Introduction
As medical knowledge evolved, the general awareness
of the hepatitis C virus (HCV) has changed since its discovery, as
it was not only considered as the etiological cause of chronic
hepatitis, but also the cause of numerous HCV-associated diseases,
particularly autoimmune diseases, such as cryoglobulinemia,
Sjörgen's syndrome and arthritis (1). After HCV-associated cryoglobulinemia
was reported for the first time, medical research has focused on
it. In the past decade, numerous studies reported that a majority
of cases of mixed cryoglobulinemia (MC) were associated with HCV
(2). Although the underlying
mechanisms have not been fully clarified, cryoglobulin formation is
at least linked to chronic HCV infection (3). A total of 20–30% of MC patients had
renal involvement, mainly due to membranoproliferative
glomerulonephritis (MPGN) (4).
Therefore, treatments of these patients have mainly focused on the
reduction of the viral load, the elimination of immune complexes
and the function of B cells. Hence, the present case study
presented a case diagnosed as MC with MPGN and its treatment, and
summarized the diagnosis, clinical manifestations and treatments of
MC with a particular focus on MC-associated renal injury.
Case report
Informed consent was provided by the patient's
husband for her inclusion in the present study. A-58-year-old woman
presented at the Department of Nephrology of the Second Hospital of
Jilin University (Changchun, China) on December 17th 2012 with
intermittent edema in the lower limbs that had persisted for eight
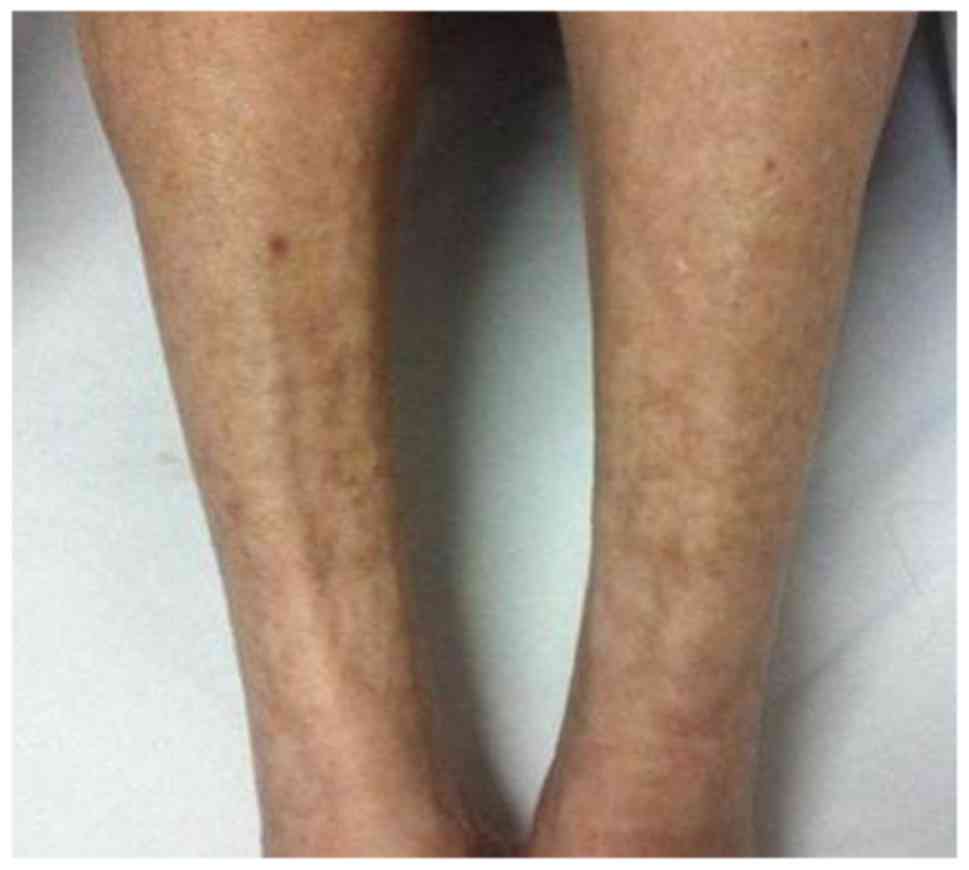
months and proteinuria for one month, while purpura on the
bilateral lower limbs had been present for five years (Fig. 1), which deteriorated in the cold. The
patient's clinical and laboratory data at first presentation are
listed in Table I. The qualitative
cryoglobulin test was positive. Renal biopsy was performed and
immunofluorescence results were as follows: Immunoglobulin
(Ig)A(+/−), IgM(+), IgG(+), complement C3(2+), complement C4(−),
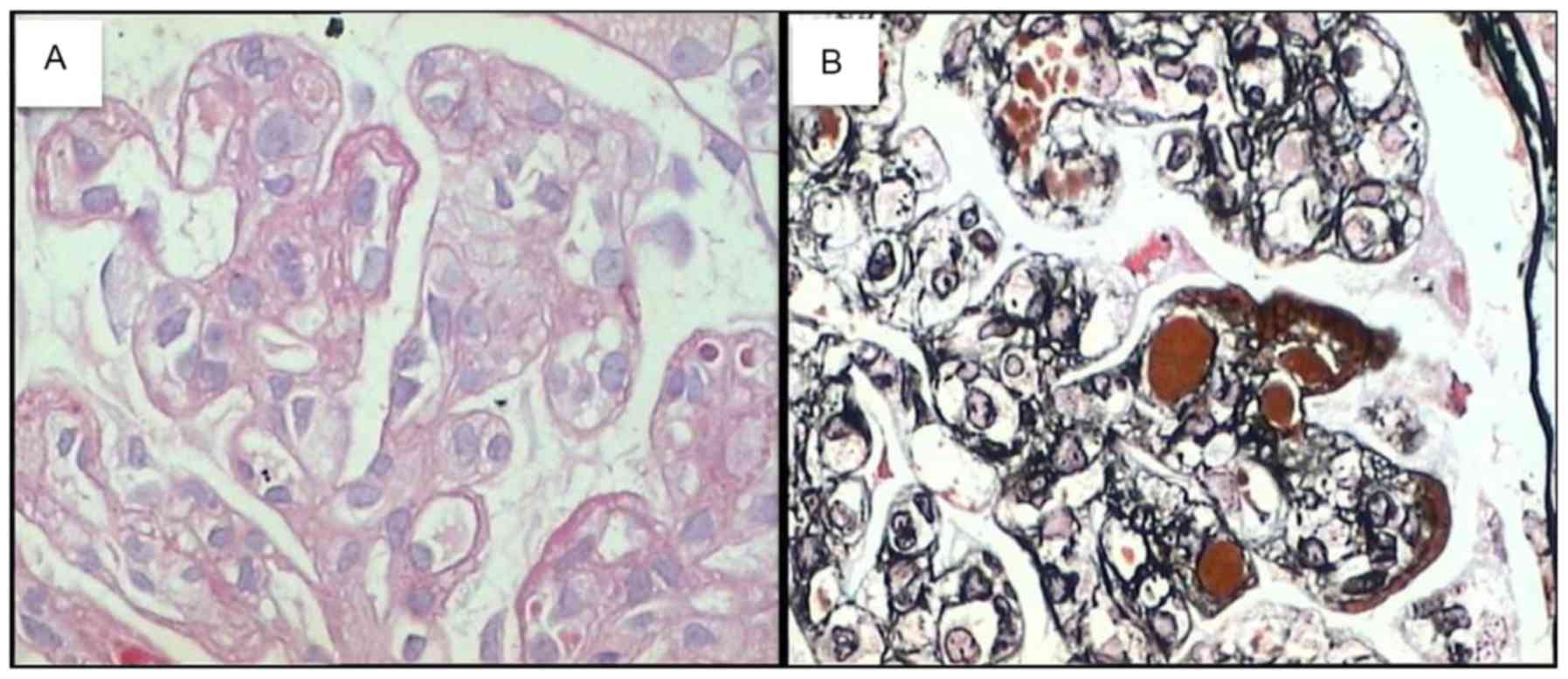
complement factor lq(+) and fibrinogen(−). Light microscopic:
Periodic Acid-Schiff staining revealed 37 intact glomeruli, parts
of which were hypertrophic, and two sclerotic gomeruli, one of
which displayed fibrous crescent formation. Glomerular mesangial
cells and endothelial cells were diffuse with severe hyperplasia.
Vacuolar degeneration of endothelial cells and microthrombosis in
capillary lumens was observed, and most of the capillary loops were
obstructed. Various renal tubular epithelial cells had degenerated
with small focal atrophy and occasional protein casts. Renal
interstitial edema, focal inflammation and inflammatory cell
infiltration were present, particularly around blood vessels. Part
of the wall of an arteriole was slightly thickened; Periodic Acid
Methenamine Silver + Masson staining revealed a diffuse double
track appearance without identifiable spikes, and endothelial and
mesangial areas contained diffuse eosinophilic complexes with red
staining (Fig. 2). The pathological
diagnosis was mesangial proliferative glomerulonephritis
(HCV-associated cryoglobulinemia) with a possibility of MPGN. Based
on all of these results and the quantity of HCV RNA, the patient
was given interferon α (500,000 IU every other day) by subcutaneous
injection from December 19th 2012 until the quantity of HCV RNA
reached below the minimum on January 30th 2013. Subsequently, the
patient was treated with prednisolone (40 mg daily) and continued
to use interferon α. In response to this treatment, the results of
continuous examinations of the liver, kidney function, blood,
routine urine, 24-h proteinuria and the load of HCV RNA remained
steady. The patient's 24-h proteinuria was reduced to 1.95 g after
three months and continued to take oral prednisolone and interferon
α without the occurrence of any edema. Therefore, prednisolone was
gradually reduced, while interferon α treatment was continued.
 | Table I.Laboratory parameters at onset. |
Table I.
Laboratory parameters at onset.
| Parameter | Value | Normal range |
|---|
| White blood cells
(×109/l) | 6.7 | 3.5–9.5 |
| Haemoglobin
(g/l) | 93 | 115–150 |
| Platelet count
(×109/l) | 207 | 125–350 |
| Creatinine
(µmol/l) | 76.9 | 44–106 |
| Proteinuria/24 h
(g) | 3.65 | <0.15 |
| Albumin (g/l) | 22.5 | 40–55 |
| HBsAg | Negative | 0–0.05 |
| Anti-HBs | Negative | Negative |
| Anti-HBc | Negative | Negative |
| HCV-RNA (IU/ml) |
1.12×107 | <1.0e2 |
| Rheumatoid factor
(IU/ml) | 459.0 | <20 |
| Complement C4
(mg/dl) | <1.67 | 16–38 |
| Complement C3
(mg/dl) | 91.6 | 79–152 |
| ANA | Negative | <1:100 |
| HIV | Negative | 0–1.0 |
| Proteinuria | 3+ | Negative |
| IgG (g/l) | 10.7 | 7.51–15.6 |
| IgM (g/l) | 3.4 | 0.46–3.04 |
| IgA (g/l) | 1.85 | 0.82–4.53 |
| Erythrocyte
sedimentation rate (mm) | 74.0 | <20 |
| ANCA | Negative | Negative |
The patient presented at our department for the
second time on August 30th 2013 due to elevated axillary
temperature (>37.2°C) for 15 days and after using interferon α;
further symptoms, including cough, expectoration, abdominal pain,
diarrhea as well as increased urinary frequency and urgency, were
not present. Clinical examination revealed an axillary temperature
of 38°C and a blood pressure of 160/80 mmHg, and findings of the
heart, lung and abdominal examination were unremarkable. The
bilateral lower limbs showed a large tract of purpura without
tenderness and edema. Laboratory parameters were as follows (normal
ranges in brackets): Albumin (40–55 g/l), 40.2 g/l; creatinine
(44–106 µmol/l), 81.8 µmol/l; blood leukocytes
(3.5–9.5109 cells/l), 18.3×109 cells/l;
hemoglobin (115–150 g/l), 108 g/l; platelets
(125–350×109 cells/l), 154×109 cells/l; urine
protein (negative), 2+; urine leucocytes [0-5/high power field
(HPF)], 81.3/HPF. Chest computed tomography showed tracheitis.
Abdominal doppler ultrasound was normal. The load of HCV was always
below the minimum value. These results were unlikely to be due to
prednisolone intake, while the use of interferon α may have caused
the fever; it was therefore discontinued and anti-inflammatory
therapy (linezolid, 0.6 g twice daily; meropenem, 1.0 g twice
daily; and micafungin, 0.15 g once daily, all intravenous) was
commenced. The temperature was consistently >39°C and returned
to normal values after 2 days. However, the whole blood cell count
decreased significantly, while serum creatinine increased and
oliguria occurred, and the patient died of multiple organ
dysfunction syndrome on November 22nd 2013.
Discussion
MC-associated glomerulonephritis is the most
important extrahepatic manifestation of chronic HCV infection, but
its prognosis is poor. Furthermore, as unified treatment guidelines
are currently lacking, a comprehensive and rigorous assessment of
each patient is required, based on which a reasonable treatment is
selected and changes of the disease require monitoring,
particularly when various drugs are used in combination.
HCV has been considered as the major etiological
cause of non-A and -B chronic hepatitis since its discovery in
1989; furthermore, clinical and experimental studies showed that
HCV may cause certain autoimmune diseases, such as MC and Sjörgen's
syndrome (1). While it has been
reported that ~1/3 of HCV-associated MC was accompanied with renal
damage (5), this rarely occurs in
China, only few case reports (6).
Tarantino et al (7)
postulated the following major clinical diagnostic criteria: i)
Cryoglobulin present in serum; ii) hematuria, proteinuria and renal
damage; and iii) renal biopsy pathology consistent with the
pathological characteristics of cryoglobulinemia nephropathy. Other
minor clinical features such as purpura and rheumatoid factor may
also be considered. The quantitative determination of cryoglobuline
is of high importance. However, due to the limitations of our
hospital, only qualitative detection was possible. Combined with
the pathological results, clinical manifestations and auxiliary
examination, the diagnosis of cryoglobulinemia was established.
As cryolobulinemia is vasculitis in its essence, it
may involve multiple organs. The deposition of cryoglobulins and
complement leads to diverse pathological manifestations, including
purpura, skin ulcers and glomerulonephritis. The most common
symptoms of MC are weakness, peripheral neuropathy, arthralgias and
purpura (Meltzer and Franklin triad), Raynaud's phenomenon, sicca
syndrome, renal involvement, lung disorders, elevated axillary
temperature >37.2°C and hematocytopenia. The frequent
manifestations of MC are peripheral neuropathy and skin ulcers,
which are barely responsive to treatments and may consequently
compromise the patient's quality of life. The association between
cryoglobulinemia and glomerulonephritis has been demonstrated
(8). In previous studies, 20% of
patients with MC were diagnosed with nephropathy, which even
increased to 35–60% during the follow-up period, and renal injury
is one of the factors associated with the worst prognosis of MC
patients (8,9). MC-associated nephropathy is clinically
characterized by hematuria, proteinuria, edema and renal failure.
The pathology is similar to MPGN, but histological examination of
renal biopsy specimens revealed cryoglobulin thrombi in the
capillaries. Clinical studies have shown that MC patients often
present with one or more subclinical signs of renal involvement,
including asymptomatic hematuria, nephrotic syndrome, nephrotic
proteinuria (<3 g/24 h), or only renal dysfunction (10). A total of 30% of patients may present
with acute nephrotic syndrome (11).
Nearly 15% of patients progressed to terminal chronic renal
failure, even requiring dialysis (12). Certain examinations are important;
the obvious reduction or undetectable levels of complement C4 are
another hallmark of the disease and is particularly useful for
disease classification, while the activity/severity of
cryoglobulinemia is rarely correlated with the complement C4 level.
However, they are not appropriate for clinical monitoring of
patients and therapeutic decision-making.
The treatment of HCV-associated MC is particularly
based on the complex pathogeny and clinical manifestations of the
disease. In fact, MC may be treated at three different levels; type
I cryoglobulinemia, type II cryoglobulinemia and type III
cryoglobulinemia. The principles for treating MC are as follows: i)
If no symptoms of cryoglobulinemia are present, no treatment is
applied; ii) secondary cryoglobulinemia is mainly managed by
treating the primary disease; iii) if only joint symptoms are
present, non-steroid anti-inflammatory drugs are given; iv)
corticosteroids and immunosuppressive agents are suitable for
patients with serious illness and visceral injury; and v) plasma
exchange for severe disease (13).
Studies have suggested that low doses of interleukin 2 are suitable
for treating HCV-associated cryoglobulinemia. The treatment of HCV
infection-associated symptoms mainly comprises anti-viral therapy,
but guidelines regarding the best anti-viral treatment of these
patients remain to be established. The current options are
pegylated interferon α and ribavirin, which are applied according
to the type and the quantity of HCV. Thus, rituximab has been used
as an agent to treat HCV-associated autoimmune diseases according
to B-cell lymphoproliferation (14–16). The
patient of the present study was a typical case of cryoglobulinemia
and renal damage secondary to HCV infection; prednisolone combined
with interferon therapy was initially effective, while multiple
organ failure and death eventually occurred. As cryoglobulinemia is
essentially vasculitis, it ultimately causes multiple organ damage,
and the prognosis of patients with renal damage caused by HCV is
therefore poor. Despite of the early treatment with prednisolone
and interferon being effective, it easily induces refractory
infections.
In conclusion, the present study found that
HCV-associated cryoglobulinemia with MPGN treated with prednisolone
and interferon α is effective; however, monitoring of the patient
focused on the drugs' effects, while side effects wereignored.
Therefore, the present case study provided experience for future
treatments and it is suggested that clinical studies are required
to identify the most appropriate treatment strategies.
References
|
1
|
Ferri C, Antonelli A, Mascia MT,
Sebastiani M, Fallahi P, Ferrari D, Pileri SA and Zignego AL:
HCV-related autoimmune and neoplastic disorders: The HCV syndrome.
Dig Liver Dis. 39 Suppl 1:13–21. 2007. View Article : Google Scholar
|
|
2
|
Dammacco F and Sansonno D: Antibodies to
hepatitis C virus in essential mixed cryoglobulinaemia. Clin Exp
Immunol. 87:352–356. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neumann AU, Lam NP, Dahari H, Gretch DR,
Wiley TE, Layden TJ and Perelson AS: Hepatitis C viral dynamics in
vivo and the antiviral efficacy of interferon-a therapy. Science.
282:103–107. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dammacco F, Sansonno D, Piccoli C, Tucci
FA and Racanelli V: The cryoglobulins: An overview. Eur J Clin
Invest. 31:628–638. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
lannuzzella F, Vaglio A and Garini G:
Management of hepatitis C virus-related mixed cryoglobulinemia. Am
J Med. 123:400–408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao LJ, Chen F, Li JG, Yin R, Zhang XH,
Huang SM and Liu F: Hepatitis C virus-related mixed
cryoglobulinemic endocapillary proliferative glomerulonephritis and
B-cell non-Hodgkin lymphoma: A case report and literature review.
Eur Rev Med Pharmacol Sci. 19:3050–3055. 2015.PubMed/NCBI
|
|
7
|
Tarantino A, De Vecchi A, Montagnino G,
Imbasciati E, Mihatsch MJ, Zollinger HU, Di Belgiojoso GB, Busnach
G and Ponticeli C: Renal disease in essential mixed
cryoglobulinaemia. Long-term follow-up of 44 patients. Q J Med.
50:1–30. 1981.PubMed/NCBI
|
|
8
|
Daghestani L and Pomeroy C: Renal
manifestations of hepatitis C infection. Am J Med. 106:347–354.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferri C, Sebastiani M, Giuggioli D,
Cazzato M, Longombardo G, Antonelli A, Puccini R, Michelassi C and
Zignego AL: Mixed cryoglobulinemia: Demographic, clinical and
serologic features and survival in 231 patients. Semin Arthritis
Rheum. 33:355–374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ozkok A and Yildiz A: Hepatitis C virus
associated glomerulopathies. World J Gastroenterol. 20:7544–7554.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Misiani R, Bellavita P, Fenili D, Borelli
G, Marchesi D, Massazza M, Vendramin G, Comotti B, Tanzi E,
Scudeller G, et al: Hepatitis C virus infection in patients with
essential mixed cryoglobulinemia. Ann Intern Med. 117:573–577.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tarantino A, Campise M, Banfi G,
Conflalonieri R, Bucci A, Montonli A, Cloasanti G, Damilano I,
D'Amico G, Minetti L, et al: Long-term predictors of survival in
essential mixed cryoglobulinemic glomerulonephritis. Kidney Int.
47:618–623. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhattarai M, Woytowitz DV, Kaldash H,
Bastacky S, Chen SS, Johnston JR and Bernardo JF: Recurrent mixed
cryoglobulinemia (MCS): A case report and literature review. R I
Med J (2013). 98:33–37. 2015.PubMed/NCBI
|
|
14
|
De Vita S and Quartuccio L: Treatment of
rheumatoid arthritis with rituximab: An update and possible
indications. Autoimmun Rev. 5:443–448. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grillo-López AJ, Hedrick E, Rashford M and
Benyunes M: Rituximab: Ongoing and future clinical development.
Semin Oncol. 29 1 Suppl 2:1–112. 2002. View Article : Google Scholar
|
|
16
|
Patel DD: B cell-ablative therapy for the
treatment of autoimmune diseases. Arthritis Rheum. 46:1984–1985.
2002. View Article : Google Scholar : PubMed/NCBI
|