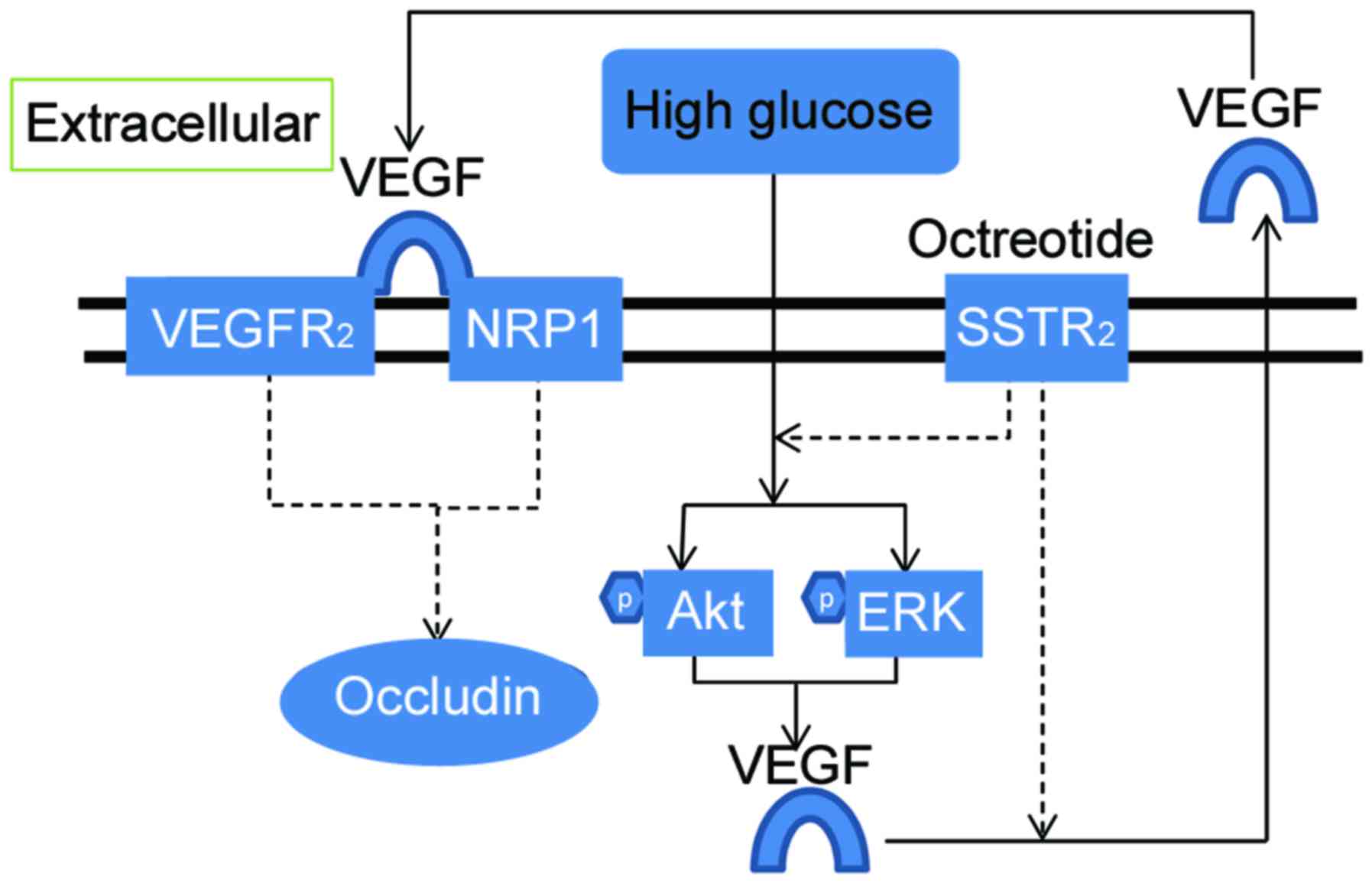
anti-angiogenesis effect via the SHP-1/STAT3 pathway (10). VEGF combined with its receptors, VEGF
receptor (VEGFR2) and neuropilin 1 (NRP1), has an
important role in the disruption of the blood-retinal barrier
(BRB), which is a core pathological change in retinal dysfunction
of DR (11–13). Tight junctions of vascular
endothelial cells are key components of BRB. Occludin, a
transmembrane protein in the tight junctions, exerts its role by
forming the permeability barrier. The high glucose conditions of DR
may lead to VEGF release and occludin degradation in cultured human
retinal endothelial cells (5).
A recent study indicated that SSTR2 was
scarcely expressed in VEGF-containing cells, and SSTR2
or VEGF barely localized in retinal vessels (10). However, under hypoxic conditions,
SSTR2 and VEGF immunoreactivity was noted in retinal
capillaries (10). This is
consistent with another study that demonstrated VEGF was released
from damaged neurons and entered vessels under hypoxic conditions
(9). Therefore, it is not clear
whether SSTR2 exists in normal vascular endothelial
cells, and whether activation of SSTR2 is able to
regulate the degradation of occludin induced by high levels of
glucose in vascular endothelial cells. The present study aimed to
elucidate this and determine whether SSTR2 modulates the
occludin degradation induced by high glucose via the
VEGF/NRP1/protein kinase B (Akt) signaling pathway.
Materials and methods
Materials
RPMI-1640 culture medium and fetal bovine serum
(FBS) were purchased from Gibco (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). D-glucose, penicillin and streptomycin were
obtained from DingGuo Biotech Co., Ltd., (Shanghai, China).
Octreotide (OCT) and cyclo-somatostatin (c-SOM; both Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) were dissolved and diluted in 0.9%
NaCl to 1 mM.
Cell culture and experimental
grouping
Rhesus monkey retinal fovea vascular endothelial
cells (RF/6A) were obtained from Wuhan Boster Biological
Technology, Ltd., (Wuhan, China). The RF/6A endothelial cells were
maintained in Dulbecco's odified Eagle medium (DMEM; Wuhan Boster
Biological Technology, Ltd.) culture medium supplemented with 10%
FBS, streptomycin (100 µg/ml) and penicillin (100 IU/ml) in a 5%
CO2 incubator at 37°C. Cells were seeded in 6-well
plates at a density of 1×105 under normal (5.6 mM) and
high glucose (30 mM) conditions, respectively. Passages 4–8 of the
cells were used in the present study. The experimental groups in
the current study were as follows: i) Control (normal conditions);
ii) high glucose (HG; 30 mM D-glucose); iii) HG + Octreotide (OCT;
1 µM); iv) HG + OCT + c-SOM (OCT, 1 µM; c-SOM, 1 µM); and v) HG +
VEGF co-receptor NRP1 antagonist (ATWLPPR: GL Biochem, Ltd.,
Shanghai China; 0.1 mM). Cells were observed and photographed with
an inverted microscope (CX40RF200; Olympus Corp., Tokyo,
Japan).
Terminal deoxynucleotidyl transferase
dUTP nick-end labeling (TUNEL)
TUNEL assay was performed using an in situ
cell apoptosis detection kit (MK1022; Boster Biological Technology,
Pleasanton, CA, USA) based on the manufacturer's protocol. RF/6A
cells grown in normal and high glucose medium for 5 days were fixed
with 4% paraformaldehyde at room temperature for 30 min. Following
washing (3 times for 2 min) with phosphate-buffered saline (PBS)
and distilled water, Proteinase K (1:200) was added to cells and
incubated at 37°C for 10 min. Cells were washed with PBS and
incubated with Labeling Buffer mixed with terminal deoxynucleotidyl
and digoxidenin-11-deoxyuridine triphosphate (both provided in the
kit) in a moist chamber at 37°C for 2 h. Cells were then washed
with PBS (3 times for 2 min) and incubated at room temperature for
30 min with anti-digoxigenin peroxidase (provided in the kit) prior
to treatment with with SABC-AP and chromogenic substrate (BCIP/NBT;
provided in the kit).
Culture media VEGF measurement
A sandwich mouse VEGF ELISA kit (EK0541; Boster
Biological Technology) was used to determine the level of VEGF
according to the protocol provided by the manufacturer. Briefly,
following collection of the cell culture fluid, with a 5-min
centrifugation at 2,000 × g (4°C), the supernatant was diluted with
sample diluent (provided in the kit) to a proportion of 1:2. From
each sample, 100 ml supernatant was transferred to wells, which
were coated with VEGF monoclonal antibody (1:100; provided in the
kit). Subsequently, the avidin-biotin-peroxidase complex, ABC and
chromogenic substrate were added in sequence according to the
manufacturer's protocol. VEGF levels were determined by the
absorbance at 450 nm using a microplate reader (Varioskan Flash;
Thermo Fisher Scientific, Inc.) and normalized to the standard
recombinant VEGF.
Western blot analysis
The culture solution was discarded and the RF/6A
cells were washed with PBS three times and lysed by
radioimmunoprecipitation lysis buffer containing EDTA-Na2, sodium
fluoride and phenylmethylsulfonyl fluoride (all 1 mM). Cell
extracts were collected and centrifuged for 5 min at 20,000 × g and
4°C. The supernatant was transferred into a 1.5-ml Eppendorf tube.
Protein concentrations were measured using a Bradford Protein Assay
kit (Beyotime Institute of Biotechnology, Co., Ltd., Haimen,
China). Each protein sample (50 µg) was separated by 10% SDS-PAGE
and transferred onto nitrocellulose membranes (EMD Millipore,
Billerica, MA, USA). Following washing in TBST (2 times for 1 min),
the membranes were blocked in 5% non-fat milk in Tris-buffered
saline with Tween-20 and incubated overnight at 4°C with the
primary antibodies, as follows: SSTR2 (Boster Biological
Technology; BA1406-2;1:400), Occludin (Santa Cruz Biotechnology,
Inc., Dallas, TX, USA; sc-8144; 1:400), Akt (Boster Biological
Technology; BA0631; 1:400), phosphorylated-Akt (p-AKT; Santa Cruz
Biotechnology, Inc.; sc-33,437; 1:500), extracellular
signaling-regulated kinase (ERK), and p-ERK (both CST Biological
Reagents Company Ltd., Shanghai, China; 9102s and 9101s; both
1:1,000). The secondary antibody was horseradish peroxidase-goat
anti-rabbit IgG (Boster Biological Technology; BA1080;
1:2,000).
Statistical analysis
Results were presented as the mean ± standard
deviation. Statistical analyses were performed by using SigmaStat
(version 3.2; Systat software, Inc., San Jose, CA, USA). Different
groups were compared using one-way analysis of variance (Turkey
test or Kruskal Wallis were used as post-hoc tests) or Student
t-test. P<0.05 was considered to determine statistically
significant differences.
Results
Observation of RF/6A cell appearance
in high glucose conditions
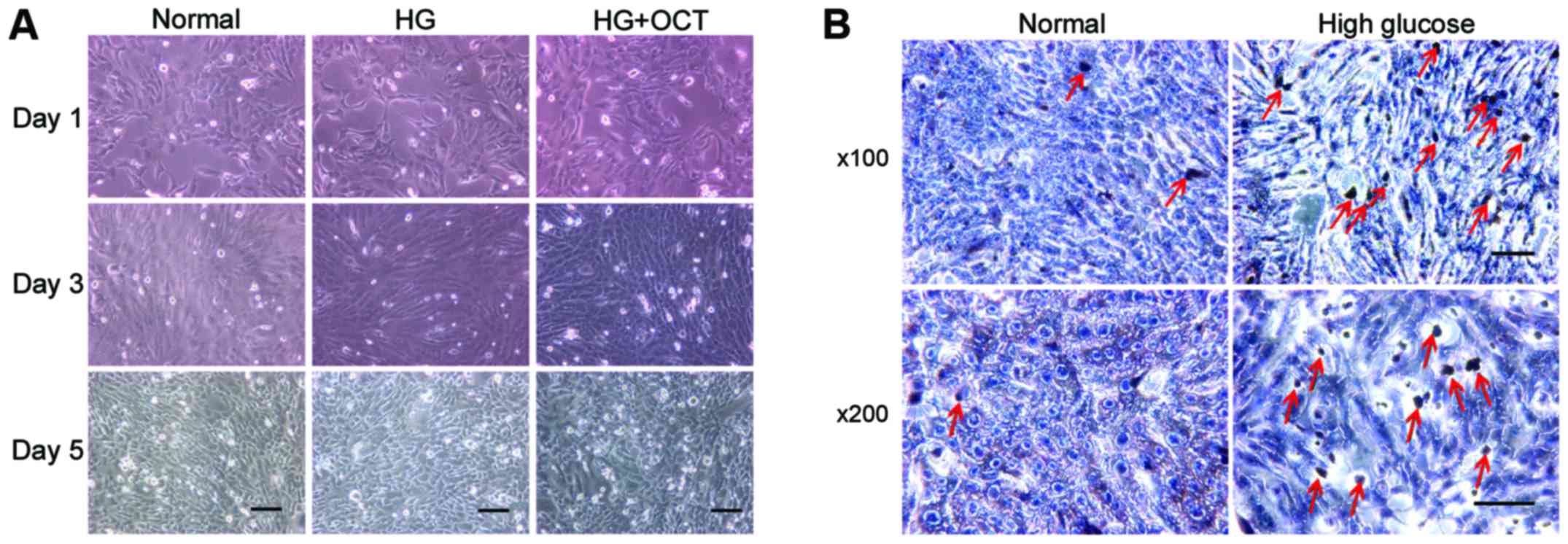
Morphological observation demonstrated that the
RF/6A cells exhibited a shuttle-like and polygonal appearance when
they were not crowded in the well. As the concentration increased,
a number of cells exhibited a cobblestone appearance. No difference
in appearance was observed between normal and high glucose
conditions (Fig. 1A). TUNEL assay
was performed on day 5 to determine whether RF/6A cells were
influenced by the high glucose conditions. The number of
apoptosis-positive cells in high glucose conditions was higher than
those exposed to normal conditions (Fig.
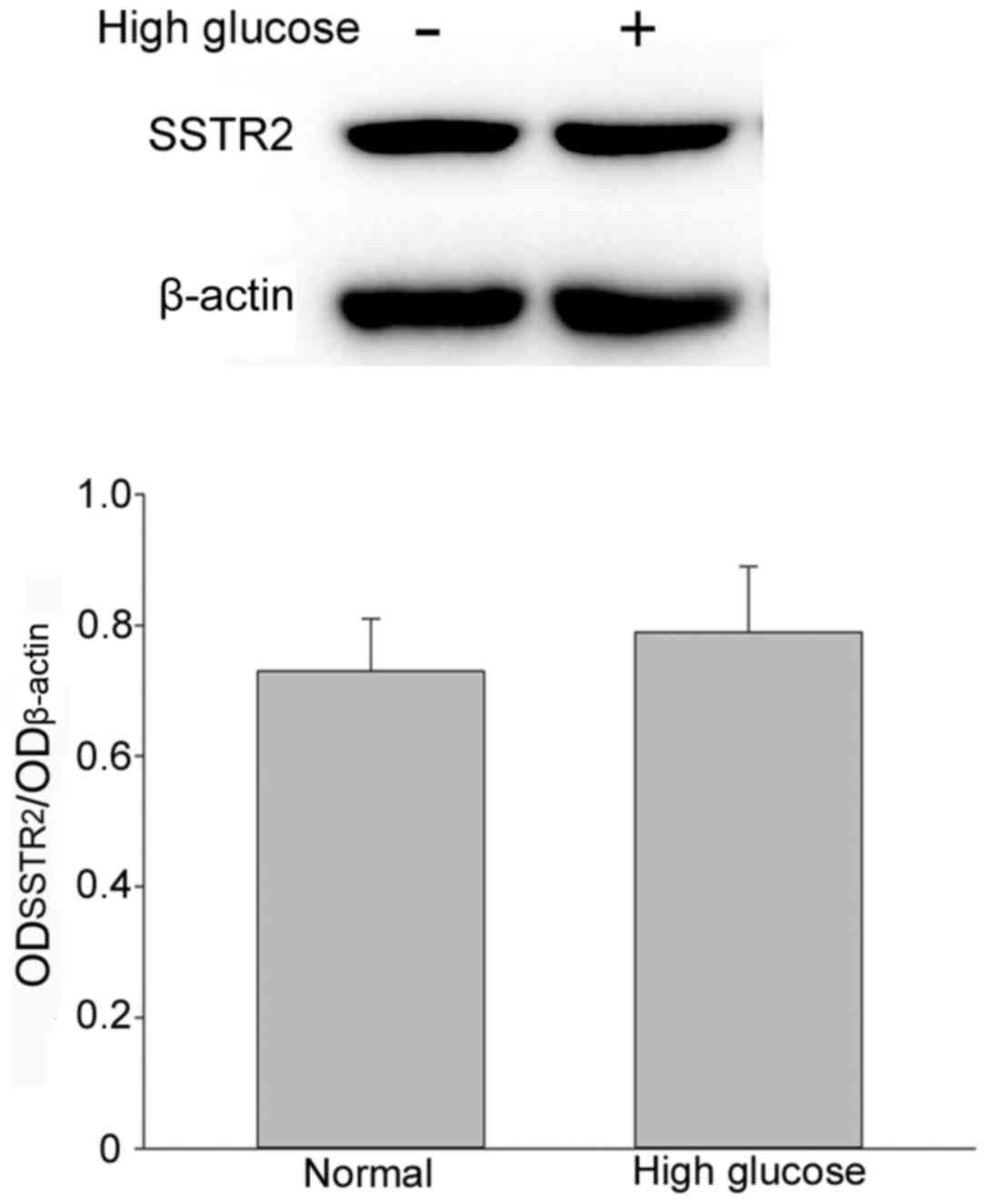
1B). Expression of SSTR2 in RF/6A cells. To evaluate the
expression of SSTR2 in RF/6A cells, western blot
analysis was performed on normal control cells and high
glucose-treated cells. SSTR2 was expressed in the RF/6A
cells both in normal and high glucose conditions. No significant
difference in expression was observed in RF/6A cells treated with
30 mM D-glucose for 5 days, as compared with the untreated cells
(Fig. 2).
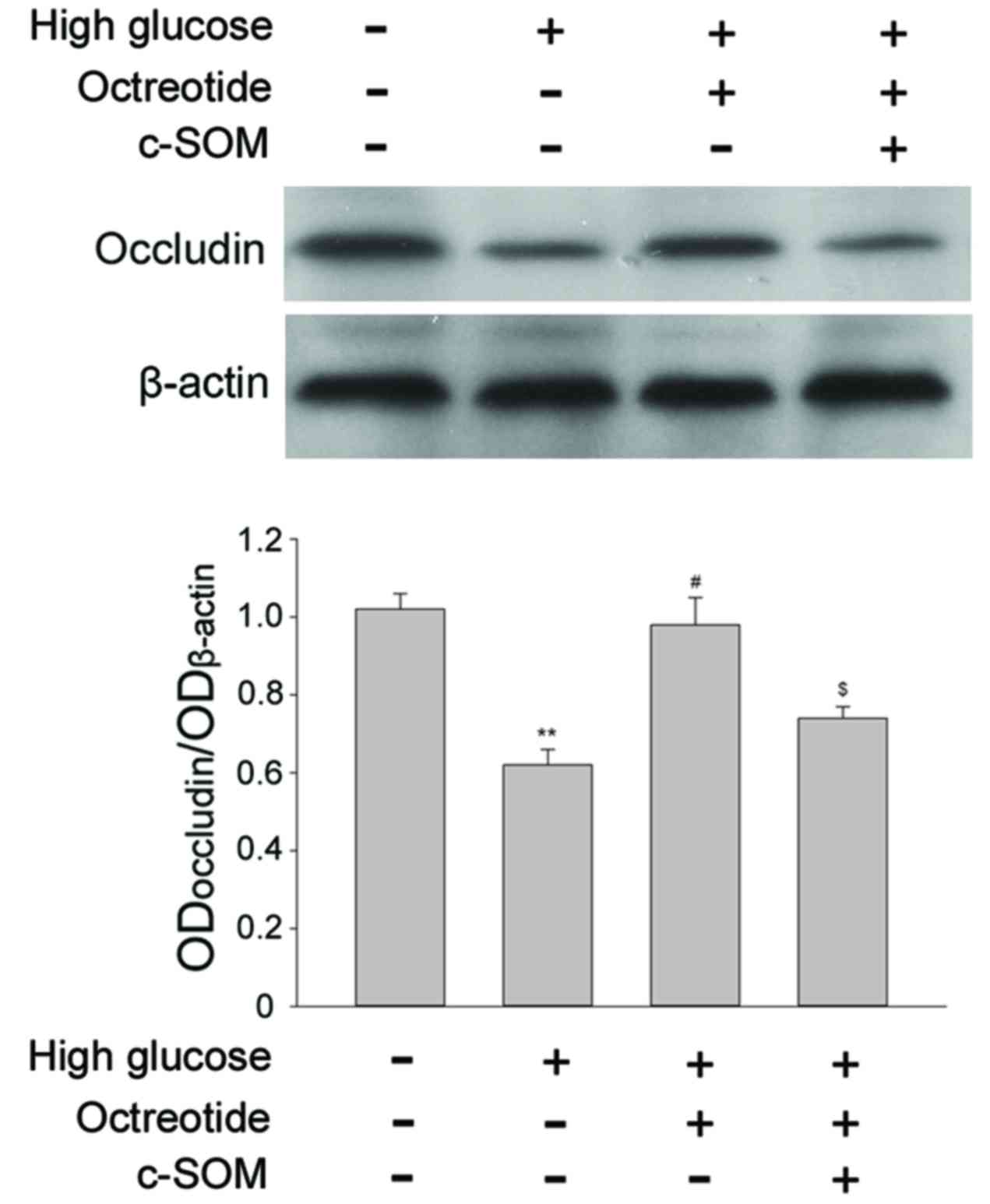
SSTR2 modulates the
degradation of occludin induced by high glucose
Occludin is a transmembrane tight junction protein
expressed in endothelial cells and is also a key factor in the
formation of the permeability barrier. Under 30 mM D-glucose
conditions, occludin expression was significantly reduced when
compared with cells exposed to normal conditions (Fig. 3; P<0.05). For RF/6A cells under
high glucose conditions, treatment with the SSTR2
agonist OCT (1 µM) restored the level of occluding, and this change
was significant when compared with the high glucose group
(P<0.05). Co-administration of SSTR2 antagonist c-SOM
(1 µM) with octreotide significantly inhibited the effect of OCT
(P<0.05). The results demonstrated that the specific
SSTR2 was involved in modulation of occludin levels in
the RF/6A cells under the high glucose conditions.
OCT inhibits the VEGF expression and
secretion involved in the activation of Akt and ERK in the high
glucose condition
A sandwich ELISA assay was performed to evaluate the
secretion level of VEGF from RF/6A cells. As presented in Fig. 4A, cells exposed to 30 mM glucose
exhibited in a significant increase in VEGF levels from day1
(P<0.05). In cells subjected to high glucose, treatment with 1
mM OCT significantly inhibited the release of VEGF into the culture
medium from day 3 (P<0.05; Fig.
4A). Expression levels of VEGF, Akt, p-Akt, ERK and p-ERK were
determined by western blot analysis. As presented in Fig. 4B and C, high glucose conditions
significantly up-regulated the expression of VEGF in RF/6A cells
(P<0.05). At the same time, the expression of p-Akt and p-ERK
also increased significantly compared with the cells under normal
conditions (P<0.05). The increase in expression of VEGF, p-Akt
and p-ERK was significantly inhibited by treatment with OCT
(P<0.05). VEGF is able to activate its downstream signaling
molecules (Akt and ERK) by binding to its membrane receptor. Thus,
these results demonstrated that OCT inhibited VEGF expression and
secretion and subsequently the autocrine response induced by a high
concentration of glucose.
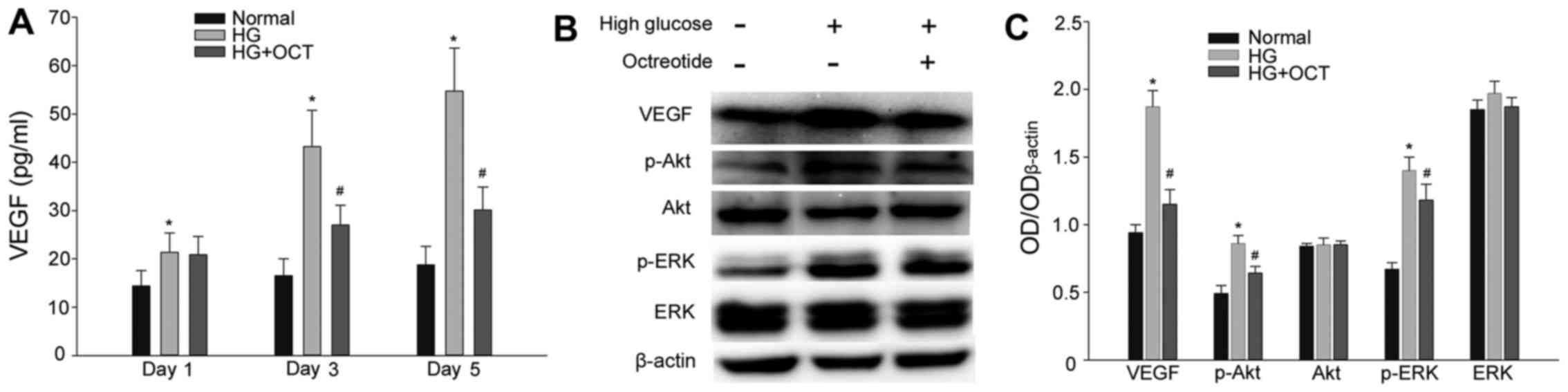 | Figure 4.Effect of HG on VEGF expression and
secretion in RF/6A cells, and the VEGF autocrine response was
inhibited by OCT. (A) HG conditions resulted in an increase of VEGF
release in a time-dependent manner. The increase of VEGF release
may be inhibited by administration of OCT. (B) Expression of VEGF,
Akt, p-Akt, ERK and p-ERK in RF/6A was evaluated by western blot
and densitometric analysis and subsequently (C) quantified. VEGF,
p-Akt and p-ERK upregulation in HG conditions was inhibited by
treatment with OCT. Each column represents the mean + standard
deviation of OD data from three samples. β-actin was used as the
loading control. *P<0.05 vs. normal control;
#P<0.05 vs. high glucose group. VEGF, vascular
endothelial growth factor; Akt, protein kinase B; ERK,
extracellular signal-related kinase; p-Akt, phosphorylated Akt;
p-ERK, phosphorylated ERK; HG, high glucose; OCT, octreotide; OD,
optical density. |
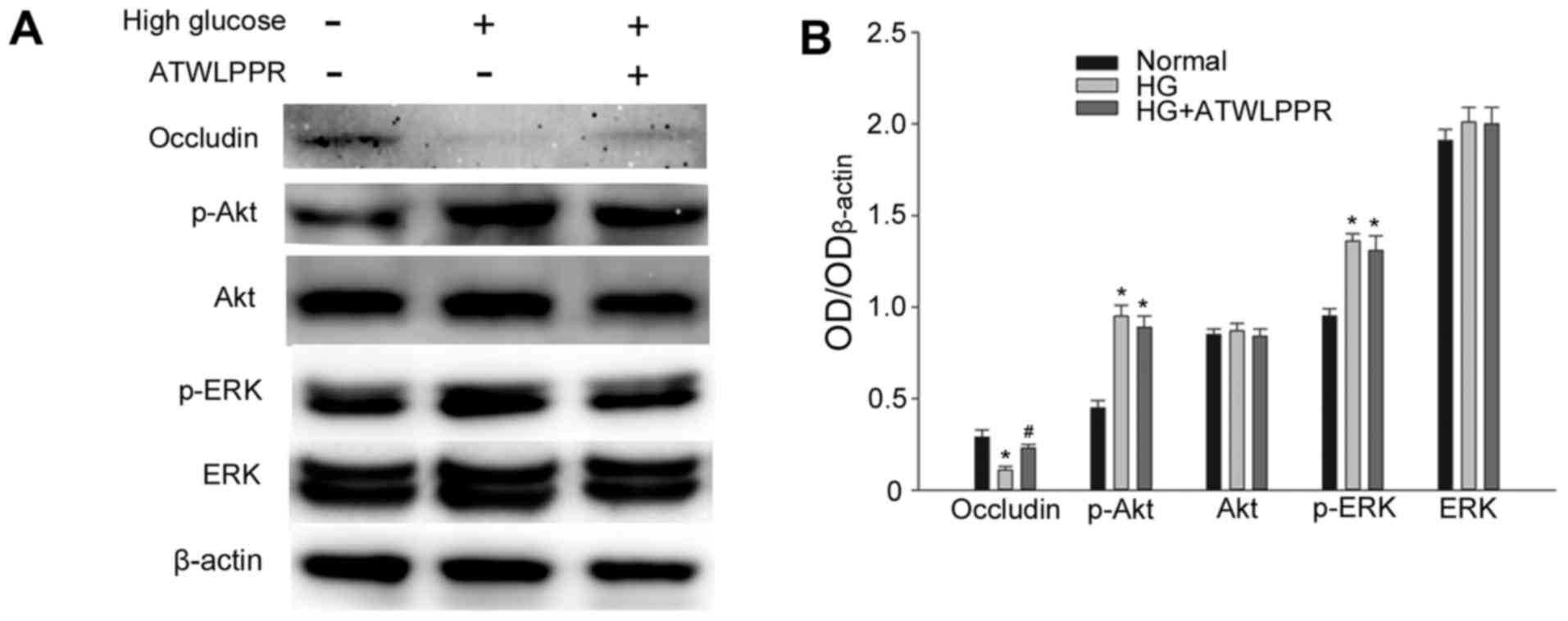
Involvement of NRP1 in the effects of
high glucose-induced occludin downregulation
VEGF is secreted from RF/6A cells and bound to its
receptors VEGFR2/NRP1, exerting its role of downstream
signal transduction. Western blot analysis indicated that treatment
with NRP1 (VEGFR2 co-receptor) inhibitor ATWLPPR (0.1
mM) significantly prevented occludin downregulation under high
glucose conditions (P<0.05; Fig.
5). p-Akt and p-ERK increased significantly in high glucose
conditions (P<0.05; Fig. 5B);
however, this was not influenced by ATWLPPR. These results indicate
that NRP1 may be involved in the modulation of the VEGF autocrine
or paracrine responses, as demonstrated in Fig. 6.
Discussion
The results of the present study indicate that
SSTR2 exists in RF/6A cells cultured in normal and high
glucose medium. Furthermore, no significant difference in
SSTR2 expression was observed using densitometric
analysis between the two culture conditions. Activation of
SSTR2 prevented occludin downregulation under high
glucose conditions and this process is involved in the increased
expression of p-Akt, p-ERK and VEGF. Administration of the NRP1
inhibitor, ATWLPPR, inhibited the decrease of occludin induced by
high glucose.
A previous study demonstrated that the
SSTR2 agonist, OCT, has anti-inflammation and
anti-oxidation effects and alleviated retinal edema in retinas
following ischemia-reperfusion (5).
It has been indicated that OCT was associated with the integrity of
blood vessel endothelium, as confirmed by previous studies
(14–16). Damage of endothelial cells is often
observed in the early stage of DR (3,17,18).
Somatostatin and its receptors are widely distributed in the retina
and serves important and complex physiological roles (7,19). The
current study focused on an endothelial cell line, RF/6A, and
determined the existence of SSTR2 initially. A previous
study assessing hypoxic retinas indicated that SSTR2
immunostaining was increasingly noted in capillaries (20). It has also been indicated that
SSTR2 and SSTR5 are expressed in
proliferative endothelial cells but not in quiescent cells
(21). Whether SSTR2 in
the endothelium serves a downstream role and modulates the
integrity of endothelium remains unknown. The results of the
present study indicated that high glucose conditions did not change
the expression level of SSTR2 in RF/6A cells. Tight
junctions among the endothelium in capillary vessels are the
functional base of BRB (3). Occludin
is an important tight junction transmembrane protein, responsible
for the permeability of the barrier (22). Tissues or cells exposed to ischemia,
hypoxia and high glucose conditions may exhibit a reduction of
tight junction proteins, such as occludin, and this process forms
the primary pathological base of BRB injury as it is demonstrated
by enhanced permeability (3,22–24). In
the current study, the condition culture medium with 30 mM glucose
led to the decreased expression of occludin in RF/6A cells and this
reduction was regulated by SSTR2. A study on the
intestinal epithelia indicated that activation of SSTR2
led to protective effects on the intestinal barrier by modulation
of the expression of tight junction proteins (25). Accumulated evidence has indicated
that the administration of somatostatin or SSTR2 agonist
had anti-inflammatory and neuroprotective effects in ischemic
retinopathy (4,5). However, whether SSTR2 acts
directly on capillaries epithelium, and the mechanism involved,
remains unclear.
It is well-known that high glucose conditions are
able to increase VEGF expression (22,26). The
current study also indicated that not only the release of, but also
the expression of VEGF in RF/6A cells was enhanced by high glucose.
VEGF is an important factor that contributes to the permeability
and the integrity of blood vessels (24). In patients with diabetes, increased
VEGF in the eyes has been implicated in elevated vascular
permeability and breakdown of BRB (27,28).
VEGF-induced permeability is mediated by the downregulation of
tight junction proteins (occludin), and the molecular mechanisms
may be associated with urokinase plasminogen activator, nitric
oxide and protein kinase C (24,29–32). The
results of the current study demonstrated that high glucose induced
an increase in VEGF expression, which may be inhibited by OCT, a
SSTR2 preferring agonist. It is therefore hypothesized
that SSTR2 regulates occludin, potentially by
controlling the expression and secretion of VEGF. The results of
the present study were consistent with other research performed in
a mouse model of retinopathy (33).
Although there remains a lack of such reports in RF/6A cells,
previous studies have demonstrated that octreotide prevents the
upregulation of VEGF induced by hypoxia (10,33).
VEGF, as a specific mitogen, has been demonstrated to have an
effective role in autocrine regulation of endothelial cells
(34,35). Thus, the VEGF autocrine response may
contribute to the vessel permeability changes in DR (22).
Akt and ERK are important cellular signal molecules
that serve their biological functions when activated by
phosphorylation. Activation of Akt may inhibit apoptosis and
promote cell survival and lumen formation (36). ERK is involved in regulating
angiogenesis (37). A high
concentration of glucose may induce oxidative stress, tumor
necrosis factor-α upregulation and apoptosis in the endothelium
(38). The results of the current
study indicated that high glucose induced an increase of p-Akt and
p-ERK in RF/6A cells. Treatment with OCT was then demonstrated to
reduce the activation of Akt and ERK. The present study was not
able to illustrate whether the effects of OCT were from the
inhibition of VEGF or other pathways, leading to anti-oxidative
stress. Numerous studies have demonstrated that Akt and ERK are
common downstream signal molecules of VEGF and its receptors
(13,24,39,40).
Increased p-ERK and p-Akt were typically observed in retinal
ischemia diseases and retinal neovascularization (41,42), and
they were also required factors for the expression of VEGF
(41,43).
NRP1 acts as a co-receptor for VEGF by forming a
complex with VEGFR2. NRP1 may promote VEGF binding and
potentiate VEGFR2 activation and intracellular signaling (44). To illustrate whether NRP1 was
involved in the high glucose-induced VEGF autocrine response and
occludin reduction, a NRP1 inhibitor, ATWLPPR was administered to
RF/6A cells. The results indicated that ATWLPPR reduced the
increase of occludin, but had no significant effects on the
activation of Akt and ERK. This was consistent with a previous
study that demonstrated NRP1 to be a required component for the
regulation of vascular permeability, induced by VEGF (40). Evans et al (44) suggested that treatment of small
interfering RNA on NRP1 in HUVEC cells did not affect the
phosphorylation of AKT and ERK mediated by VEGF. This was in
accordance with the results of the present study. Activation of Akt
and ERK was unaffected by the inhibition of NRP1, this was
potentially due to lower thresholds of the receptors activity.
In conclusion, the results of the present study
demonstrated that SSTR2 in RF/6A cells prevented the
high glucose-induced decrease of occludin. NRP1, increased
expression of VEGF, and activation of Akt and ERK were involved in
this process. This may account for the regulating mechanisms of OCT
on vascular permeability in diabetes or high glucose-mediated VEGF
autocrine or paracrine response.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (grant no. 31300884) and Science and
Technology Development Project of Henan Province (grant no.
162300410233 and 162300410036).
References
|
1
|
Ciulla TA, Amador AG and Zinman B:
Diabetic retinopathy and diabetic macular edema: Pathophysiology,
screening, and novel therapies. Diabetes Care. 26:2653–2664. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Klein R, Klein BE, Moss SE and
Cruickshanks KJ: The wisconsin epidemiologic study of diabetic
retinopathy: XVII. The 14-year incidence and progression of
diabetic retinopathy and associated risk factors in type 1
diabetes. Ophthalmology. 105:1801–1815. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang C, Wang H, Nie J and Wang F:
Protective factors in diabetic retinopathy: Focus on blood-retinal
barrier. Discov Med. 18:105–112. 2014.PubMed/NCBI
|
|
4
|
Hernández C, García-Ramírez M, Corraliza
L, Fernández-Carneado J, Farrera-Sinfreu J, Ponsati B,
González-Rodríguez A, Valverde AM and Simó R: Topical
administration of somatostatin prevents retinal neurodegeneration
in experimental diabetes. Diabetes. 62:2569–2578. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang J, Sun Z, Shen J, Wu D, Liu F, Yang
R, Ji S, Ji A and Li Y: Octreotide protects the mouse retina
against ischemic reperfusion injury through regulation of
antioxidation and activation of NF-κB. Oxid Med Cell Longev.
2015:9701562015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hernández C, Simó-Servat O and Simó R:
Somatostatin and diabetic retinopathy: Current concepts and new
therapeutic perspectives. Endocrine. 46:209–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cervia D, Casini G and Bagnoli P:
Physiology and pathology of somatostatin in the mammalian retina: A
current view. Mol Cell Endocrinol. 286:112–122. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cristiani R, Petrucci C, Dal Monte M and
Bagnoli P: Somatostatin (SRIF) and SRIF receptors in the mouse
retina. Brain Res. 936:1–14. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cervia D, Catalani E, Dal Monte M and
Casini G: Vascular endothelial growth factor in the ischemic retina
and its regulation by somatostatin. J Neurochem. 120:818–829. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mei S, Cammalleri M, Azara D, Casini G,
Bagnoli P and Dal Monte M: Mechanisms underlying somatostatin
receptor 2 down-regulation of vascular endothelial growth factor
expression in response to hypoxia in mouse retinal explants. J
Pathol. 226:519–533. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deissler HL, Lang GK and Lang GE: Capacity
of aflibercept to counteract VEGF-stimulated abnormal behavior of
retinal microvascular endothelial cells. Exp Eye Res. 122:20–31.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gelfand MV, Hagan N, Tata A, Oh WJ,
Lacoste B, Kang KT, Kopycinska J, Bischoff J, Wang JH and Gu C:
Neuropilin-1 functions as a VEGFR2 co-receptor to guide
developmental angiogenesis independent of ligand binding. Elife.
3:e037202014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Herzog B, Pellet-Many C, Britton G,
Hartzoulakis B and Zachary IC: VEGF binding to NRP1 is essential
for VEGF stimulation of endothelial cell migration, complex
formation between NRP1 and VEGFR2, and signaling via FAK Tyr407
phosphorylation. Mol Biol Cell. 22:2766–2776. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Celiker U, Ilhan N, Ozercan I, Demir T and
Celiker H: Octreotide reduces ischaemia-reperfusion injury in the
retina. Acta Ophthalmol Scand. 80:395–400. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rauca C, Schäfer K and Höllt V: Effects of
somatostatin, octreotide and cortistatin on ischaemic neuronal
damage following permanent middle cerebral artery occlusion in the
rat. Naunyn Schmiedebergs Arch Pharmacol. 360:633–638. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen L, Wang L, Zhang X, Cui L, Xing Y,
Dong L, Liu Z, Li Y, Zhang X, Wang C, et al: The protection by
octreotide against experimental ischemic stroke: Up-regulated
transcription factor Nrf2, HO-1 and down-regulated NF-κB
expression. Brain Res. 1475:80–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rojas M, Zhang W, Xu Z, Lemtalsi T,
Chandler P, Toque HA, Caldwell RW and Caldwell RB: Requirement of
NOX2 expression in both retina and bone marrow for diabetes-induced
retinal vascular injury. PLoS One. 8:e843572013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moran E, Ding L, Wang Z, Cheng R, Chen Q,
Moore R, Takahashi Y and Ma JX: Protective and antioxidant effects
of PPARα in the ischemic retina. Invest Ophthalmol Vis Sci.
55:4568–4576. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cervia D and Casini G: The neuropeptide
systems and their potential role in the treatment of mammalian
retinal ischemia: A developing story. Curr Neuropharmacol.
11:95–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dal Monte M, Ristori C, Videau C, Loudes
C, Martini D, Casini G, Epelbaum J and Bagnoli P: Expression,
localization, and functional coupling of the somatostatin receptor
subtype 2 in a mouse model of oxygen-induced retinopathy. Invest
Ophthalmol Vis Sci. 51:1848–1856. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adams RL, Adams IP, Lindow SW, Zhong W and
Atkin SL: Somatostatin receptors 2 and 5 are preferentially
expressed in proliferating endothelium. Br J Cancer. 92:1493–1498.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Spoerri PE, Afzal A, Li Calzi S, Shaw LC,
Cai J, Pan H, Boulton M and Grant MB: Effects of VEGFR-1, VEGFR-2,
and IGF-IR hammerhead ribozymes on glucose-mediated tight junction
expression in cultured human retinal endothelial cells. Mol Vis.
12:32–42. 2006.PubMed/NCBI
|
|
23
|
Muthusamy A, Lin CM, Shanmugam S, Lindner
HM, Abcouwer SF and Antonetti DA: Ischemia-reperfusion injury
induces occludin phosphorylation/ubiquitination and retinal
vascular permeability in a VEGFR-2-dependent manner. J Cereb Blood
Flow Metab. 34:522–531. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harhaj NS, Felinski EA, Wolpert EB,
Sundstrom JM, Gardner TW and Antonetti DA: VEGF activation of
protein kinase C stimulates occludin phosphorylation and
contributes to endothelial permeability. Invest Ophthalmol Vis Sci.
47:5106–5115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li X, Wang Q, Xu H, Tao L, Lu J, Cai L and
Wang C: Somatostatin regulates tight junction proteins expression
in colitis mice. Int J Clin Exp Pathol. 7:2153–2162.
2014.PubMed/NCBI
|
|
26
|
Zhao B, Cai J and Boulton M: Expression of
placenta growth factor is regulated by both VEGF and hyperglycaemia
via VEGFR-2. Microvasc Res. 68:239–246. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aiello LP, Bursell SE, Clermont A, Duh E,
Ishii H, Takagi C, Mori F, Ciulla TA, Ways K, Jirousek M, et al:
Vascular endothelial growth factor-induced retinal permeability is
mediated by protein kinase C in vivo and suppressed by an orally
effective beta-isoform-selective inhibitor. Diabetes. 46:1473–1480.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Amin RH, Frank RN, Kennedy A, Eliott D,
Puklin JE and Abrams GW: Vascular endothelial growth factor is
present in glial cells of the retina and optic nerve of human
subjects with nonproliferative diabetic retinopathy. Invest
Ophthalmol Vis Sci. 38:36–47. 1997.PubMed/NCBI
|
|
29
|
Behzadian MA, Windsor LJ, Ghaly N, Liou G,
Tsai NT and Caldwell RB: VEGF-induced paracellular permeability in
cultured endothelial cells involves urokinase and its receptor.
FASEB J. 17:752–754. 2003.PubMed/NCBI
|
|
30
|
Qaum T, Xu Q, Joussen AM, Clemens MW, Qin
W, Miyamoto K, Hassessian H, Wiegand SJ, Rudge J, Yancopoulos GD
and Adamis AP: VEGF-initiated blood-retinal barrier breakdown in
early diabetes. Invest Ophthalmol Vis Sci. 42:2408–2413.
2001.PubMed/NCBI
|
|
31
|
Wu HM, Yuan Y, Zawieja DC, Tinsley J and
Granger HJ: Role of phospholipase C protein kinase C, and calcium
in VEGF-induced venular hyperpermeability. Am J Physiol.
276:H535–H542. 1999.PubMed/NCBI
|
|
32
|
Joussen IS, Poulaki V, Tsujikawa A, Qin W,
Qaum T, Xu Q, Moromizato Y, Bursell SE, Wiegand SJ, Rudge J, et al:
Suppression of diabetic retinopathy with angiopoietin-1. Am J
Pathol. 160:1683–1693. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dal Monte M, Ristori C, Cammalleri M and
Bagnoli P: Effects of somatostatin analogues on retinal
angiogenesis in a mouse model of oxygen-induced retinopathy:
Involvement of the somatostatin receptor subtype 2. Invest
Ophthalmol Vis Sci. 50:3596–3606. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Imaizumi T, Itaya H, Nasu S, Yoshida H,
Matsubara Y, Fujimoto K, Matsumiya T, Kimura H and Satoh K:
Expression of vascular endothelial growth factor in human umbilical
vein endothelial cells stimulated with interleukin-1alpha-an
autocrine regulation of angiogenesis and inflammatory reactions.
Thromb Haemost. 83:949–955. 2000.PubMed/NCBI
|
|
35
|
Simorre-Pinatel V, Guerrin M, Chollet P,
Penary M, Clamens S, Malecaze F and Plouet J: Vasculotropin-VEGF
stimulates retinal capillary endothelial cells through an autocrine
pathway. Invest Ophthalmol Vis Sci. 35:3393–3400. 1994.PubMed/NCBI
|
|
36
|
Lee MJ, Thangada S, Claffey KP, Ancellin
N, Liu CH, Kluk M, Volpi M, Sha'afi RI and Hla T: Vascular
endothelial cell adherens junction assembly and morphogenesis
induced by sphingosine-1-phosphate. Cell. 99:301–312. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Amin MA, Volpert OV, Woods JM, Kumar P,
Harlow LA and Koch AE: Migration inhibitory factor mediates
angiogenesis via mitogen-activated protein kinase and
phosphatidylinositol kinase. Circ Res. 93:321–329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nacci C, Tarquinio M and Montagnani M:
Molecular and clinical aspects of endothelial dysfunction in
diabetes. Intern Emerg Med. 4:107–116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Breslin JW, Pappas PJ, Cerveira JJ, Hobson
RW II and Durán WN: VEGF increases endothelial permeability by
separate signaling pathways involving ERK-1/2 and nitric oxide. Am
J Physiol Heart Circ Physiol. 284:H92–H100. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Becker PM, Waltenberger J, Yachechko R,
Mirzapoiazova T, Sham JS, Lee CG, Elias JA and Verin AD:
Neuropilin-1 regulates vascular endothelial growth factor-mediated
endothelial permeability. Circ Res. 96:1257–1265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ackah E, Yu J, Zoellner S, Iwakiri Y,
Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, et
al: Akt1/protein kinase Balpha is critical for ischemic and
VEGF-mediated angiogenesis. J Clin Invest. 115:2119–2127. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bullard LE, Qi X and Penn JS: Role for
extracellular signal-responsive kinase-1 and −2 in retinal
angiogenesis. Invest Ophthalmol Vis Sci. 44:1722–1731. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jin J, Yuan F, Shen MQ, Feng YF and He QL:
Vascular endothelial growth factor regulates primate
choroid-retinal endothelial cell proliferation and tube formation
through PI3K/Akt and MEK/ERK dependent signaling. Mol Cell Biochem.
381:267–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Evans IM, Yamaji M, Britton G, Pellet-Many
C, Lockie C, Zachary IC and Frankel P: Neuropilin-1 signaling
through p130Cas tyrosine phosphorylation is essential for growth
factor-dependent migration of glioma and endothelial cells. Mol
Cell Biol. 31:1174–1185. 2011. View Article : Google Scholar : PubMed/NCBI
|