Introduction
The occurrence, development and metastasis of tumors
are closely related to angiogenesis due to the fact that blood
vessels provide the necessary oxygen supply, nutrition, metabolite
and avenue for metastasis to maintain the rapid growth of tumors
(1–3). Angiogenesis, which is the generation of
novel blood vessels, occurs by two completely different processes
(4–6). In the first, the required vascular
endothelial cells arise from the sprouting of existing blood
vessels. In the second, they are derived from recruited endothelial
precursor cells, which are a type of blast cell with the potential
to differentiate into clonal endothelial cells in vitro as
well as participate in cardiovascular generation in vivo
(7). On their surface, they
characteristically express cluster of differentiation (CD)34,
vascular endothelial growth factor receptor 2 (VEGFR2) or kinase
domain receptor (8,9).
A large number of basic and clinical studies have
indicated that the number of endothelial progenitor cells (EPCs) is
closely related to tumor size, prognosis and therapy response
(10–13). Evidence from animal models suggests
that the EPC level in the peripheral circulation has some relevance
to tumor volume (13). The number of
EPCs in circulation has been identified to alter with anti-tumor
and anti-angiogenesis therapies. Igreja et al (14) suggested that the EPC level in the
peripheral blood of patients with lymphoma was related to the
efficacy of the therapy. This hypothesis was supported by the fact
that the EPC level in patients with complete remission decreased,
while EPC levels continued to rise or did not change in those with
partial remission or no response to therapy. In addition, it was
revealed that tumor size and angiogenesis were associated with the
number of EPCs in lymph nodes. Ho et al (15) indicated that, in patients with
advanced non-surgically treated hepatocellular carcinoma (HCC), the
EPC level in circulation was significantly higher compared with
patients with resectable HCC, suggesting that the number of EPCs in
the peripheral circulation may be used to determine the prognosis
of HCC patients.
Currently, EPC detection methods include clone
counting and characteristic index-based flow cytometry, of which
the latter may be divided into dual-platform counting and
single-platform counting (14,16,17).
Dual-platform counting, which involves two parallel tubes and two
devices, exhibits large variations in results. Conversely,
single-platform counting uses commercialized fluorescent
microspheres, which are expensive and easily adhere. Artificially
synthesized fluorescent microspheres have a different sedimentation
rate than cells, leading to unreliable results (18–20). In
our previous study, CFSE-labeled cells were used to replace
commercial fluorescent microspheres (21). We suggested that these
fluorescence-labeled cells were stable and did not easily adhere;
thus, the test results were reliable (21).
Due to the clinical value of EPCs, establishing an
improved complete EPC counting method is crucial. The present study
used single-platform flow cytometry technology with CFSE-labeled
cell fluorescent microspheres as the internal control to determine
the number of EPCs in peripheral blood and subsequently verify the
reliability of this technology from a biological standpoint.
Furthermore, this recently developed technology was used to detect
the changes in EPC number following tumor anti-angiogenic therapy.
Subsequently, the clinical value of using CFSE-labeled cell
microspheres with single-platform flow cytometry for determining
EPC number in peripheral blood was verified.
Materials and methods
Preparation and identification of
artificial cell microspheres
A total of 50 µg (1 vial) of CFSE (Molecular Probes;
Thermo Fisher Scientific Inc., Waltham, MA, USA) was dissolved in
18 µl of dimethyl sulfoxide to prepare the original solution with a
final concentration of 5 mmol, which was stored at −20°C.
Subsequently, 1 g of paraformaldehyde (PFA, Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) was dissolved in 90 ml of distilled water
and 10 ml of 10X phosphate-buffered saline (PBS) was added to
prepare a 1% PFA solution. The THP-1 human acute leukemia cell line
(Cell Bank of Shanghai Institute, Shanghai, China) was cultured in
RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS; GE
Heathcare Life Sciences, Chalfont, UK). THP-1 cells were washed
with PBS three times and resuspended to a concentration of
1×106 cells/ml. Subsequently, 1 µl CFSE was added for
each ml of cell suspension (to a final concentration of 5 µmol/l)
followed by incubation at 37°C for 10 min. The original medium was
added until a volume that was five times the original volume was
achieved to terminate the marking procedure. The mixture was placed
in an ice bath between 0 and 8°C for 5 min, followed by washing
three times with fresh medium. Cells were resuspended in PBS
supplemented with 1% PFA, with a cell concentration of
1×106 cells/ml and stored at 4°C until subsequent use.
Non-marked THP-1 cells were used as a control. The prepared cell
mixture, with artificial cell microspheres, was subsequently
evaluated using flow cytometry.
Single-platform flow cytometry for
determining the number of EPCs in peripheral blood
A total of 10 ml of human peripheral blood
(anticoagulated with 1.8 mg/ml EDTA-K2) obtained from healthy
volunteers was harvested for the extraction of mononuclear cells
and the sample was divided into four parts, with respective volumes
of 5, 2.5, 1.25 and 0.625 ml. Samples underwent negative selection,
in which CD45 antibody-coated magnetic beads (Dynabeads; Thermo
Fisher Scientific, Inc.) were added and the mixture was subjected
to a magnetic field to adsorb cells that were able to bind the CD45
antibody-coated magnetic beads, thus removing non-EPC components.
CD34, CD133 and KDR, commonly used membrane markers to define EPCs,
were detected in cells by flow cytometry as described previously
(4). Subsequently, the target cells
were pre-treated with an Fc-receptor-blocking reagent (Miltenyi
Biotec GmbH, Bergisch-Gladbach, Germany) to prevent non-specific
binding and were incubated with an APC-conjugated-CD34 antibody
(cat. no. 340441; 1:167; BD Biosciences, San Jose, CA, USA), a
phycoerythrin-conjugated anti-KDR antibody (cat. no. FAB357p;
1:100; R&D Systems, Inc., Minneapolis, MN, USA) and a
phycoerythrin-conjugated anti-CD133 antibody (cat. no. 130-080-801;
1:100; Miltenyi Biotec GmbH) at 4°C for 40 min. A total of 10,000
CFSE-labeled microspheres were added to the test sample and washed
with PBS three times. The sample was thoroughly mixed before
counting. Red blood cells were lysed with ammonium chloride (BD
Biosciences, San Jose, CA, USA) and a total of 106
events were recorded on a FACS Calibur cytometer (BD Biosciences).
Data were analyzed with CellQuest software (version 5.2.1; BD
Biosciences).
The absolute number of cells inside the test sample
(ND) was calculated using the following formula: Absolute number of
cells=target cell number/number of cell microspheres × added number
of cell microspheres.
Identifying EPCs and determining the
number of EPCs in peripheral blood
A total of 10 ml of human peripheral blood was
collected and divided into four parts, with volumes of 5, 2.5, 1.25
or 0.625 ml. A single karyoplast was obtained by the density
centrifugation method and planted onto a human fibronectin-coated
culture plate and cultured in M199 medium (Invitrogen; Thermo
Fisher Scientific, Inc.) supplemented with 20% FBS, 10 ng/ml VEGF,
100 ng/ml penicillin and 100 ng/ml streptomycin for 2 days.
Following culturing for 2 days, the mature endothelial cells had
adhered to the wall and the non-wall-adherent cells were collected
and re-planted onto human fibronectin-coated culture plates for
final counting. The medium was changed once every 3 days and the
non-wall-adherent cells were washed off with PBS 7 days later.
Subsequently, 2.4 mg/l of
1,1′-dioctadecy1-3,3,3′3′-tetramethyl-indocarbocyanin
perchlorate-labeled-acetylated-low density lipoprotein (Thermo
Fisher Scientific, Inc.) was added to the cultured cells, followed
by incubation at 37°C in an atmosphere containing 5% CO2
for 12 h. Cells were fixed with 2% PFA for 30 min, followed by
washing with D-Hank's solution (GE Healthcare Life Sciences) twice.
A total of 10 µg/ml fluorescein isothiocyanate-Ulex Europaeus
Agglutinin-I (Sigma-Aldrich; Merck KGaA) was added and the mixture
was incubated at 37°C for 1 h. Cells were observed under a
fluorescence microscope (magnification, ×40; CKX53; Olympus
Corporation, Tokyo, Japan) and cells with positive dual-staining
were considered to be differentiated EPCs.
The number of clones was determined under a
microscope and the number of EPCs was calculated using the
following formula: EPC concentration=number of colonies/original
collected blood volume.
Detecting the changes in EPC number in
the peripheral blood of patients with cancer prior to and following
the administration of anti-angiogenic agents
A total of 20 patients with solid tumors (10 cases
of liver cancer, 6 cases of osteosarcoma and 4 cases of stomach
cancer) were selected according to the standards for clinical
treatment with anti-angiogenic agents. Patients were enrolled
between March 2014 and February 2015 and were aged 25 to 59 years
old, with a male: female ratio of 3:2. Patients had no underlying
conditions, history of surgery or allergies. The inclusion criteria
were as follows: Clear diagnosis of solid tumors, no myocardial
infarction and intracranial hemorrhage within a month, no organ
infarction and deep venous thrombosis, no significant systemic
infection, no chemotherapy radiotherapy history nearly a month and
no other cancer treatment, including targeting medical treatment.
The exclusion criteria were: Neutrophil count
<1.5×109/l or platelet count
<100×109/l, women of childbearing age who serum
pregnancy test was positive or long-term use of immunosuppressive
agents after organ transplantation. The Ethics Committee of Gongli
Hospital approved the study protocol, and written informed consent
was obtained from all participating subjects. A total of 20 ml
blood was harvested prior to and following treatment and the
testing method was the same as described above.
The absolute number of cells in the test sample (ND)
was calculated using the following formula: Absolute number of
cells=target cell number/number of cell microspheres × added number
of cell microspheres. The method for the in vitro clonogenic
counting assay was the same as described above.
Statistical analysis
Data were analyzed using Statistical Package for the
Social Sciences (SPSS) software (version 13.0; SPSS Inc., Chicago,
IL, USA). One-way analysis of variance with Dunnet's post test was
used for statistical evaluation of significant differences among
the groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Preparation and identification of
artificial cell fluorescent microspheres
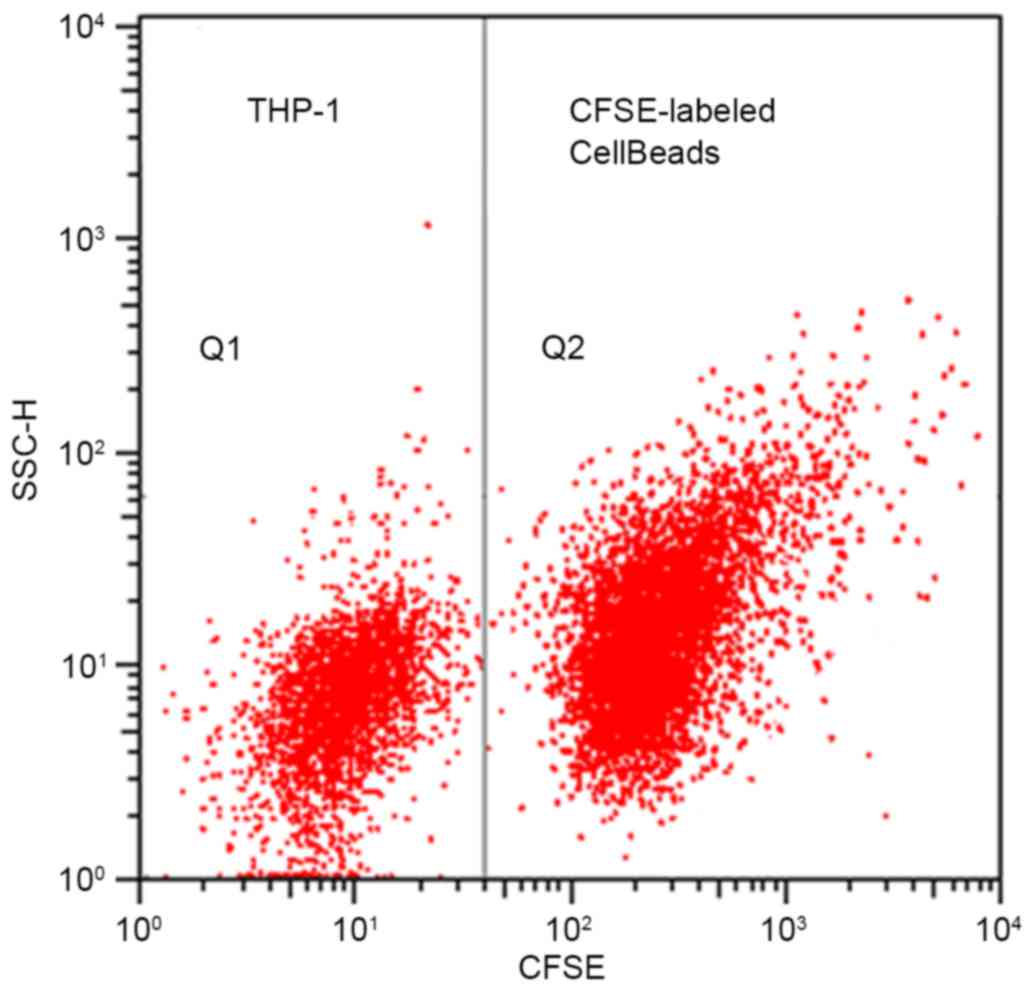
The cell fluorescent microspheres exhibited strong
homogeneous fluorescence (Fig. 1;
Q2) and the average fluorescence intensity was strong. Thus,
CFSE-labeled cell beads were easily distinguished from non-labeled
THP-1 cells (Fig. 1; Q1) and with
maintained fluorescence, indicating that the obtained cell
microspheres were feasible for the intended application.
Detection of EPCs in peripheral blood
using single-platform flow cytometry
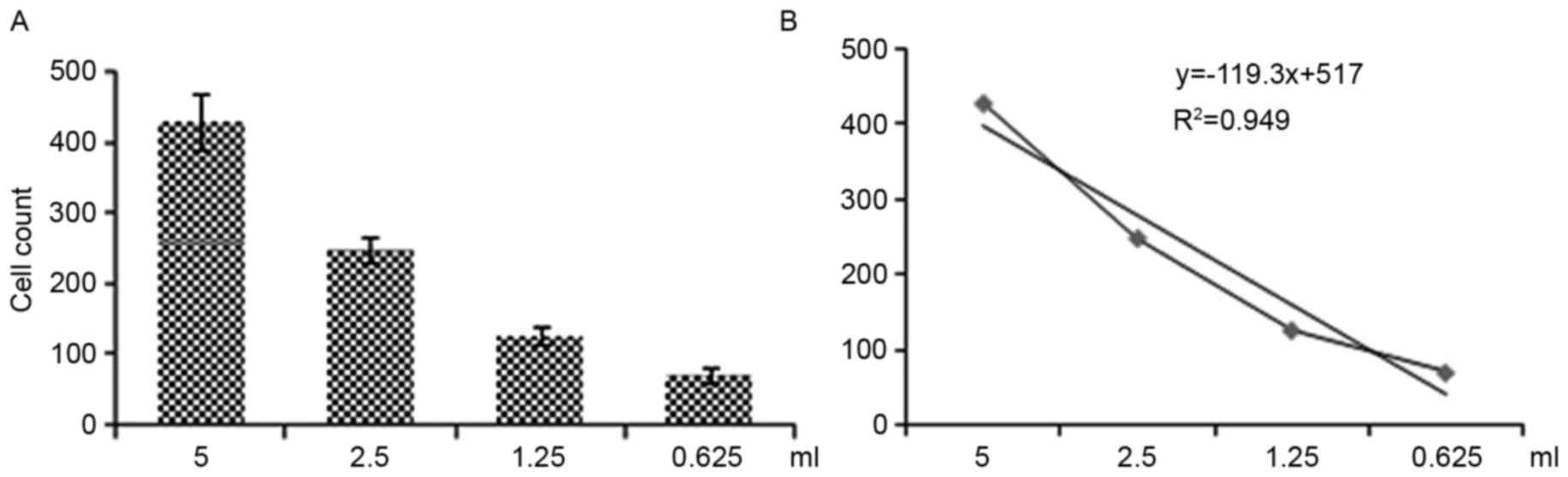
Regression curves for EPC number changed according
to the reduction in the original blood sample volume (Fig. 2), indicating that this method was
able to determine the number of EPCs in peripheral blood.
Detection of EPCs in peripheral blood
using an in vitro clonogenic counting assay
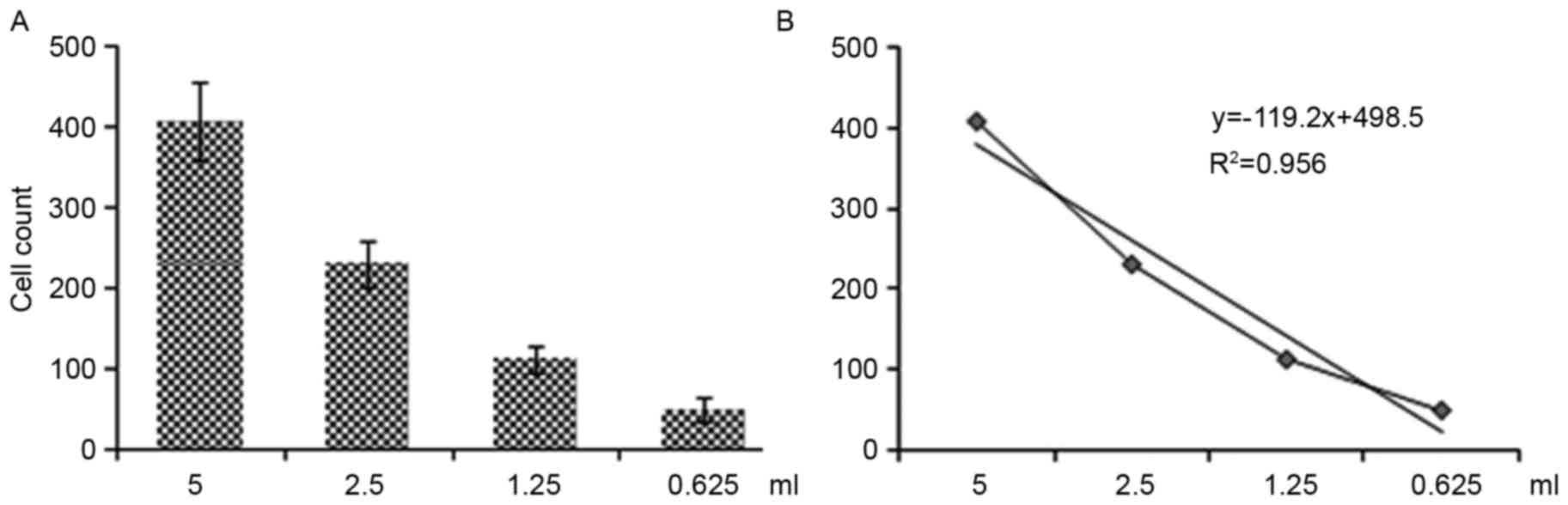
Regression curves for EPC number altered according
to the reduction in the original blood sample volume (Fig. 3), indicating that this method was
able to determine the number of EPCs in peripheral blood. When
comparing Figs. 2 and 3, there is a clear consistency between
these two detection methods, indicating that single-platform flow
cytometry may be used to feasibly and accurately determine the
number of EPCs in peripheral blood.
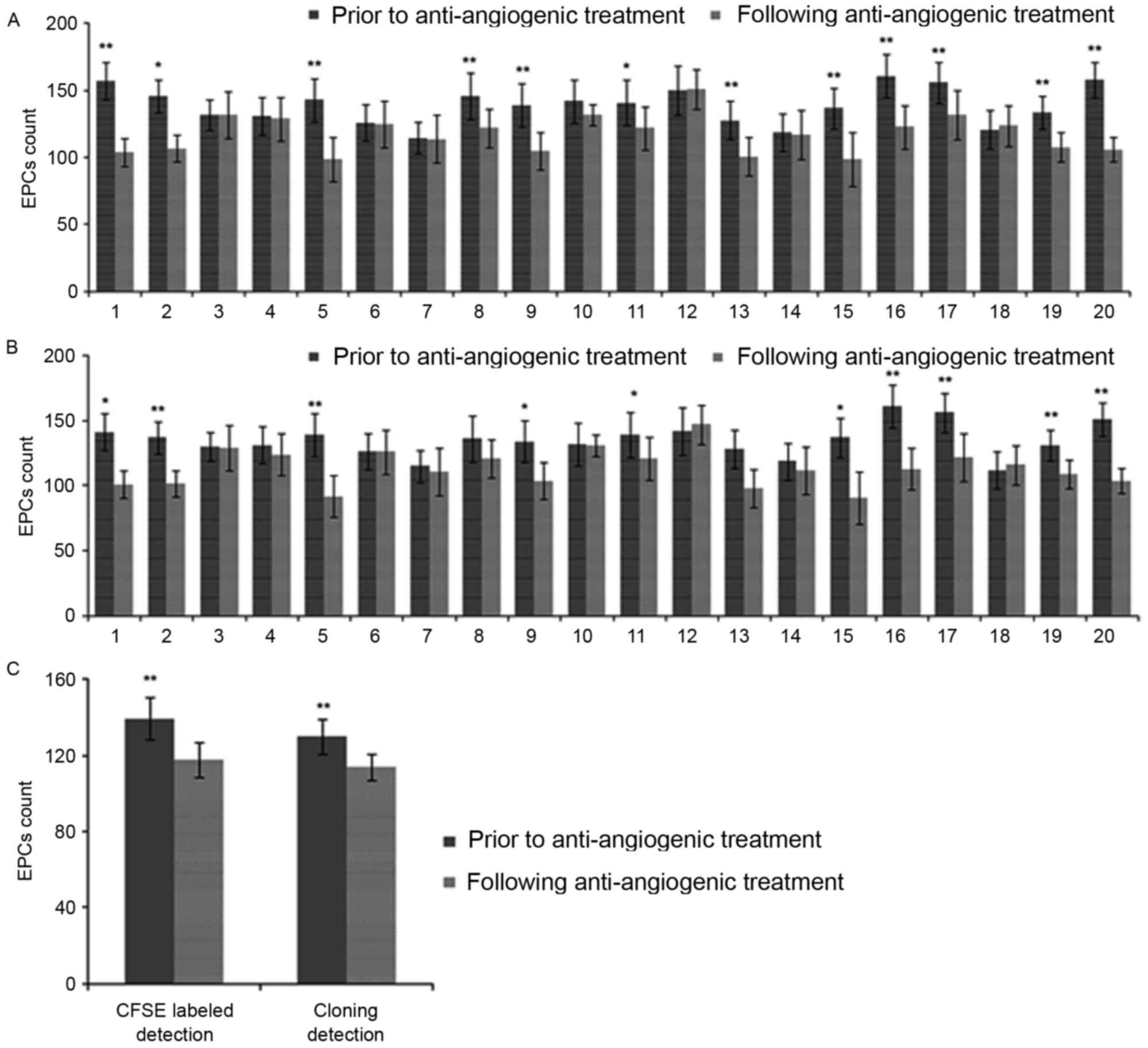
EPCs in the peripheral blood of patients with cancer
were counted prior to and following anti-angiogenic agent
administration, using single-platform flow cytometry and the in
vitro clonogenic counting assay. Changes in the EPC number in
the peripheral blood of patients with cancer prior to and following
the administration of anti-angiogenic agents were measured using
single-platform flow cytometry (Fig.
4A). Following anti-angiogenic agent administration, the EPC
number was reduced when compared with the number prior to
anti-angiogenic agent administration and in 12 patients this was
statistically significant (P<0.05; Fig. 4A). In addition, the results using the
in vitro clonogenic counting assay were consistent with the
flow cytometry results and 10 patients exhibited a significantly
decreased EPC number following anti-angiogenic agent administration
(P<0.05; Fig. 4B). Overall, the
data indicated that the anti-angiogenic treatment was able to
significantly reduce the number of EPCs in peripheral blood
(P<0.01; Fig. 4C).
Discussion
Current methods for determining EPC number may be
divided into two categories. The first uses in vitro
culture, in which cell differentiation is induced and cell clones
that are formed are characterized as endothelial cells and counted.
The second uses targeting to detect EPC-specific surface markers
and this category may be divided into flow cytometry (16,17,22,23) and
gene expression-based polymerase chain reaction (PCR) quantitative
detection (24,25).
Although these two categories of detection methods
have a large number of applications, they are essentially basic
research methods and are difficult to apply to routine clinical
testing, predominantly due to the following: Clone counting,
although currently recognized as the most widely used EPC detection
method, has the disadvantages of requiring time-consuming cell
culturing, highly technical methods and is considered expensive;
and flow cytometry, although it directly targets the indicators, is
time consuming and requires expensive commercial fluorescent
microspheres for quantitative analysis (26,27). In
addition, the physical properties of these commercial microspheres
are different from those of the cells, thus leading to unreliable
results and the operators are required to constantly adjust to
novel detection processes. Furthermore, the PCR-based method, which
detects specific indicators, has poor specificity among its
shortcomings (28). The present
study used single-platform flow cytometry with microspheres
prepared according to the method recently reported in Cytometry A,
regarding the use of the fluorescent dye, CFSE, to uniformly label
leukemia cells and impart them with fluorescence (21). A known number of fluorescent cells
were added to the test specimens as an internal reference for
detection by single-platform flow cytometry, allowing the
determination of the number of other, undetected cells in the
specimens. In our previous study, CFSE-labeled cells were used to
replace commercial fluorescent microspheres (21). In addition, they may be clearly
distinguished from cells not labeled with fluorescence and have the
same density and uniform sedimentation rate as the test cells;
thus, they may be used as an internal reference for quantitative
analysis and the results will be reliable (21). Therefore, once EPCs were
immunolabeled, the newly constructed cell fluorescent microspheres
were added, resulting in a quantitative detection, with improved
accuracy, of EPCs in human peripheral blood, with reduced testing
costs (1/5-1/6 of the current commercial microspheres). However,
further studies are required to determine whether there are
alternative cells or indicators, such as blood cells and dyes, that
are more suitable than leukemia cells and CFSE for cell fluorescent
microspheres, respectively.
In the present study, specific CD34, VEGFR-2 and
CD133 antibodies were used to label EPC cells; however, no specific
cell surface marker has been identified that is able to completely
distinguish EPCs from hematopoietic cells. Previous results have
indicated that mesenchymal stem cell-associated
CD34−/VE-cadherin−/AC133+/Flk-1+
multipotent adult progenitor cells (MAPCs) may be converted to
CD34+/VE-cadherin−/AC133−/Flk+
angioblasts by the action of VEGF and that these cells may be
further differentiated into mature endothelial cells (23,29,30) and
become involved in tumor angiogenesis and wound healing. The cell
surface markers of EPCs have not been fully elucidated and there
continues to be large variances in the reported quantities of EPCs
present in the circulation and uncertainty regarding the best
enrichment and isolation methods (31,32).
Thus, the specific phenotype that may distinguish EPCs from
hematopoietic cells or mature endothelial cells still requires
further exploration, which provides the motivation for continued
progression in the method described in the present study.
The number of EPCs is closely related to tumors due
to the fact that tumor growth requires angiogenesis (33). Once a tumor reaches a size of 3 mm,
the tumor cannot survive unless novel blood vessels are produced
(5,6). Tumors are able to secrete specific
factors that stimulate the bone marrow to increase EPC generation
and to mobilize the generated EPCs into the peripheral blood, thus
enriching local tumors with EPCs that participate in the formation
of novel blood vessels (4,25,34).
Therefore, EPCs are an important indicator of tumor growth and
prognosis and determining the number of EPCs has important clinical
significance for patients with cancer. The present study determined
the content of EPCs in the peripheral blood of patients with
cancer. Compared with the results of an in vitro clonogenic
counting assay, the accuracy of our method was reasonable.
Small arterial lesions may cause long-term high
blood pressure, leading to tissue ischemia of important target
organs such as the heart, brain and other organs (3). Endothelial dysfunction is caused by the
destruction of the dynamic balance between endothelial injury and
repair, and hypertension and endothelial dysfunction enhance one
another. A previous study found that EPC was able to differentiate
into mature endothelial cells to repair damaged endothelial cells
(35). Therefore, monitoring the
number of EPCs may have important clinical significance for
cardiovascular and cerebrovascular diseases. Therefore, monitoring
the number of EPCs may have important clinical significance for
cardiovascular and cerebrovascular diseases. The present study
demonstrated that single-platform flow cytometry based on
CFSE-labeled cell microspheres has unique advantages in determining
the number of EPCs, overcomes the shortcomings of other methods and
was objective and accurate. This method may be widely used in
clinical practice for fast and accurate analysis of EPCs in
peripheral blood.
Acknowledgements
The present study was funded by the Natural Science
Foundation of Shanghai Science and Technology Committee (grant no.
11ZR1433000), the Foundation of Key Disciplines in Health Systems
of Pudong New Dist. (grant no. PWZx2014-03) and the Foundation of
Discipline Leader in Health Systems of Pudong New District (grant
no. PWRd2014-02).
References
|
1
|
Hida K, Maishi N, Torii C and Hida Y:
Tumor angiogenesis-characteristics of tumor endothelial cells. Int
J Clin Oncol. 21:206–212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lopes-Bastos BM, Jiang WG and Cai J:
Tumour-endothelial cell communications: Important and indispensable
mediators of tumour angiogenesis. Anticancer Res. 36:1119–1126.
2016.PubMed/NCBI
|
|
3
|
Giuliano S and Pagès G: Mechanisms of
resistance to anti-angiogenesis therapies. Biochimie. 95:1110–1119.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Silván U, Diez-Torre A, Bonilla Z, Moreno
P, Díaz-Núñez M and Aréchaga J: Vasculogenesis and angiogenesis in
nonseminomatous testicular germ cell tumors. Urol Oncol.
33:268.e17–e28. 2015. View Article : Google Scholar
|
|
5
|
Vacca A and Ribatti D: Angiogenesis and
vasculogenesis in multiple myeloma: Role of inflammatory cells.
Recent Results Cancer Res. 183:87–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang HS, Feng YJ and Yao LQ: Angiogenesis,
vasculogenesis, and vasculogenic mimicry in ovarian cancer. Int J
Gynecol Cancer. 19:605–610. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shchors K and Evan G: Tumor angiogenesis:
Cause or consequence of cancer? Cancer Res. 67:7059–7061. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Duda DG, Cohen KS, Scadden DT and Jain RK:
A protocol for phenotypic detection and enumeration of circulating
endothelial cells and circulating progenitor cells in human blood.
Nat Protoc. 2:805–810. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ge YZ, Wu R, Lu TZ, Xin H, Yu P, Zhao Y,
Liu H, Xu Z, Xu LW, Shen JW, et al: Circulating endothelial
progenitor cell: A promising biomarker in clinical oncology. Med
Oncol. 32:3322015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de la Puente P, Muz B, Azab F and Azab AK:
Cell trafficking of endothelial progenitor cells in tumor
progression. Clin Cancer Res. 19:3360–3368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fuereder T, Wacheck V, Strommer S, Horak
P, Gerschpacher M, Lamm W, Kivaranovic D and Krainer M: Circulating
endothelial progenitor cells in castration resistant prostate
cancer: A randomized, controlled, biomarker study. PLoS One.
9:e953102014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lau CK, Yang ZF, Ho DW, Ng MN, Yeoh GC,
Poon RT and Fan ST: An Akt/hypoxia-inducible
factor-1alpha/platelet-derived growth factor-BB autocrine loop
mediates hypoxia-induced chemoresistance in liver cancer cells and
tumorigenic hepatic progenitor cells. Clin Cancer Res.
15:3462–3471. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ling CC, Ng KT, Shao Y, Geng W, Xiao JW,
Liu H, Li CX, Liu XB, Ma YY, Yeung WH, et al: Post-transplant
endothelial progenitor cell mobilization via CXCL10/CXCR3 signaling
promotes liver tumor growth. J Hepatol. 60:103–109. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Igreja C, Fragoso R, Caiado F, Clode N,
Henriques A, Camargo L, Reis EM and Dias S: Detailed molecular
characterization of cord blood-derived endothelial progenitors. Exp
Hematol. 36:193–203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ho JW, Pang RW, Lau C, Sun CK, Yu WC, Fan
ST and Poon RT: Significance of circulating endothelial progenitor
cells in hepatocellular carcinoma. Hepatology. 44:836–843. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ishida Y, Kimura A, Nosaka M, Kuninaka Y,
Shimada E, Yamamoto H, Nishiyama K, Inaka S, Takayasu T,
Eisenmenger W and Kondo T: Detection of endothelial progenitor
cells in human skin wounds and its application for wound age
determination. Int J Legal Med. 129:1049–1054. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lanuti P, Rotta G, Almici C, Avvisati G,
Budillon A, Doretto P, Malara N, Marini M, Neva A, Simeone P, et
al: Endothelial progenitor cells, defined by the simultaneous
surface expression of VEGFR2 and CD133, are not detectable in
healthy peripheral and cord blood. Cytometry A. 89:259–270. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brunck ME, Andersen SB, Timmins NE,
Osborne GW and Nielsen LK: Absolute counting of neutrophils in
whole blood using flow cytometry. Cytometry A. 85:1057–1064. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ngoma A, Saito S, Ohto H, Ikeda K, Yasuda
H, Kawabata K, Kanno T, Kikuta A, Mochizuki K and Nollet KE: CD34+
cell enumeration by flow cytometry: A comparison of systems and
methodologies. Arch Pathol Lab Med. 135:909–914. 2011.PubMed/NCBI
|
|
20
|
Kleine TO, Nebe CT, Löwer C, Lehmitz R,
Kruse R, Geilenkeuser WJ and Dorn-Beineke A: Modifications of
haematology analyzers to improve cell counting and leukocyte
differentiating in cerebrospinal fluid controls of the joint German
society for clinical chemistry and laboratory medicine. Cytometry
A. 75:688–691. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao FF, Xu LM, Peng B, Xie QH, Uzan G and
Zhang DH: A routinely applicable way for using FCM in cell
enumeration with CFSE-labeled CellBeads as internal standard.
Cytometry A. 75:975–978. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morita R, Sato K, Nakano M, Miura H, Odaka
H, Nobori K, Kosaka T, Sano M, Watanabe H, Shioya T and Ito H:
Endothelial progenitor cells are associated with response to
chemotherapy in human non-small-cell lung cancer. J Cancer Res Clin
Oncol. 137:1849–1857. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mund JA, Ingram DA, Yoder MC and Case J:
Endothelial progenitor cells and cardiovascular cell-based
therapies. Cytotherapy. 11:103–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mehra N, Penning M, Maas J, Beerepoot LV,
van Daal N, van Gils CH, Giles RH and Voest EE: Progenitor marker
CD133 mRNA is elevated in peripheral blood of cancer patients with
bone metastases. Clin Cancer Res. 12:4859–4866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Steurer M, Kern J, Zitt M, Amberger A,
Bauer M, Gastl G, Untergasser G and Gunsilius E: Quantification of
circulating endothelial and progenitor cells: Comparison of
quantitative PCR and four-channel flow cytometry. BMC Res Notes.
1:712008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harrison GM, Bennett AJ, Moody M, Read GF
and Williams PE: Use of formalin-fixed, propidium iodide-stained
human leukocytes as a standard for enumerating CD4+ T lymphocytes
in a single-platform assay. Clin Diagn Lab Immunol. 8:397–401.
2001.PubMed/NCBI
|
|
27
|
Houston JP, Naivar MA and Freyer JP:
Digital analysis and sorting of fluorescence lifetime by flow
cytometry. Cytometry A. 77:861–872. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Churro C, Pereira P, Vasconcelos V and
Valério E: Species-specific real-time PCR cell number
quantification of the bloom-forming cyanobacterium Planktothrix
agardhii. Arch Microbiol. 194:749–757. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pradhan KR, Mund JA, Claussen HL,
Gosiengfiao YC, Radulescu VC, Ballard JJ, Liu Z, Vik TA and Case J:
A pilot study of circulating endothelial and hematopoietic
progenitor cells in children with sarcomas. J Pediatr Hematol
Oncol. 37:443–448. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rusak M, Radzikowska U,
Glowinska-Olszewska B, Dobrenko E, Piotrowska-Jastrzebska J,
Dabrowska M, Bodzenta-Lukaszyk A, Bossowski A and Moniuszko M:
Endothelial progenitor cell levels in juvenile idiopathic arthritis
patients: Effects of anti-inflammatory therapies. Pediatr Rheumatol
Online J. 13:62015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tagawa S, Nakanishi C, Mori M, Yoshimuta
T, Yoshida S, Shimojima M, Yokawa J, Kawashiri MA, Yamagishi M and
Hayashi K: Determination of early and late endothelial progenitor
cells in peripheral circulation and their clinical association with
coronary artery disease. Int J Vasc Med. 2015:6742132015.PubMed/NCBI
|
|
32
|
Fox A, Smythe J, Fisher N, Tyler MP,
McGrouther DA, Watt SM and Harris AL: Mobilization of endothelial
progenitor cells into the circulation in burned patients. Br J
Surg. 95:244–251. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Calzi S, Neu MB, Shaw LC, Kielczewski
JL, Moldovan NI and Grant MB: EPCs and pathological angiogenesis:
When good cells go bad. Microvasc Res. 79:207–216. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takahashi T, Kalka C, Masuda H, Chen D,
Silver M, Kearney M, Magner M, Isner JM and Asahara T: Ischemia-
and cytokine-induced mobilization of bone marrow-derived
endothelial progenitor cells for neovascularization. Nat Med.
5:434–438. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ekholm L, Kahlenberg JM, Helmers S
Barbasso, Tjärnlund A, Yalavarthi S, Zhao W, Seto N, Betteridge Z,
Lundberg IE and Kaplan MJ: Dysfunction of endothelial progenitor
cells is associated with the type I IFN pathway in patients with
polymyositis and dermatomyositis. Rheumatology (Oxford).
55:1987–1992. 2016. View Article : Google Scholar : PubMed/NCBI
|