Introduction
The sea cucumber, Holothuria nobilis Selenka
(H. nobilis Selenka), is a spiny skinned invertebrate with a
wide distribution, spreading from the Fujian Dongshan Ocean to the
Xisha Islands in China. H. nobilis Selenka is rich in
biological compounds, including proteins, polysaccharides, vitamins
and triterpene glycoside. Of particular interest is triterpene
glycoside, the predominant secondary metabolite of the sea cucumber
with a range of biological and pharmacological activities,
including antitumor, antimicrobial and anti-inflammatory properties
(1). For example, impatienside A is
a type of triterpene glycoside isolated from the sea cucumber
Holothuria impatiens (2).
Impatienside A has been reported to be effective in the treatment
of various tumor cell lines, including HCT-116, A549 and HepG2
(2). The structure of impatienside A
is similar to that of bivittoside D and the glycoside shows greater
cytotoxicity than other potent anticancer drugs, including
etoposide (V-16) (2). Intercedenside
D-I, also a cytotoxic triterpene glycoside, is extracted from the
sea cucumber Mensamaria intercedens and inhibits the
proliferation of many human tumor cell lines (3). Another key glycoside is sulfated
saponin philinopside, a potential angiogenesis inhibitor isolated
from the sea cucumber Pentacta quandrangulari that shows
anti-angiogenic and antitumor activities (4).
Saponins are a main type of triterpene and are
predominantly found in the sea cucumber (5,6). Three
novel triterpene glycosides of the saponin category, namely
nobilisides A, B and C, were isolated from the sea cucumber H.
nobilis Selenka. Nobilisides A and C are non-sulfated
monoglycosides, while nobiliside B is a sulfated diglycoside.
Nobilisides A, B and C possess 22,25-epoxy side chains and
nobiliside A contains two conjugated double bonds [22 E, 24-diene
and 7,9(11)-diene], a structure
seldom found in other glycosides. All three glycosides show
substantial cytotoxicity against many tumor cell lines (7).
This cytotoxicity was validated in the present
study. Saponins were extracted from the concentrated liquid of
H. nobilis Selenka and demonstrated inhibitory activity
against human leukemic cell line K562, human leukemia cell line
U937, human lung cancer cell line A-549, human cervix carcinoma
cell line HeLa, human breast cancer cell line MCF-7 and human liver
carcinoma cell line HepG2.
Materials and methods
Materials
The human tumor cells lines (K562, U937, A-549,
HeLa, MCF-7 and HepG2) were purchased from the Shanghai Institute
of Cell Biology (Shanghai, China). Gibco™ RPMI-1640 medium was
purchased from Thermo Fischer Scientific, Inc. (Waltham, MA, USA).
Fetal bovine serum (FBS) was purchased from Shanghai Lanji Co.,
Ltd. (Shanghai, China). Neutral protease was from Nanning Pangbo
Biological Engineering Co., Ltd. (Nanning, China).
H. nobilis Selenka. H. nobilis Selenka was
collected in June 2012 in Fujian Dongshan Ocean (Fujian, China) and
identified by Professor Liao Yulin from Qingdao Ocean University
(Qingdao, China). The species was cultured in the Zhejiang
Pharmaceutical College (Fuzhou, China).
Saponin extraction from H. nobilis
Selenka
Saponin was extracted from H. nobilis Selenka
and its presence was detected as previously described, with slight
modifications (8). H. nobilis
Selenka (50 g) was washed, chopped and digested with 2% neutral
protease (v/v). Insoluble materials were discarded via filtration.
The digested solution was added to 30% v/v ethanol made from 95%
ethanol at 4°C for 24 h, then centrifuged at 3,800 × g for 10 min.
The supernatants were mixed with 60% ethanol (v/v) of 95% at 4°C
for 24 h and centrifuged at 3,800 × g for 10 min. The supernatant
was concentrated to 20% of its original volume through evaporation
of water and ethanol, and washed three times with 50 ml diethyl
ether (>98%) to remove fat content via a separator funnel. The
supernatant was further isolated three times with 150 ml of
water-saturated butanol. Alcohol was evaporated and saponins were
extracted into n-butanol. The 20 ml n-butanol fraction with
saponins was loaded onto a silica gel (200–300 mesh, 0.45 g/ml;
Qingdao Haiyang Chemical Co., Ltd., Qingdao, China) column, prior
to elution with 1,484 g/ml chloroform, 0.791 g/ml methanol and
water (7.5:2.5:1). The eluents (10 ml) were loaded onto a Zobax SB
C-18 type ODS reverse phase HPLC column (Agilent Technologies,
Inc., Santa Clara, CA, USA; mobile phase composition: 25:75:0.01
acetonitrile/water/acetic acid (v/v/v), at a flow rate of 2
ml/min), and eluted with 80% methanol (v/v) at the flow rate of 1.0
ml/min. Standard sample saponin (catalog no. 47036; Sigma-Aldrich,
Merck Millipore, Darmstadt, Germany) was used as an internal
control. A purified compound was obtained.
Structure determination
The molecular weight and chemical structure of the
final product was identified by gas chromatography-mass
spectrometry (GC-MS; Fisons Gas Chromatograph Model GC8000 series
8035 with MD800 Quadrupole mass spectrometer; SpectraLab
Scientific, Inc., Ontario, Canada). In the present study,
electrospray ionization-mass spectrometry (ESI-MS) of
[M+Na]+ions at M/Z 891 and ESI-MS of
[M-Na]−ions at M/Z 845 was conducted. The GC column was
a AB-35MS fused silica capillary column with dimensions 30×0.25×0.5
mm. The GC conditions were as following: An injection port
temperature of 250°C, an initial column temperature held isothermal
at 100°C before increase to 250°C at 6°C/min, then maintained at
250°C for 10 min. The ion source and interface temperatures were
200 and 250°C, respectively. Helium gas (180°C; 7.63 psi) was used
as the carrier gas at a speed of 1 ml/min. Spectra were obtained in
the electron ionization mode with 70-eV.
The chemical structure of the final product was
analyzed by a 600 MHz Varian Inova Nuclear Magnetic Resonance (NMR)
spectrometer (Varian, Inc., Palo Alto, CA, USA). The purified
saponin was dissolved in 500 ml of 10 mM NaN3, 1 mM
EDTA, 50 mM K3PO4, 0.1 M NaCl containing
H2O/D2O. NMR spectra were compared to
deuterated methanol (CD30D; catalog no. 151974; Sigma-Aldrich,
Merck Millipore) signals at δ 3.30 (1H) and 49.00 (13C). The
samples were dissolved in dimethyl sulfoxide-d6 (DMSO-d6; catalog
no. 547239) or deuterated chloroform (CDCl3; catalog no. 441333)
(both from Sigma-Aldrich, Merck Millipore) and/or CD3OD, dependent
on sample solubility. The observed chemical shift (δ) values are
presented as ppm and the coupling constant (J) values in H/Z.
A total of 10 mg purified saponin was mixed with KBr
salt. Infrared spectra were analyzed as KBr pellets on a 100 FTIR
spectrometer (Thermo Fisher Scientific, Inc.), between 4,000–400
cm−1.
xCELLigence Real-Time Cell Analysis
(RTCA)
K562, U937, A-549, HeLa, MCF-7 and HepG2 cell lines
were cultured in RPMI 1640 medium with 10% FBS at 37°C and 5%
CO2. Cells were sub-cultured every 2 days and harvested
at the exponential growth phase. Cells were then seeded at a
concentration of 5×104 cells/well into 100 µl medium and
different concentrations of purified saponin (dissolved in 2%
DMSO), all within 96-cell micro-plates (#CLS3595; Merck Millipore,
Darmstadt, Germany) and incubated for 24 h at 37°C and 5%
CO2. Different concentrations (20, 30, 40 and 50 mM) of
doxorubicin hydrochloride (D4035; Merck Millipore) were used as
controls as reported previously (9).
Each sample was measured in the E-plate 96 of a xCELLigence-system
(catalog no. 05232368001; ACEA Biosciences, Inc., San Diego, CA,
USA). Half maximal inhibitory concentration (IC50)
values were defined as the inhibition of cell lines by the novel
compound. Using the aforementioned method, the inhibitory effects
of 0.5 µg/ml purified saponin (the concentration used to measure
inhibition), on all cell lines, were measured for 24 h.
Apoptosis assay
The tumor cell lines were cultured at a density of
1×105 cells/ml with 0.5 µg/ml of purified saponin for 24
h. Cells were washed two times with phosphate-buffered saline (PBS)
and re-suspended in 250 µl binding buffer (catalog no. 556547; BD
Biosciences, San Jose, CA, USA), 5 µl Annexin V-FITC (#A9210) and
10 µl propidium iodide (#81845) (both from Merck Millipore). The
mixture was incubated at 25°C for 5 min in the dark and measured
using a fluorescence microscope (#BX-50; Olympus Corporation,
Tokyo, Japan). A Dako Cyan flow cytometer (Agilent Technologies,
Inc.) was used for quantification.
Statistical analysis
The data from different groups were analyzed using
Student's t-test via the SPSS 20.0 software (IBM SPSS, Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Structure determination of purified
compounds
Purified saponin was obtained as previously
described (10). The chemical
characteristics of the purified compound include a solid state of a
colorless crystal powder at room temperature, a melting point of
212.4–214.4°C and positive Liebermann-Burchard and molish
reactions. Table I shows the
13CNMR and 1HNMR chemical shifts along with
the nuclear overhauser effect spectroscopy (NOESY) and the
heteronuclear multiple-bond correlation spectroscopy (HMBC)
correlations for the aglycone moiety of purified saponin nobiliside
D. Table II shows the
13CNMR and 1HNMR chemical shifts and NOESY
and HMBC correlations for the sugar moiety of nobiliside D (in
pyridine-d5:D2O, 4:1, 600/150 MHz). IR
Spectrum analysis demonstrated that the purified saponin exhibited
the following characteristics: Max-3418, Strong Broad Peak
(Hydroxy), 1762 (Carbonyl), 1637 (Double Bond), 1253, 1069.
 | Table I.13C NMR and 1H
NMR chemical shifts and NOESY and HMBC correlations for the aglycon
moiety of nobiliside D. |
Table I.
13C NMR and 1H
NMR chemical shifts and NOESY and HMBC correlations for the aglycon
moiety of nobiliside D.
| Position | δC | δH (m,
J in Hz) | NOESY | HMBC |
|---|
| 1 | 36.4 | 1.38 (1H, m, α), 1.86
(1H, m, β) |
|
|
| 2 | 27.4 | 1.76 (1H, m, α), 2.02
(1H, m, β) | H-3α, H3-30,
H3-19 |
|
| 3 | 88.8 | 3.14 (1H, dd, 4.2,
12.0, α) | H-xyl1, H-5α,
H3-31 |
C:xyl1 |
| 4 | 40.1 |
|
|
|
| 5 | 52.8 | 1.00 (1H, d, 10.2,
α) | H-3α, H3-31 |
|
| 6 | 28.4 | 2.02 (2H, m) |
|
|
| 7 | 21.2 | 1.49 (1H, m, α), 1.74
(1H, m, β) |
|
|
| 8 | 41.0 | 3.36 (1H, t, 9.6,
β) | H-7β, H-6β, H-15β,
H3-19 |
|
| 9 | 153.9 |
|
|
|
| 10 | 39.8 |
|
|
|
| 11 | 115.6 | 5.62 (1H, d, 4.2,
β) | H-12β, H-1β,
H3-19 | C:10,8,13,12 |
| 12 | 71.6 | 4.99 (1H, dd, 5.4,
12.0, β) | H-11β | C:14,9 |
| 13 | 58.9 |
|
|
|
| 14 | 46.0 |
|
|
|
| 15 | 36.9 | 1.82 (1H, m, α),
1.40(1H, m, β) | H-8β | C:17 |
| 16 | 35.6 | 2.41 (1H, ddd, α),
2.97 (1H, dd, 6.0, 14.4, β) | H-15α,
H3-32 H-15β | C:14,17 |
| 17 | 89.8 |
|
|
|
| 18 | 174.6 |
|
|
|
| 19 | 22.7 | 1.40 (3H, s) | H-1β, H-2β, H3-30,
H-8β, H-11β | C:1,10,5,9 |
| 20 | 86.7 |
|
|
|
| 21 |
18.9 | 1.76 (3H, s) |
| C:22,17,20 |
| 22 |
80.7 | 4.32 (1H, m,
α) | H-16β | C:21,20,17 |
| 23 |
28.0 | 1.45 (2H, m) |
| C:24,25 |
| 24 |
38.5 | 1.64 (2H, m) |
| C:25,27 |
| 25 |
81.4 |
|
|
|
| 26 |
28.7 | 1.20 (3H, s) |
| C:27,25,24 |
| 27 |
27.1 | 1.19 (3H, s) |
| C:26,24,25 |
| 30 |
16.8 | 1.13 (3H, s) | H3-19 | C:31,3,4,5 |
| 31 |
28.2 | 1.28 (3H, s) | H-3α, H-5 | C:30,3,4,5 |
| 32 |
20.4 | 1.65 (3H, s) | H-16α | C:13,14,8,15 |
 | Table II.13C NMR and 1H
NMR chemical shifts and NOESY and HMBC correlations for the sugar
moiety of nobiliside D. |
Table II.
13C NMR and 1H
NMR chemical shifts and NOESY and HMBC correlations for the sugar
moiety of nobiliside D.
| Sugar | Position | δC | δH (m,
J in Hz) | NOESY | HMBC |
|---|
| Xyl | 1 | 105.3 | 4.71 (1H, d,
7.2) | H-3, H-3′, H-5′,
H-1″ | C-3, C-1″ |
|
| 2 |
83.4 | 4.04 (1H, m) |
| C-1′,C-3′,C-1″
H-5′ |
|
| 3 |
75.4 | 4.28 (1H, m) |
| C-2′, C-4′ |
|
| 4 |
76.0 | 5.10 (1H, m) |
| C-2′, C-3′ |
|
| 5 |
64.2 | 3.73 (1H, m, α),
4.75 (1H, dd, 4.8, 11.4, β) | H-1′, H-4′ | C-4′, C-1′
C-4′ |
| Xyl (repeat) | 1 | 105.9 | 4.83 (1H, d,
7.2) | H-3′, H-5″,
H-2′ | C-2′ |
|
| 2 |
76.8 | 4.02 (1H, m) |
| C-3′, C-1″ |
|
| 3 |
77.7 | 4.22 (1H, m) |
| C-2′ |
|
| 4 |
69.9 | 3.68 (1H, m) |
|
|
|
| 5 |
65.2 | 3.74 (1H, m, α),
4.55 (1H, dd, 4.2, 10.8, β) |
|
|
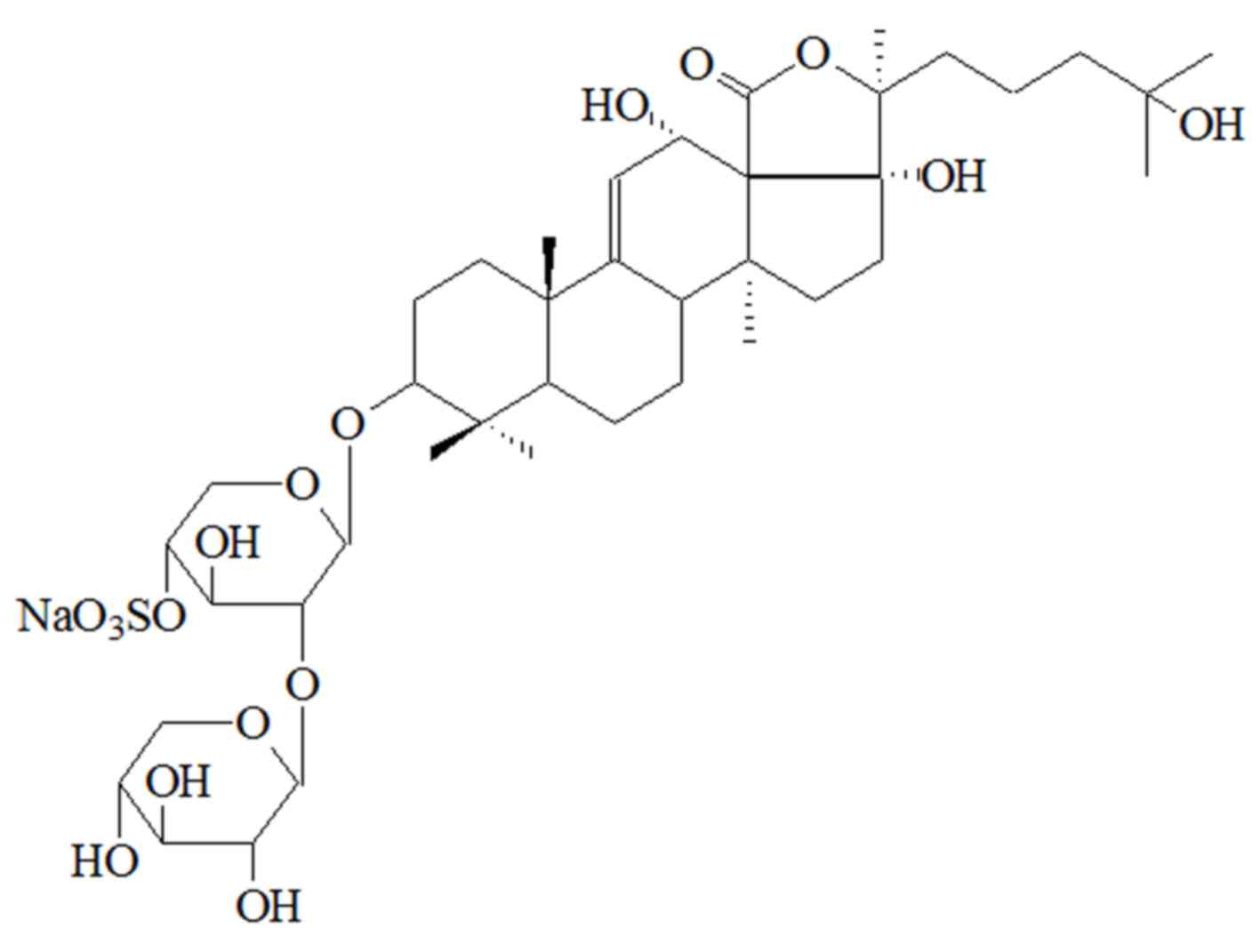
The 13CNMR and 1HNMR results
indicate that the purified compound is nobiliside D (11), a novel type of saponin with molecular
formula C40H61O17SNa and chemical
name 3-O-[-β-D-xylopyranosyl(1–2)-4′-O-sulfonate-β-D-xylopyranosyl-alkoxy-9-ene-3β,
12α, 17α, 25β-four alcohol. The structure of nobiliside D is shown
in Fig. 1.
Antitumor activity
xCELLigence-system was used to test the cytotoxic
effects of nobiliside D on the human tumor cell lines K562, U937,
A-549, HeLa, MCF-7 and HepG2, as described previously. As shown in
Table III, nobiliside D had strong
inhibitory effects on tumor lines K562 and MCF-7, with
IC50 values of 0.83±0.14 and 0.82±0.11 µg/ml,
respectively, although all the inhibitory effects were lower than
that of doxorubicin hydrochloride, except for MCF-7.
 | Table III.Antitumor activity of nobiliside D on
different tumor cell lines. |
Table III.
Antitumor activity of nobiliside D on
different tumor cell lines.
| Tumor cell
lines | Nobiliside
IC50 (µg/ml) | Doxorubicin
hydrochloride IC50 (µg/ml) |
|---|
| A549 | 2.43±0.16 | 1.58±0.45 |
| HeLa | 2.90±0.21 | 1.74±0.16 |
| K562 | 0.83±0.14 | 0.01±0.00 |
| MCF-7 | 0.82±0.11 | 1.32±0.18 |
| U937 | 2.97±0.21 | 0.01±0.02 |
| HepG2 | 1.43±0.08 | 0.38±0.04 |
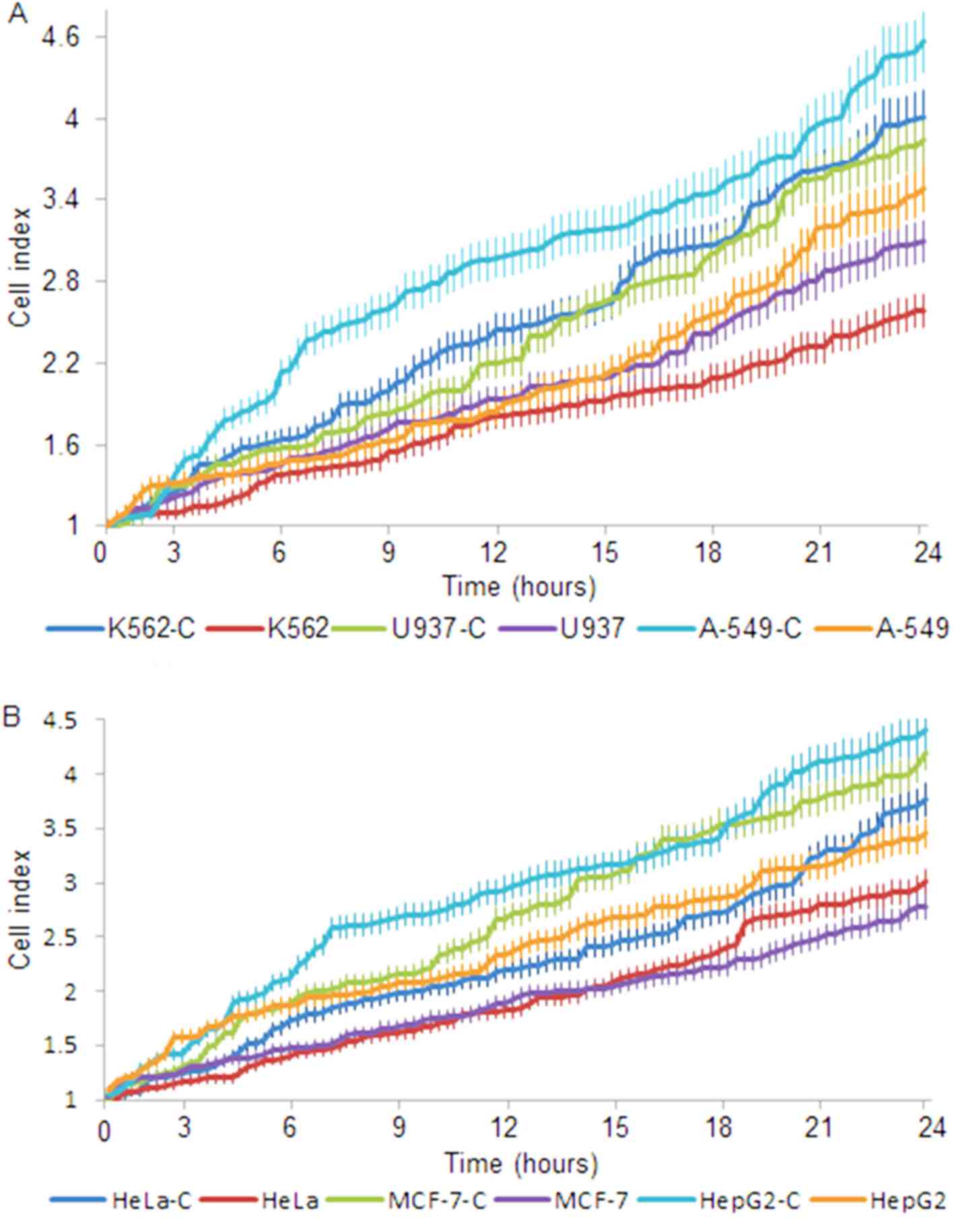
The growth rate of all cell lines was affected by
nobiliside D (Fig. 2). The growth
rate of K562, U937 and A-549 cells compared with controls (K562-C,
U937-C and A-549-C) was markedly decreased after 1 day of culture
(Fig. 2A, P<0.05). The growth
rate of HeLa, MCF-7 and HepG2 cells compared with controls (HeLa-C,
MCF-7-C and HepG2-C) was also markedly different after 1 day of
culture (Fig. 2B, P<0.05). The
results indicate that nobiliside D has varying inhibitory effects
on the different tumor cell lines and that therapy utilizing
nobiliside D is potentially most suitable for the treatment of
human leukemia and breast cancer.
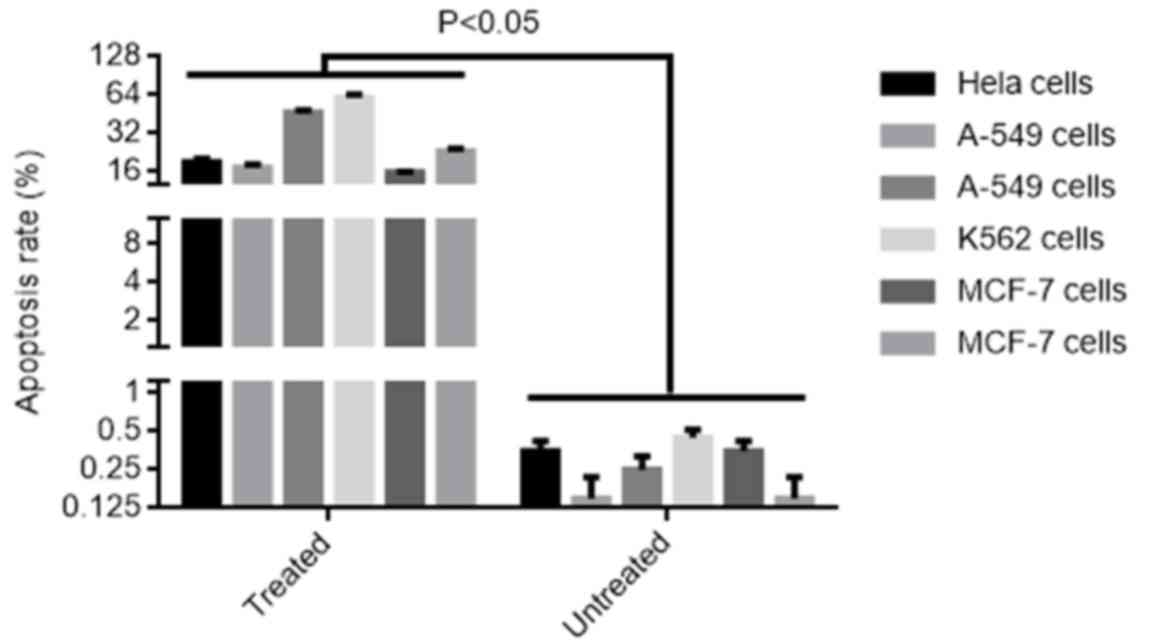
Nobiliside D promoted apoptosis of
human cell lines
A greater apoptotic rate was observed in human tumor
cell lines HeLa, A-549, K562, MCF-7, U937 and HepG2 treated by
nobiliside D (0.5 µg/ml) for 24 h compared to un-treated cells
(P<0.05). 18.2% of Hela cells, 16.4% of A-549 cells, 45.8% of
K562 cells, 58.7% of MCF-7 cells, 15.6% of U937 cells and 22.4% of
HepG2 cells were apoptotic, while only less than 0.5% of untreated
control cells were apoptoic (Fig.
3). Additionally, dead cells were identified in all cell line
cultures, as previously described (12). The percentages of dead cells were
approximately 0.6, 0.9, 2.6. 3.4, 0.7 and 2.0% dead cells for HeLa,
A-549, K562, MCF-7, U937 and HepG2 cells, respectively.
Discussion
The present study demonstrated that nobiliside D can
be extracted from the sea cucumber H. nobilis Selenka. In
contrast to the typical polysaccharide extraction protocol using an
alcohol precipitation method, extraction of nobiliside D is based
on its solubility in specific concentrations of alcohol. Therefore,
nobiliside D was extracted from filtrate using 60% ethanol (v/v),
enabling precipitation of the compound. The structure of nobiliside
D was subsequently identified by NMR spectroscopy and analysis of
the spectra of the active fractions. Additionally, the nobiliside D
extracted from H. nobilis Selenka was identified using
ESI-MS and NMR analysis. The novel compound was identified as the
saponin family member nobiliside D. This extraction method achieves
simultaneous extraction from H. nobilis Selenka, as well as
improving the extraction efficiency and shortening the time of
purification.
Saponins are established to have anti-tumor activity
(13,14). Saponins are the main metabolites of
sea cucumber (5,6) and currently >10 types of saponin
have been isolated and purified (15,16). The
abundance of novel compounds from sea cucumber suggests potential
for biopharmaceutical applications (17–20).
However, the saponin family member nobiliside D has been seldom
isolated and its activity remains unknown. The present study
indicates nobiliside D has clear inhibitory activities on the tumor
cell lines tested and may offer potential for development as a
novel drug for the treatment of various cancers.
However, studies are currently limited. For example,
the detailed molecular mechanisms of the functioning of nobiliside
D, which is a novel triterpenoid saponin, remain to be elucidated.
In addition, there are numerous natural products from H.
nobilis Selenka with anti-tumor activities and the activity of
nobiliside D should be compared with these other compounds.
Finally, the adverse effects of nobiliside D were not investigated,
as these experiments would require in vivo models.
Collectively, the GC-MS and NMR analyses indicate
that the novel compound nobiliside D was isolated from the sea
cucumber H. nobilis Selenka in the present study. Nobiliside
D demonstrated antiproliferative activities against the human tumor
cell lines tested, particularly with human leukemic cell line K562
and breast cancer cell line MCF-7. The compound inhibits
proliferation of these cells by promoting cellular apoptosis. The
results suggest that nobiliside D could be developed as a potential
drug for the treatment of breast cancer and human leukemia.
However, the present study was limited to the cellular level and
therefore, to verify the results, the compound requires testing in
an animal model or preclinical trial. Further studies are required
to enable full use of the therapeutic natural products of sea
cucumber in the future.
Acknowledgements
The present study was financially supported by
grants from the Zejiang Province Natural Science Foundation awarded
to Jia-Jia Zhang (grant no. Y2110082), the Ningbo Research Funding
of Industrial Projects awarded to Jia-Jia Zhang (grant no.
2012B10059), the Ningbo Municipal Natural Science Foundation
awarded to Ke-Qi Zhu (grant no. 2012A610258, and the Traditional
Chinese medicine Research Fund of Zhejiang Province awarded to
Qi-Ke Zhu (grant no. 2011ZB125).
References
|
1
|
Aminin DL, Menchinskaya ES, Pisliagin EA,
Silchenko AS, Avilov SA and Kalinin VI: Anticancer activity of sea
cucumber triterpene glycosides. Mar Drugs. 13:1202–1223. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun P, Liu BS, Yi YH, Li L, Gui M, Tang
HF, Zhang DZ and Zhang SL: A new cytotoxic lanostane-type
triterpene glycoside from the sea cucumber Holothuria impatiens.
Chem Biodivers. 4:450–457. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zou Z, Yi Y, Wu H, Yao X, Du L, Jiuhong W,
Liaw CC and Lee KH: Intercedensides D-I, cytotoxic triterpene
glycosides from the sea cucumber Mensamaria intercedens Lampert. J
Nat Prod. 68:540–546. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tong Y, Zhang X, Tian F, Yi Y, Xu Q, Li L,
Tong L, Lin L and Ding J: Philinopside A, a novel marine-derived
compound possessing dual anti-angiogenic and anti-tumor effects.
Int J Cancer. 114:843–853. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu S, Ye X, Chen L, Xie X, Zhou Q, Lian XY
and Zhang Z: Cytotoxic and anti-colorectal tumor effects of
sulfated saponins from sea cucumber Holothuria moebii.
Phytomedicine. 22:1112–1119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu S, Ye X, Huang H, Peng R, Su Z, Lian XY
and Zhang Z: Bioactive sulfated saponins from sea cucumber
Holothuria moebii. Planta Med. 81:152–159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu J, Yi YH, Tang HF, Wu HM, Zou ZR and
Lin HW: Nobilisides A-C, three new triterpene glycosides from the
sea cucumber Holothuria nobilis. Planta Med. 72:932–935. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mona MH, Omran NE, Mansoor MA and
El-Fakharany ZM: Antischistosomal effect of holothurin extracted
from some Egyptian sea cucumbers. Pharm Biol. 50:1144–1150. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shanskij YD, Ershov YA and Pechennikov VM:
A method for evaluation of therapeutic dose of doxorubicin
hydrochloride using breast tumor cell culture MCF-7. Bull Exp Biol
Med. 148:464–467. 2009.(In English, Russian). View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu X, Ye W, Mo Z, Yu B, Zhao S, Wu H, Che
C, Jiang R, Mak TC and Hsiao WL: Five new Ocotillone-type saponins
from Gynostemma pentaphyllum. J Nat Prod. 67:1147–1151. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Honey-Escandón M, Arreguín-Espinosa R,
Solís-Marín FA and Samyn Y: Biological and taxonomic perspective of
triterpenoid glycosides of sea cucumbers of the family
Holothuriidae (Echinodermata, Holothuroidea). Comp Biochem Physiol
B Biochem Mol Biol. 180:16–39. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Imir N, Aydemir E, Simsek E, Gokturk RS,
Yesilada E and Fiskin K: Cytotoxic and immunomodulatory effects of
Ebenus boissieri Barbey on breast cancer cells. Genet Mol Res.
15:2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Man S, Chai H, Qiu P, Liu Z, Fan W, Wang J
and Gao W: Turmeric enhancing anti-tumor effect of Rhizoma paridis
saponins by influencing their metabolic profiling in tumors of H22
hepatocarcinoma mice. Pathol Res Pract. 211:948–954. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weng A, Thakur M, Beceren-Braun F, Bachran
D, Bachran C, Riese SB, Jenett-Siems K, Gilabert-Oriol R, Melzig MF
and Fuchs H: The toxin component of targeted anti-tumor toxins
determines their efficacy increase by saponins. Mol Oncol.
6:323–332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bahrami Y and Franco CM: Structure
elucidation of new acetylated saponins, Lessoniosides A B, C, D and
E and non-acetylated saponins, Lessoniosides F and G, from the
viscera of the sea cucumber Holothuria lessoni. Mar Drugs.
13:597–617. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bahrami Y, Zhang W, Chataway T and Franco
C: Structure elucidation of five novel isomeric saponins from the
viscera of the sea cucumber Holothuria lessoni. Mar Drugs.
12:4439–4473. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cui C, Cui N, Wang P, Song S, Liang H and
Ji A: Sulfated polysaccharide isolated from the sea cucumber
Stichopus Japonicus against PC12 hypoxia/reoxygenation injury by
inhibition of the MAPK signaling pathway. Cell Mol Neurobiol.
35:1081–1092. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang Z, Sun J and Xu Z: Beneficial effects
of rhodotorula sp. C11 on growth and disease resistance of juvenile
japanese spiky sea cucumber Apostichopus Japonicus. J Aquat Anim
Health. 27:71–76. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nguyen TH and Kim SM: Alpha-Glucosidase
inhibitory activities of fatty acids purified from the internal
organ of sea cucumber Stichopus japonicas. J Food Sci.
80:H841–H847. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo Y, Ding Y, Xu F, Liu B, Kou Z, Xiao W
and Zhu J: Systems pharmacology-based drug discovery for marine
resources: An example using sea cucumber (Holothurians). J
Ethnopharmacol. 165:61–72. 2015. View Article : Google Scholar : PubMed/NCBI
|