Introduction
Globally, lung cancer is the leading cause of
cancer-associated mortality in men and the second leading cause of
cancer-associated mortality in women (1). Recurrence and metastasis are the
biggest obstacles to effective lung cancer treatment. Although
treatments for lung cancer have improved over the past few decades,
the 5-year survival rate is only ~16% (2). Thus, it is important to identify
biomarkers associated with lung cancer metastasis in order to
improve the therapeutic strategies available.
Tumor metastasis is a complex process consisting of
multiple biological steps (3)
including increased motility, invasion into surrounding tissue,
intravasation, entry and survival in the circulation, extravasation
and eventual colonization of a distant site (4–6). The
epithelial-mesenchymal transition (EMT) is a well-coordinated
process that induces metastasis in epithelial cancer (7,8).
Glutathione S-transferase A 1 (GSTA1) is an isoform of GST
primarily involved in the detoxification of electrophilic compounds
by undergoing conjugation with glutathione (9,10).
Previous studies have demonstrated that the altered expression of
GST genes increases the risk of prostate cancer and hepatocellular
carcinoma (11–14). Recently, Pan et al (15) identified the potential of GSTA1 in
the early diagnosis and treatment of lung cancer. It was determined
that the expression of GSTA1 in lung cancer tissues and cells was
higher than in healthy tissues and cells, and that GSTA1
suppression inhibits the proliferation of lung cancer cells.
However, to the best of our knowledge, the potential role of GSTA1
in lung cancer metastasis has not yet been investigated.
It is estimated that ~85% of total lung cancer cases
occur in tobacco smokers (16).
Nicotine is the major addictive component of tobacco smoke and many
of the carcinogens in tobacco smoke are derivatives of nicotine
(17). Numerous studies have
demonstrated that nicotine promotes the growth and metastasis of
lung tumors (18–20).
In the present study, GSTA1-small interfering RNA
was transfected into A549 cells to knock down GSTA1 expression, and
the effect of GSTA1 on the viability, invasion and adhesion of lung
cancer cells was investigated in the presence of nicotine in
vitro. Furthermore, the effect of GSTA1 on EMT, a process
strongly associated with lung cancer metastasis, was examined by
western blot analysis.
Materials and methods
Cell culture and transfection
The A549 cell line was obtained from the American
Type Culture Collection (Manassas, VA, USA) and cultured in
Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% newborn calf
serum (NBCS; Invitrogen; Thermo Fisher Scientific, Inc.). Cells
were maintained at 37°C in a humidified atmosphere containing 5%
CO2. A549 cells were seeded (1×105) in DMEM
and Nicotine (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was
used to treat A549 cells at the concentrations of 0.01, 0.1, 1 and
10 µM at 37°C for 24 h. The concentration of nicotine was selected
by assessing which nicotine concentration exhibited the maximum
effect on GSTA1 expression for subsequent experiments. Cells were
treated with nicotine for 6, 12, 24 and 48 h in the preliminary
experiments, and 24 h was selected as the duration following
treatment with 10 µM nicotine, as together they had the maximum
effect on GSTA1 expression for the subsequent experiments.
GSTA1-small interfering RNA (siRNA) was synthesized by Sangon
Biotech Co., Ltd. (Shanghai, China). A scramble siRNA (Sangon
Biotech Co., Ltd.) was used as the control. A549 cells were
transfected with siRNA (1 µM) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's instructions. The cells used in this study were
divided into four groups: PBS + Scr group (cells were transfected
with scramble siRNA and treated with 1 µl PBS), PBS + Si group
(cells were transfected with GSTA1-siRNA and treated with 1 µl
PBS), Nicotine + Scr group (cells were transfected with scramble
siRNA and treated with 10 µM nicotine) and Nicotine + Si group
(cells were transfected with GSTA1-siRNA and treated with 10 µM
nicotine). The PBS + Scr group was used as the control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from A549 cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA (1
µg) was converted into cDNA using a First Strand cDNA Synthesis kit
(Fermentas; Thermo Fisher Scientific, Inc.) following the
manufacturer's instructions. The following primers were used in the
present study: GSTA1, forward, 5′-GGCTGCAGCTGGAGTAGAGT-3′ and
reverse 5′-GCAAGCTTGGCATCTTTTTC-3′ and β-actin, forward,
5′-AGAGCTACGAGCTGCCTGAC-3′ and reverse 5′-AGCACTGTGTTGGCGTACAG-3′.
qPCR was performed using a SYBR Green PCR kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.) in a 7300 Sequence Detection system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The reaction
was performed over 40 cycles at 95°C for 30 sec, 59°C for 30 sec
and 72°C for 30 sec. All reactions were performed in triplicate.
Levels of GSTA1 mRNA were normalized to those of β-actin, as an
internal control using the 2−ΔΔCq method (21).
Western blot analysis
Total protein was extracted using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China) according to the manufacture's
protocol. Briefly, the cells were incubated with the lysis buffer
at room temperature for 5 min. Then cell lysates were centrifuged
at 13,000 × g for 5 min at room temperature and supernatants were
harvested. Equal amounts of total protein (20 µg) were separated
using 12% SDS-PAGE and subsequently transferred onto nitrocellulose
membranes (EMD Millipore, Billerica, MA, USA). Following blocking
with 3% bovine serum albumin (Sigma-Aldrich; Merck KGaA) at 4°C
overnight, membranes were incubated with keratin rabbit polyclonal
antibody (1:400; cat. no. 41723; Signalway Antibody Inc., College
Park, MD, USA), GSTA1 monoclonal antibody (1:500; cat. no.
sc-100546), E-cadherin rabbit polyclonal antibody (1:500; cat. no.
sc-7870), vimentin monoclonal antibody (1:800; cat. no. sc-373717),
N-cadherin mouse monoclonal antibody (1:500; cat. no. sc-393933)
and β-actin monoclonal antibody (1:1,000; cat. no. sc-130301; all
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight.
Membranes were subsequently incubated with horseradish
peroxidase-labeled goat anti-mouse (1:2,000; cat. no. sc-2005) or
goat anti-rabbit antibodies (1:2,000; cat. no. sc-2004; both Santa
Cruz Biotechnology, Inc.) at 37°C for 1 h. Immunoreactive bands
were detected using an enhanced chemiluminescence detection kit
(Pierce; Thermo Fisher Scientific, Inc.). ImageJ version 1.37
software (National Institutes of Health, Bethesda, MD, USA) was
used for quantification, and the experiments were independently
performed three times.
MTT assay
An MTT assay was performed to determine cell
viability. A549 cells were plated into 96-well plates at the
density of 1×104/well, and allowed to grow at 37°C for
24, 48, 72 and 96 h. Subsequently, they were incubated with MTT
solution (1 mg/ml; Sigma-Aldrich; Merck KGaA) at 37°C for 4 h.
Dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA) was added to each
well to solubilize the formazan product. Control cells were
incubated with the dimethyl sulfoxide alone. The absorbance of each
sample at 570 nm was measured using a microplate reader (Multiskan
Ascent, Thermo Fisher Scientific, Inc.).
Cell invasion assay
The invasion of A549 cells was measured using a
Transwell-Matrigel assay. Transwell inserts (Corning, Inc.,
Corning, NY, USA) were precoated with Matrigel matrix (BD
Biosciences, Franklin Lakes, NJ, USA) at 37°C for 30 min. Cells
were trypsinized and plated into the upper chambers at a
concentration of 3×104 cells/ml in serum-free DMEM. Cell
medium containing 10% NBCS was added to the lower chambers. The
chambers were incubated at 37°C overnight and a cotton swab was
used to remove non-invasive cells. The invasive cells were then
fixed in 95% ethanol at room temperature for 15 min followed by
staining in hematoxylin for 10 min. The invaded cells were observed
under an inverted microscope (XDS-200D; Caikon Optical Instrument
Co., Ltd, Shanghai, China) at a magnification of ×400, and counted
in 10 randomly selected fields in three independent
experiments.
Cell adhesion assay
The 96-well plates were precoated with fibronectin
(Sigma-Aldrich; Merck KGaA) and then blocked with 1% bovine serum
albumin at 37°C for 2 h. A total of 3×104 A549 cells in
serum-free DMEM were seeded into each well and incubated at 37°C
for 2 h. Following washing with phosphate-buffered saline, cells
were fixed in 4% paraformaldehyde (Shanghai Solarbio Bioscience
& Technology Co., Ltd., Shanghai, China) at room temperature
for 30 min. Subsequently, cells were stained with 0.5% crystal
violet (Beyotime Institute of Biotechnology) followed by incubation
with 2% SDS at room temperature for 1 min. The absorbance at 570 nm
was measured using a microplate reader.
Statistical analysis
All the data are presented as the mean ± standard
deviation. Statistical analysis was performed using SPSS 19.0
statistical software (SPSS, Inc., Chicago, IL, USA). One-way
analysis of variance followed by the least significant difference
test was used for comparison of multiple groups and P<0.05 was
considered to indicate a statistically significant difference.
Results
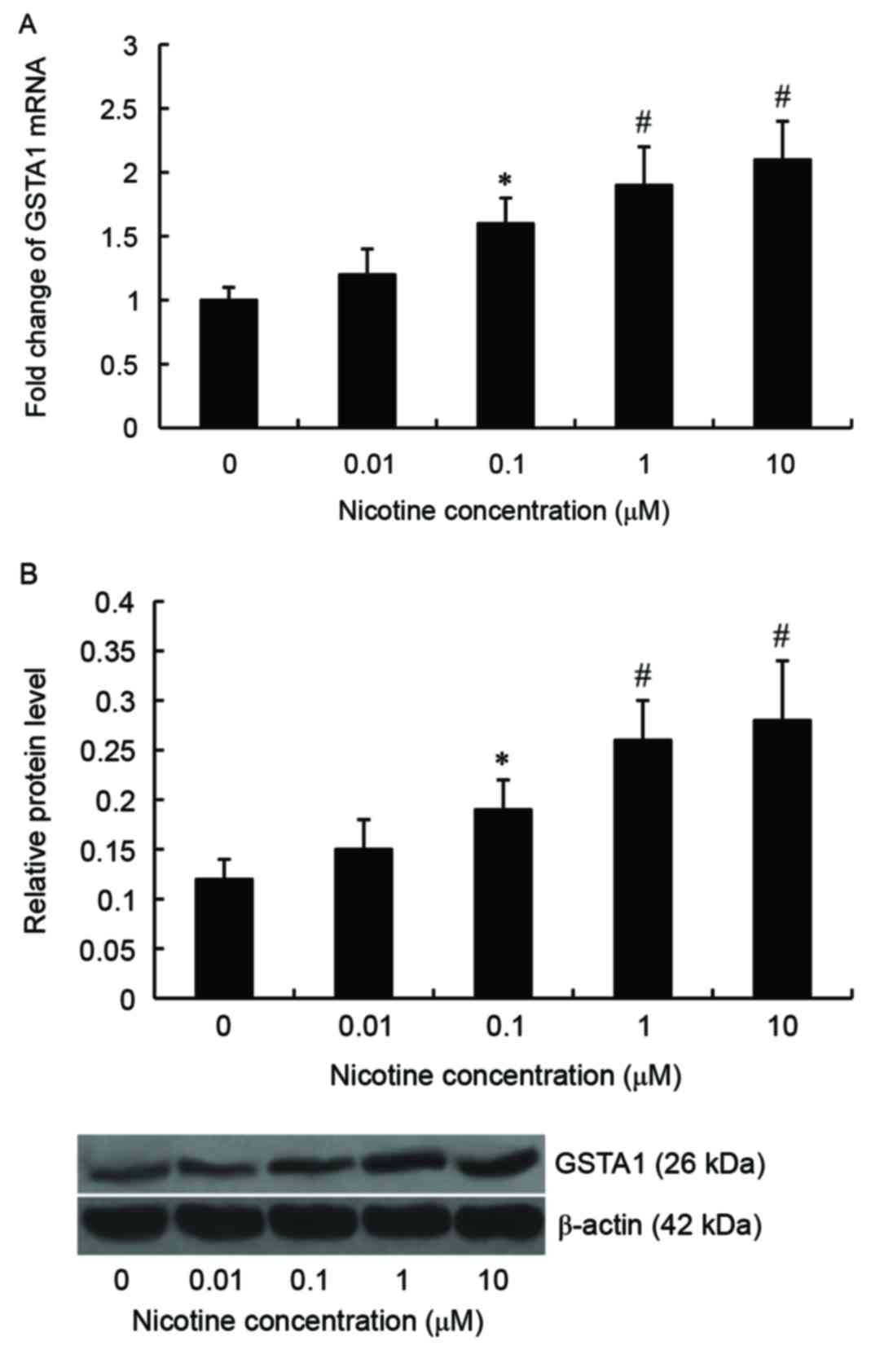
mRNA and protein expression of GSTA1
is induced by nicotine in A549 cells
A549 cells were treated with 0, 0.01, 0.1, 1 or 10
µM nicotine for 24 h. Subsequently, levels of GSTA1 mRNA and
protein were examined by RT-qPCR and western blot analysis,
respectively. Results from RT-qPCR demonstrated that levels of
GSTA1 mRNA were elevated in A549 cells following nicotine
stimulation and the maximum level was reached at 10 µM. Compared
with controls, levels of GSTA1 mRNA were significantly increased
following treatment with 0.1 (P<0.05), 1 (P<0.01) and 10 µM
(P<0.01) nicotine (Fig. 1A).
Similar results were obtained following western blot analysis
(Fig. 1B). We treated cells with
nicotine for 6, 12, 24 and 48 h in the preliminary experiments, and
6 and 12 h of incubation was demonstrated to exhibit no effect on
GSTA1 expression. Following preliminary assessments, 10 µM nicotine
was selected for use in subsequent experiments, as the
concentration of nicotine that exhibited the maximum effect on
GSTA1 expression to treat cells, following 24 h of incubation, for
the subsequent experiments.
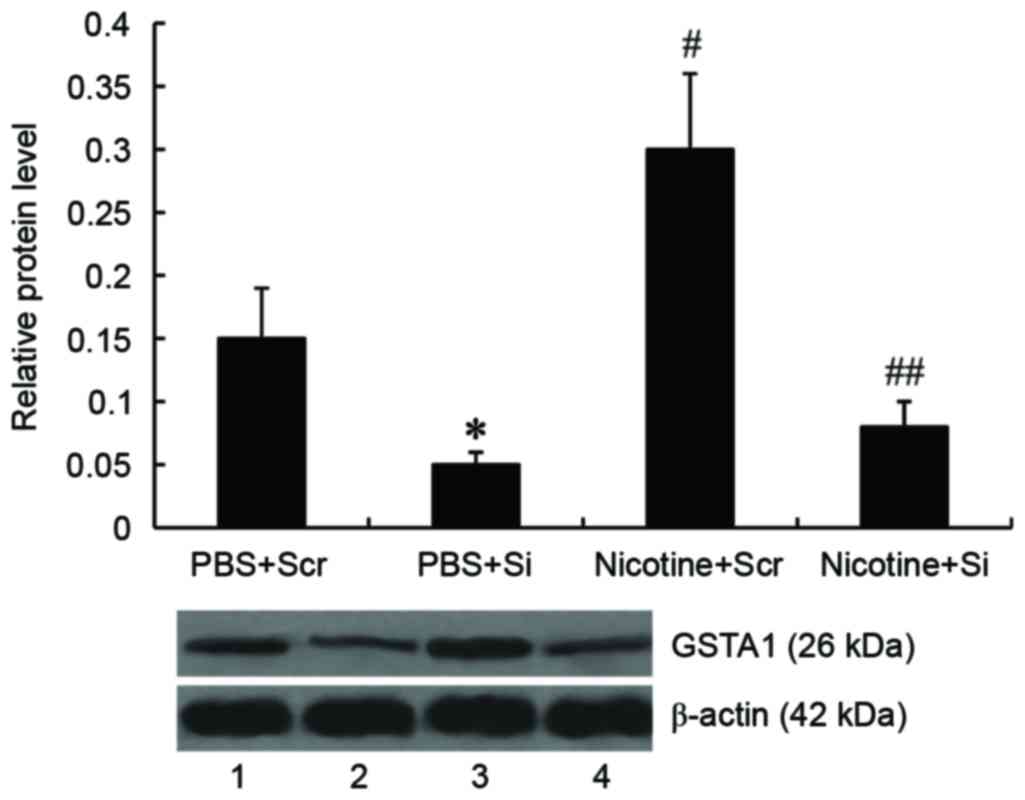
GSTA1 suppression inhibits the
nicotine-induced increased viability of A549 cells
To knock down GSTA1 expression, A549 cells were
transfected with GSTA1-siRNA; scramble siRNA was used as a control.
It was demonstrated by western blot analysis that levels of GSTA1
protein were significantly decreased in A549 cells following
GSTA1-siRNA transfection (P<0.05). Treatment with nicotine
significantly increased GSTA1 expression compared with controls
(P<0.01), however, this increase in GSTA1 expression by nicotine
was abrogated following transfection with GSTA1-siRNA (P<0.01;
Fig. 2).
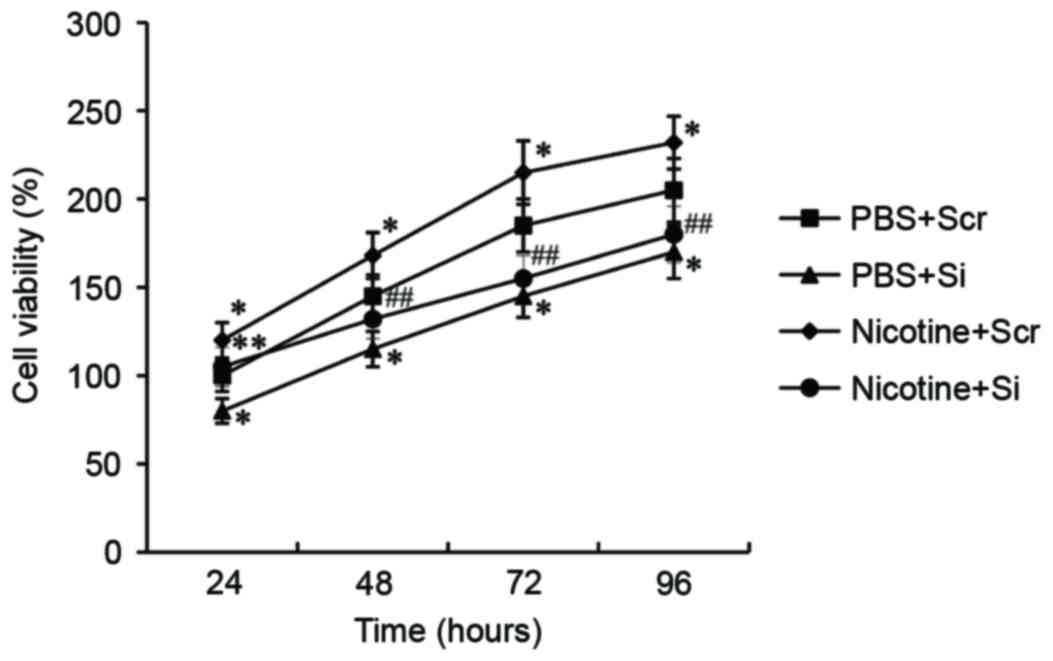
To investigate the role of GSTA1 on cell viability,
A549 cells transfected with GSTA1-siRNA were treated with 10 µM
nicotine for 24 h and an MTT assay was performed. As presented in
Fig. 3, nicotine stimulation
significantly increased the viability of A549 cells transfected
with scramble siRNA compared with untreated A549 cells (P<0.05).
However, viability was significantly reduced following transfection
with GSTA1-siRNA in nicotine-treated cells compared with the
nicotine-treated scramble-siRNA cells (P<0.01) and in untreated
cells transfected with GSTA1-siRNA compared with untreated cells
transfected with scramble siRNA (P<0.05).
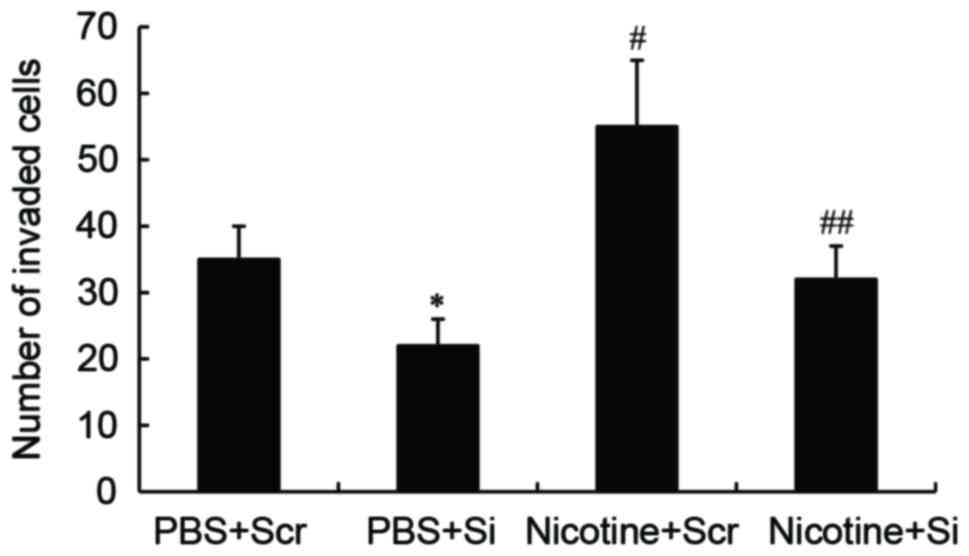
GSTA1 suppression inhibits the
nicotine-induced invasion abilities of A549 cells
A Transwell-migration assay was performed to
determine whether GSTA1 affects cell invasion. In PBS-treated
cells, GSTA1 knockdown significantly decreased the cell invasion
ability (P<0.05) compared with cells transfected with scramble
siRNA. The invasion ability of A549 cells significantly increased
following nicotine treatment compared with PBS-treated A549 cells
(P<0.01). However, nicotine-treated cells transfected with
GSTA1-siRNA exhibited significantly decreased invasive ability
compared with nicotine-treated cells transfected with scramble
siRNA (P<0.01; Fig. 4).
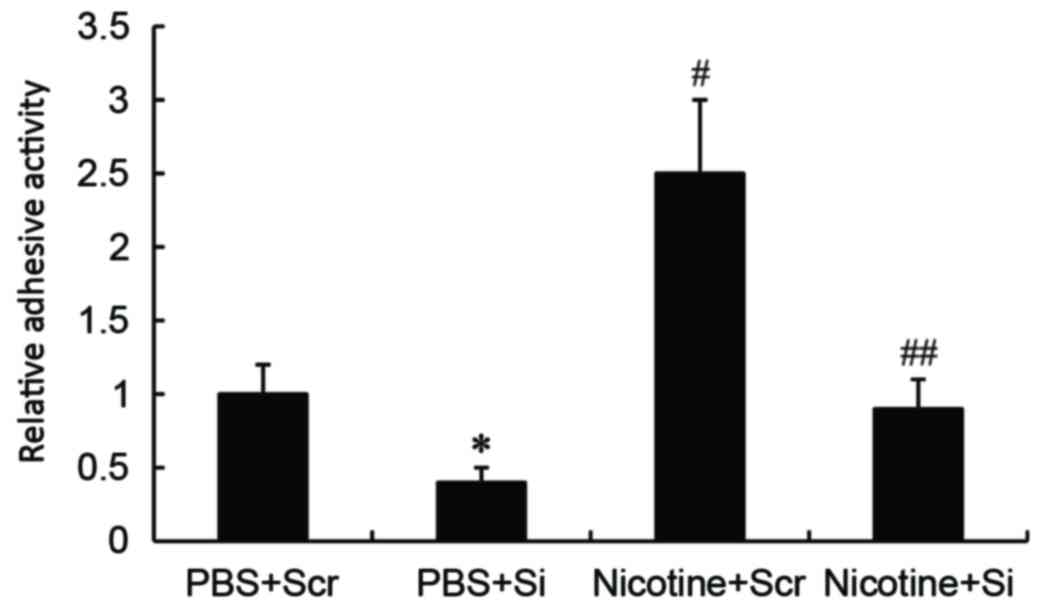
GSTA1 suppression inhibits the
nicotine-induced adhesion abilities of A549 cells
Cell adhesion assays demonstrated that significantly
increased adhesion activity was observed in nicotine-stimulated
A549 cells compared with PBS-treated cells transfected with
scramble siRNA (P<0.01). However, GSTA1-siRNA knockdown
significantly inhibited the adhesion activity of nicotine treated
(P<0.01) and untreated (P<0.05) A549 cells compared with
scramble siRNA nicotine-treated and untreated cells, respectively
(Fig. 5).
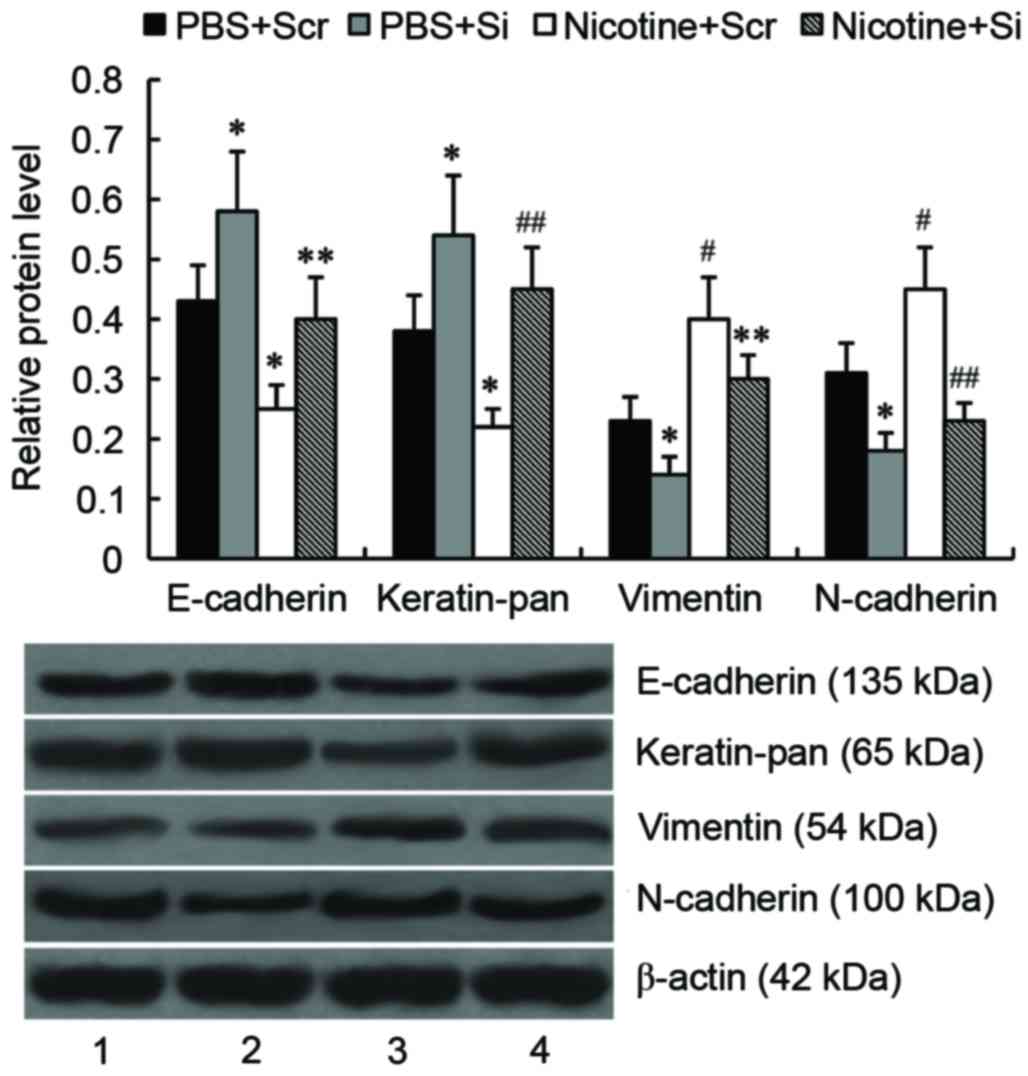
GSTA1 suppression inhibits
nicotine-induced EMT of A549 cells
To investigate the effect of GSTA1 on EMT in A549
cells, the expression of the epithelial markers E-cadherin and
keratin, and the mesenchymal markers vimentin and N-cadherin, were
determined by western blot analysis. As presented in Fig. 6, compared with untreated cells
transfected with scramble siRNA, levels of E-cadherin and keratin
were significantly downregulated (P<0.05), whereas levels of
vimentin and N-cadherin were significantly upregulated (P<0.01)
in nicotine-treated cells transfected with scramble siRNA.
Following GSTA1 knockdown, levels of E-cadherin and keratin were
significantly increased (P<0.05), whereas levels of vimentin and
N-cadherin were significantly reduced (P<0.05) compared with
untreated cells transfected with scramble siRNA. In
nicotine-treated A549 cells, levels of E-cadherin (P<0.05) and
keratin (P<0.01) were significantly increased and levels of
vimentin (P<0.05) and N-cadherin (P<0.01) were significantly
reduced compared with nicotine-treated cells transfected with
scramble siRNA.
Discussion
Glutathione S-transferases are a multigene family of
phase II isoenzymes that catalyze the conjugation of a variety of
carcinogens, environmental toxins and endogenous compounds with
glutathione (22). Polymorphisms
associated with the altered expression of these genes may affect
the metabolism of carcinogens and chemotherapeutic agents (23), resulting in an increased risk of
various malignances (24–30). Previous studies have demonstrated
that GSTA1 overexpression protects tumor cells from doxorubicin and
reactive oxygen species-induced apoptosis (31–34).
GSTA1 also serves a role in regulating the proliferation of Caco-2
cells (35). Recently, it has been
demonstrated that GSTA1 promotes the proliferation of lung cancer
cells (15). In the present study,
it was demonstrated that the results from the MTT assay were
consistent with those from a previous study (15). Furthermore, the present study
demonstrated that GSTA1 suppression inhibited lung cancer cell
invasion and adhesion, indicating that GSTA1 is associated with
lung cancer metastasis.
Although there is insufficient evidence to classify
nicotine as a carcinogen, nicotine has tumor-promoting properties.
It has been demonstrated that nicotine promotes tumor growth and
metastasis by inducing EMT, cell migration and invasion,
angiogenesis, as well as suppressing apoptosis induced by drugs or
radiation (36–38). In the present study, nicotine was
used to treat A549 cells, and it was determined that the expression
of GSTA1 was induced by nicotine. To determine whether GSTA1 was
involved in mediating the effect of nicotine on lung cancer cells,
GSTA1 was knocked down in A549 cells by siRNA. The results
indicated that nicotine-induced lung cancer cell viability,
invasion and adhesion were suppressed following GSTA1
knockdown.
It has been suggested that the EMT is the driving
force of metastasis (7). During EMT,
epithelial cells lose their apical and basolateral polarity, break
cell-cell attachment and transform into mesenchymal cells (39). Notably, the EMT is reversible and the
mesenchymal-epithelial transition is a reversion back towards the
epithelial phenotype (40).
E-cadherin and keratin are key markers of the epithelial phenotype
and the loss of these proteins leads to the loss of cell junctions
and the promotion of metastasis (41,42).
Vimentin and N-cadherin are mesenchymal cell markers and have
crucial roles in cellular migration (43,44). In
the present study, GSTA1 affected the expression of EMT markers of
A549 lung cancer cells. In addition, the present findings indicated
that the nicotine-induced EMT was reversed following GSTA1
knockdown. These results suggest that the effect of GSTA1 on EMT
may explain its effect on lung cancer metastasis.
In conclusion, the present study demonstrated that
GSTA1 promotes lung cancer cell invasion and adhesion. In addition,
the present results suggest that nicotine increases the viability,
invasion and adhesion abilities of A549 lung cancer cells and that
this effect is mediated by GSTA1. GSTA1 exerts its effect on lung
cancer cell metastasis by promoting the EMT. The present study
suggests that GSTA1 serves a potential role GSTA1 in lung cancer
metastasis and therefore may be a novel target for lung cancer
treatment.
References
|
1
|
Torre LA, Siegel RL and Jemal A: Lung
Cancer Statistics. Adv Exp Med Biol. 893:1–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nguyen DX, Bos PD and Massagué J:
Metastasis: From dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fidler IJ: The pathogenesis of cancer
metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brabletz T, Hlubek F, Spaderna S,
Schmalhofer O, Hiendlmeyer E, Jung A and Kirchner T: Invasion and
metastasis in colorectal cancer: Epithelial-mesenchymal transition,
mesenchymal-epithelial transition, stem cells and beta-catenin.
Cells Tissues Organs. 179:56–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hayes JD, Flanagan JU and Jowsey IR:
Glutathione transferases. Annu Rev Pharmacol Toxicol. 45:51–88.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khan MS, Khan MK, Siddiqui MH and Arif JM:
An in vivo and in silico approach to elucidate the
tocotrienol-mediated fortification against infection and
inflammation induced alterations in antioxidant defense system. Eur
Rev Med Pharmacol Sci. 15:916–930. 2011.PubMed/NCBI
|
|
11
|
Lee WH, Morton RA, Epstein JI, Brooks JD,
Campbell PA, Bova GS, Hsieh WS, Isaacs WB and Nelson WG: Cytidine
methylation of regulatory sequences near the pi-class glutathione
S-transferase gene accompanies human prostatic carcinogenesis. Proc
Natl Acad Sci USA. 91:pp. 11733–11737. 1994; View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Niu D, Zhang J, Ren Y, Feng H and Chen WN:
HBx genotype D represses GSTP1 expression and increases the
oxidative level and apoptosis in HepG2 cells. Mol Oncol. 3:67–76.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang YJ, Chen Y, Ahsan H, Lunn RM, Chen
SY, Lee PH, Chen CJ and Santella RM: Silencing of glutathione
S-transferase P1 by promoter hypermethylation and its relationship
to environmental chemical carcinogens in hepatocellular carcinoma.
Cancer Lett. 221:135–143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reszka E, Wasowicz W and Gromadzinska J:
Genetic polymorphism of xenobiotic metabolising enzymes, diet and
cancer susceptibility. Br J Nutr. 96:609–619. 2006.PubMed/NCBI
|
|
15
|
Pan XD, Yang ZP, Tang QL, Peng T, Zhang
ZB, Zhou SG, Wang GX, He B and Zang LQ: Expression and function of
GSTA1 in lung cancer cells. Asian Pac J Cancer Prev. 15:8631–8635.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hecht SS: Cigarette smoking: Cancer risks,
carcinogens, and mechanisms. Langenbecks Arch Surg. 391:603–613.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guingab-Cagmat J, Bauzo RM, Bruijnzeel AW,
Wang KK, Gold MS and Kobeissy FH: Methods in tobacco abuse:
Proteomic changes following second-hand smoke exposure. Methods Mol
Biol. 829:329–348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Davis R, Rizwani W, Banerjee S, Kovacs M,
Haura E, Coppola D and Chellappan S: Nicotine promotes tumor growth
and metastasis in mouse models of lung cancer. PLoS One.
4:e75242009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grozio A, Catassi A, Cavalieri Z, Paleari
L, Cesario A and Russo P: Nicotine, lung and cancer. Anticancer
Agents Med Chem. 7:461–466. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cattaneo MG, Codignola A, Vicentini LM,
Clementi F and Sher E: Nicotine stimulates a serotonergic autocrine
loop in human small-cell lung carcinoma. Cancer Res. 53:5566–5568.
1993.PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vos RM and Van Bladeren PJ: Glutathione
S-transferases in relation to their role in the biotransformation
of xenobiotics. Chem Biol Interact. 75:241–265. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Watson MA, Stewart RK, Smith GB, Massey TE
and Bell DA: Human glutathione S-transferase P1 polymorphisms:
Relationship to lung tissue enzyme activity and population
frequency distribution. Carcinogenesis. 19:275–280. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stoehlmacher J, Park DJ, Zhang W, Groshen
S, Tsao-Wei DD, Yu MC and Lenz HJ: Association between glutathione
S-transferase P1, T1, and M1 genetic polymorphism and survival of
patients with metastatic colorectal cancer. J Natl Cancer Inst.
94:936–942. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Howells RE, Dhar KK, Hoban PR, Jones PW,
Fryer AA, Redman CW and Strange RC: Association between
glutathione-S-transferase GSTP1 genotypes, GSTP1 over-expression,
and outcome in epithelial ovarian cancer. Int J Gynecol Cancer.
14:242–250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wenzlaff AS, Cote ML, Bock CH, Land SJ and
Schwartz AG: GSTM1, GSTT1 and GSTP1 polymorphisms, environmental
tobacco smoke exposure and risk of lung cancer among never smokers:
A population-based study. Carcinogenesis. 26:395–401. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
White DL, Li D, Nurgalieva Z and El-Serag
HB: Genetic variants of glutathione S-transferase as possible risk
factors for hepatocellular carcinoma: A HuGE systematic review and
meta-analysis. Am J Epidemiol. 167:377–389. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kilburn L, Okcu MF, Wang T, Cao Y,
Renfro-Spelman A, Aldape KD, Gilbert MR and Bondy M: Glutathione
S-transferase polymorphisms are associated with survival in
anaplastic glioma patients. Cancer. 116:2242–2249. 2010.PubMed/NCBI
|
|
29
|
Sergentanis TN and Economopoulos KP: GSTT1
and GSTP1 polymorphisms and breast cancer risk: A meta-analysis.
Breast Cancer Res Treat. 121:195–202. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu Z, Zhu H, Luk JM, Wu D, Gu D, Gong W,
Tan Y, Zhou J, Tang J, Zhang Z, et al: Clinical significance of
SOD2 and GSTP1 gene polymorphisms in Chinese patients with gastric
cancer. Cancer. 118:5489–5496. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sharma A, Patrick B, Li J, Sharma R,
Jeyabal PV, Reddy PM, Awasthi S and Awasthi YC: Glutathione
S-transferases as antioxidant enzymes: Small cell lung cancer (H69)
cells transfected with hGSTA1 resist doxorubicin-induced apoptosis.
Arch Biochem Biophys. 452:165–173. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang Y, Sharma R, Sharma A, Awasthi S and
Awasthi YC: Lipid peroxidation and cell cycle signaling:
4-hydroxynonenal, a key molecule in stress mediated signaling. Acta
Biochim Pol. 50:319–336. 2003.PubMed/NCBI
|
|
33
|
Cheng JZ, Singhal SS, Sharma A, Saini M,
Yang Y, Awasthi S, Zimniak P and Awasthi YC: Transfection of mGSTA4
in HL-60 cells protects against 4-hydroxynonenal-induced apoptosis
by inhibiting JNK-mediated signaling. Arch Biochem Biophys.
392:197–207. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao T, Singhal SS, Piper JT, Cheng J,
Pandya U, Clark-Wronski J, Awasthi S and Awasthi YC: The role of
human glutathione S-transferases hGSTA1-1 and hGSTA2-2 in
protection against oxidative stress. Arch Biochem Biophys.
367:216–224. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Adnan H, Quach H, MacIntosh K, Antenos M
and Kirby GM: Low levels of GSTA1 expression are required for
Caco-2 cell proliferation. PLoS One. 7:e517392012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Egleton RD, Brown KC and Dasgupta P:
Nicotinic acetylcholine receptors in cancer: Multiple roles in
proliferation and inhibition of apoptosis. Trends Pharmacol Sci.
29:151–158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dasgupta P, Rizwani W, Pillai S, Kinkade
R, Kovacs M, Rastogi S, Banerjee S, Carless M, Kim E, Coppola D, et
al: Nicotine induces cell proliferation, invasion and
epithelial-mesenchymal transition in a variety of human cancer cell
lines. Int J Cancer. 124:36–45. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Heeschen C, Jang JJ, Weis M, Pathak A,
Kaji S, Hu RS, Tsao PS, Johnson FL and Cooke JP: Nicotine
stimulates angiogenesis and promotes tumor growth and
atherosclerosis. Nat Med. 7:833–839. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lim J and Thiery JP:
Epithelial-mesenchymal transitions: Insights from development.
Development. 139:3471–3486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hay ED and Zuk A: Transformations between
epithelium and mesenchyme: Normal, pathological, and experimentally
induced. Am J Kidney Dis. 26:678–690. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Patel NA, Patel PS and Vora HH: Role of
PRL-3, Snail, Cytokeratin and Vimentin expression in epithelial
mesenchymal transition in breast carcinoma. Breast Dis. 35:113–127.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kokkinos MI, Wafai R, Wong MK, Newgreen
DF, Thompson EW and Waltham M: Vimentin and epithelial-mesenchymal
transition in human breast cancer-observations in vitro and in
vivo. Cells Tissues Organs. 185:191–203. 2007. View Article : Google Scholar : PubMed/NCBI
|