Introduction
Postmenopausal women are prone to develop
postmenopausal osteoporosis (PM-OP), which is caused by estrogen
deficiency after menopause (1). The
primary pathologic change caused by PM-OP is bone collagen loss as
a result of estrogen depletion, which subsequently stimulates
imbalanced bone remodeling (2).
Osteoblasts synthesize type I collagen, which composes the primary
organic matrix of bone (3).
Transforming growth factor (TGF)-β1 has an important role in cell
proliferation, differentiation and apoptosis, as well as bone
collagen synthesis (4). The TGF-β1
signaling pathway is initiated by ligands that bind to TGF-β
receptors, which activate the transfer of intracellular mothers
against decapentaplegic homolog (Smad) proteins into the nucleus
and transmit the signal (5). Once
TGF-β receptors are activated by ligands via the phosphorylation of
Smad family members, TGF-β receptor type I (TβRI) initiates signal
transmission (6). At present, eight
types of Smad proteins have been identified in mammals (7) Smads are divided into three categories
based on their structure and function: Receptor-regulated Smads,
which include Smad2 and Smad3; common-mediator Smads, which include
Smad4; and inhibitory Smads, which include Smad6 and Smad7
(8,9). Increased TGF-β1 expression is
accompanied by increased Smad2, Smad3 and Smad4 expression, as well
as cell proliferation in in vitro cultured osteoblasts
(10). Research regarding Smad7 is
comparatively limited. Type I collagen is an important gene for
determining bone mineral density and osteoporosis predisposition
(11). Human bone is primarily
composed of collagen, which determines bone strength and quality
(12). Type I collagen is the most
abundant type of collagen found in bone and constitutes ~95% of the
entire collagen content of bone (13).
Osteoblasts are a vital type of cells in bone tissue
and are important for the maintenance of normal bone mass and bone
formation. The function of osteoblasts is associated with
proliferation, differentiation and mineralization in the bone
formation process and in alternative bone structure-associated
processes (14). Osteoblast
proliferation and type I collagen secretion may be either increased
or reduced through the TGF-β/Smad signaling pathway (10). Smad7 is an important factor in the
negative feedback loop of the TGF-β/Smad signaling pathway and has
an inhibitory effect on TGF-β, activin and the bone morphogenetic
protein signaling pathway (15).
A previous study has demonstrated that inadequate
secretion of ovarian hormones is the primary cause of osteoporosis
(16). A number of studies have
suggested that the inhibitory mechanism of estrogen in osteoporosis
occurs through the proliferation of osteoblasts and the promotion
of collagen secretion (17–19). Phytoestrogens exist in a large
variety of plants and are structurally similar to estrogen
(20) Previous studies have
demonstrated that phytoestrogens improve and protect mineralized
skeletal bone remodeling (21,22).
Fructus Psoraleae, which is the fruit of Psoralea
corylifolia L., is a principal traditional Chinese medicine and
is included in the Chinese Pharmacopoeia (23,24).
Isopsoralen is a derivative of Fructus Psoraleae and is considered
one of the active components of the herb (25). Psoralen exhibits estrogenic activity
that significantly promotes MCF-7 cell proliferation (26).
Isopsoralen is the active ingredient of psoralen,
and the present study examined the effect of isopsoralen on
pre-osteoblasts. Isopsoralen is a type of phytoestrogen that
promotes cultured MC3T3-E1 cell proliferation and differentiation
(27). However, at present, the
underlying mechanisms by which isopsoralen promotes the TGF-β
signaling pathway in osteoblasts in vitro has not been
reported. The present study aimed to observe the effect of
different concentrations of isopsoralen on MC3T3-E1 cell viability,
TGF-β1 reporter gene activity and type I collagen and Smad7 protein
expression. Additionally, the mechanism by which the phytoestrogen,
isopsoralen, prevents and treats primary osteoporosis was
investigated. The results of the present study reveal the
mechanisms of the functional estrogen-like effects of isopsoralen
and have applications to clinical gynecological medicine,
particularly for postmenopausal women with osteoporosis.
Materials and methods
Experimental cells
Murine pre-osteoblast cells (MC3T3-E1) were cultured
in DMEM (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS; Hyclone; GE
Healthcare Life Sciences) in an atmosphere containing 5%
CO2 at 37°C. MC3T3-E1 were purchased from the Cell
Center of the Institute of Basic Medical Sciences Chinese Academy
of Medical Sciences (Beijing, China).
Primary reagents and drugs
Isopsoralen was obtained from the National
Institutes for Food and Drug Control (Beijing, China). Dulbecco's
modified Eagle's medium (DMEM) and FBS were purchased from Hyclone
(GE Healthcare Life Sciences). The following reagents were also
utilized: RNA extraction reagent and TRIzol reagent (both from
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA);
GoldView nucleic acid stain (Promega Corp., Madison, WI, USA);
diethylpyrocarbonate (DEPC) water, 6X DNA loading buffer and 5X
Tris-acetate-EDTA solution (SBS Genetech Co., Ltd., Beijing,
China); upstream and downstream primers (Invitrogen; Thermo Fisher
Scientific, Inc.); M-MLV reverse transcriptase (200 U/µl), M-MLVRT
5X reaction buffer and RNA enzyme inhibitors (all from Promega
Corp.); Taq DNA polymerase 3 U/µl, 10X reaction buffer (with
MgCl2), and 10 mM dNTPs (pH 7.5; all from Takara
Biotechnology Co., Ltd., Dalian, China); and isopropyl alcohol,
chloroform, anhydrous ethanol (analytically pure), streptomycin
sulfate, penicillin, (all from Beyotime Institute of Biotechnology,
Haimen, China). Anti-Smad7 (sc-11392) and anti-GAPDH (sc-365062)
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Polyclonal horseradish peroxidase-conjugated
goat anti-rabbit (ZB-2308) and rabbit anti-mouse (ZB-2305)
immunoglobulins were obtained from Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd. (Beijing, China).
Cell viability assay
The effect of isopsoralen on MC3T3-E1 cell viability
was detected by the MTT method. MC3T3-E1 cells were seeded in
96-well plates at density of ~3×103 cells/well. When
cells adhered to the plate, serum-free DMEM was added and the cells
were incubated at 37°C in a humidified atmosphere containing 5%
CO2 for 24 h. After 24 h in serum-free culture medium,
cells were cultured in DMEM with 10% FBS containing 0, 0.1, 0.01,
0.001 or 0.0001 µM isopsoralen for 72 h at 37°C in an atmosphere
containing 5% CO2. Subsequently, 4 mg/ml MTT solution in
PBS was added to each well. Following incubation for 4 h at 37°C
with 5% CO2, dimethyl sulfoxide was added to each well
to dissolve the formazan crystals. MTT reduction was determined by
measuring the absorbance of each well at 490 nm using a microplate
spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Luciferase reporter gene assay
(CAGA)12-Luc-reporter plasmid along with an internal
control (pRL-TK vector; Promega Corporation) were transfected into
HEK293T cells using Vigofect (Vigorous Inc., Beijing, China),
according to the manufacturer's instructions. After 24-h
transfection, HEK293T were stimulated with different concentrations
(0, 0.1, 0.01, 0.001 or 0.0001 µM) of isopsoralen. Isopsoralen
stimulation lasted for 12 h and then reporter activity was
determined by the Dual-Luciferase Assay System (Vigorous Inc.). The
Luciferase activity was measured using a Luminescence Counter Top
Count NXT (Packard Instrument Company, Inc., Meriden, CT, USA).
Firefly luciferase activity was normalized against Renilla
luciferase activity.
Cell grouping
A total of 2×105 cells were transferred
into five plates (60-mm cell culture dishes). Waste liquid was
aspirated from each plate when the cells reached 60% confluence.
Subsequently, 3 ml DMEM supplemented with 10% FBS and penicillin
(100 U/ml) and streptomycin (100 U/ml) was added and all cells were
incubated in an incubator at 37°C in an atmosphere containing 5%
CO2. Synchronization was performed for 24 h to cause
cell quiescence at the G0 stage. The cells in each plate were
divided into the following five groups: The control (0 µM
isopsoralen), 0.1 µM (0.1 µM isopsoralen), 0.01 µM (0.01 µM
isopsoralen), 0.001 µM (0.001 µM isopsoralen) and 0.0001 µM (0.0001
µM isopsoralen) groups. The control group was cultured in DMEM
medium supplemented with 10% FBS. The other 4 groups were cultured
in DMEM supplemented with 10% FBS containing the respective
concentrations of isopsoralen. Each group was incubated at 37°C
with 5% CO2. Each group of cells were stimulated by
isopsoralen for 72 h in a 60-mm plastic dish and then the total RNA
and protein were extracted and used for RT-PCR or western
blotting.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total cellular RNA was prepared from MC3T3-E1 cells
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
The concentration and purity of total RNA were evaluated by
measuring the 260/280 nm ratios from the cells following 72-h
incubation at 37°C with 5% CO2. Total RNA (5 µg) was
denatured at 70°C for 5 min and then rapidly chilled on ice. Each
RT reaction mixture contained 5 µg total RNA, 1 µl oligo (dT) 15
primer (SBS Genetech Co., Ltd., Beijing, China), 1 µl dNTPs, 4 µl
5X M-MLV buffer, 1 µl RNA enzyme inhibitors and 20 µl DEPC water.
The reaction mixture was mixed by centrifugation at 300 × g for 5
sec, incubated at 42°C for 60 min, then at 95°C for 5 min, cooled
on ice for 5 min and stored at −20°C. The final reaction mixture
for cDNA PCR included 36.5 µl DEPC water, 5 µl 10X reaction buffer,
2 µl upstream and downstream primers of each target, 1 µl dNTPs, 3
µl reverse transcription product and 0.5 µl Taq DNA polymerase (3
U/µl). The above reaction solution was incubated in a PCR machine,
and the following PCR reaction conditions were employed: 94°C for 5
min; 28 cycles of 94°C for 30 sec, 59°C for 30 sec and 72°C for 45
sec; followed by 10 min at 72°C. The primers used for PCR
amplification were as follows: Type 1 collagen forward,
5′-GACGCCATCAAGGTCTACTG-3′ and reverse, 5′-ACGGGAATCCATCGGTCA-3′
(product size 154 bp) and GAPDH forward, 5′-GCATTGTGGAAGGGCTCA-3′
and reverse, 5′-GGGTAGGAACACGGAAGG-3′ (product size 207 bp)
(28). The above primers were
synthesized by Invitrogen (Thermo Fisher Scientific, Inc.).
Experiments were performed in triplicate and GAPDH was considered
the internal reference.
Semi-quantitative analysis of the
amplification product
The PCR products (10-µl volume for each product)
were analyzed by 1% agarose gel electrophoresis and stained with
GoldView. The optical density of the electrophoretic bands was
scanned using an (AlphaImager 1220; Alpha Innotech, San Leandro,
CA, USA). The ratio of the electrophoretic band optical density of
collagen and of GAPDH were obtained to determine the relative mRNA
expression level.
Western blot analysis
Total protein was extracted from the cells following
72-h incubation at 37°C with 5% CO2. To extract the
cytoplasmic protein, 200 µl cytosolic lysate was added to each
culture and cultures were placed on ice for 30 min. Cells were
detached with a cell scraper, transferred into a pre-cooled
Eppendorf (EP) tube and centrifuged at 8,000 × g for 10 min at 4°C.
The supernatant was immediately transferred into a pre-cooled EP
tube and mixed with an equal volume of buffer solution. The sample
was subsequently boiled at 100°C for 10 min and then stored at
−20°C. A total of 10 µl supernatant was used to determine the
lysate protein concentration via the BCA kit (Beyotime Institute of
Biotechnology) and the rest of supernatant was stored at −80°C.
Aliquots of denatured protein liquid (30 µg) were
loaded and separated by SDS-PAGE (10% separation gel, 4%
concentrated gel). The proteins from the gel were then blotted onto
a nitrocellulose membrane (EMD Millipore, Billerica, MA, USA). The
membrane was then incubated overnight at 4°C. Anti-Smad7
(1:500-1,000; sc-11392) and anti-GAPDH (1:1,000; sc-365062) primary
antibodies were added to the blots and incubated at 4°C overnight.
Once the membrane was washed three times for 10 min each time in
Tris-buffered saline with Tween-20 (TBST), secondary antibodies
polyclonal horseradish peroxidase-conjugated goat anti-rabbit
(ZB-2308) and goat anti-mouse immunoglobulins (ZB-2305; Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd.) were subsequently
added and the membrane was incubated at 37°C for 1 h. The membrane
was washed again using the 1X TBST buffer for 10 min three times
and then stained with enhanced chemiluminescence reagent (Amersham
Biosciences Corp., Piscataway, NJ, USA), and exposed to X-ray film.
Image J analysis software 1.42q (National Institutes of Health,
Bethesda, MA, USA) was used to conduct the image analysis.
Statistical analysis
Data are presented as mean ± standard error. Three
independent biological repeats were performed for each treatment.
Single factor variance analysis was used to analyze all
experimental data via the statistical software package SPSS 13.0
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Detection of the effect of isopsoralen
on MC3T3-E1 cell viability using the MTT method
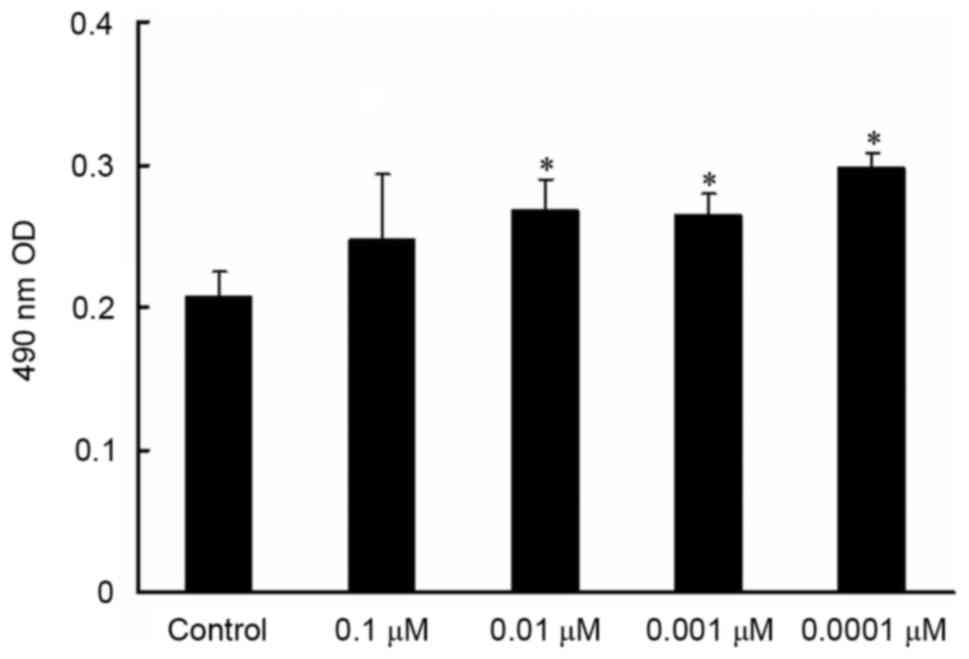
MTT assay revealed that MC3T3-E1 cell viability
exhibited a dose-dependent increase with decreasing isopsoralen
concentrations and that MC3T3-E1 cell viability was significantly
increased following treatment with 0.01, 0.001 and 0.0001 µM
isopsoralen compared with the control group (P<0.05; Fig. 1).
Effect of isopsoralen on the relative luciferase
activity of the TGF-β1 reporter gene. Compared with the control
group, 0.01, 0.001 and 0.0001 µM isopsoralen treatment was capable
of significantly increasing luciferase activity and activating the
TGF-β1 reporter gene (P<0.05; Fig.
2). There was no significant difference between 0.01, 0.001 and
0.0001 µM isopsoralen treatment groups on the relative luciferase
activity (Fig. 2).
Effect of isopsoralen on type I
collagen mRNA expression levels in pre-osteoblast cells
RT-PCR assays revealed the effect of isopsoralen on
MC3T3-E1 cells after 72 h compared with the control group. Doses of
0.01, 0.001 and 0.0001 µM isopsoralen significantly promoted type I
collagen mRNA expression levels in MC3T3-E1 cells after 72 h of
stimulation compared with the control group (P<0.05; Fig. 3).
Effect of isopsoralen on Smad7 protein
expression levels in pre-osteoblast cells
Western blot analysis results revealed that, after
MC3T3-E1 cells were treated with various concentrations of
isopsoralen for 72 h, Smad7 protein expression levels were
significantly decreased following treatment with 0.01, 0.001 and
0.0001 µM isopsoralen compared with the control group (P<0.05;
Fig. 4).
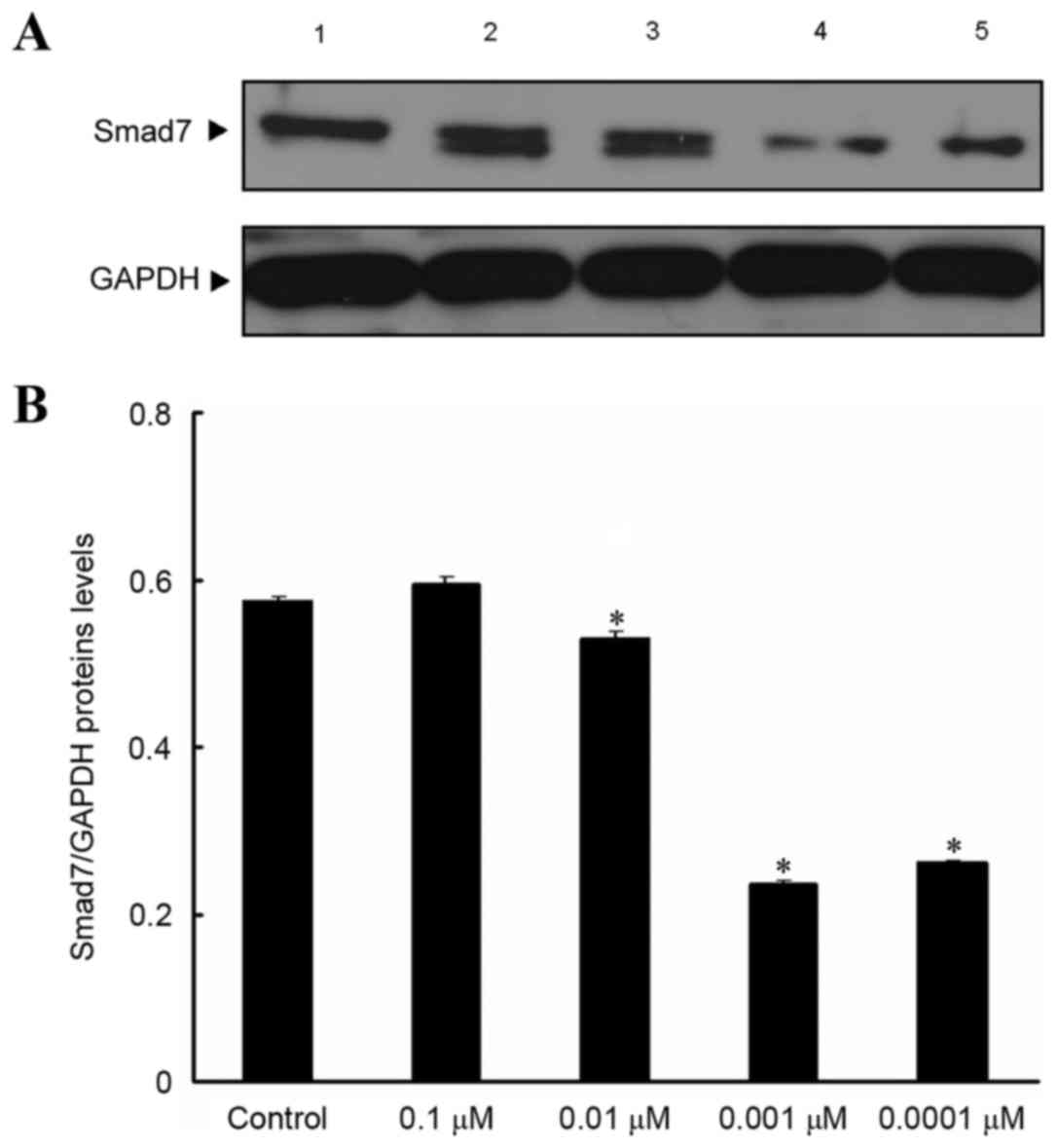 | Figure 4.Effect of isopsoralen on Smad7
protein expression levels in in pre-osteoblast cells. (A) Smad7
protein expression levels in MC3T3-E1 cells were detected by
western blot analysis after stimulation with, 0, 0.1, 0.01, 0.001
or 0.0001 µM isopsoralen for 72 h. (B) The relative protein
expression levels were determined. *P<0.05 vs. control group.
Smad, mothers against decapentaplegic homolog; 1, control group; 2,
0.1 µM isopsoralen group; 3, 0.01 µM isopsoralen group; 4, 0.001 µM
isopsoralen group; 5, 0.0001 µM isopsoralen group. |
Discussion
Estrogen protects bones by promoting calcitonin
secretion, inhibiting bone resorption, facilitating intestinal
calcium absorption and accelerating bone proliferation (29). A previous study indicated that hip
fractures caused by PM-OP are associated with aging and declining
levels of estrogen, with declining estrogen levels being the
primary cause (30). Hormone
replacement therapy (HRT) has a positive effect on treating PM-OP,
as shown by years of clinical practice (31–33).
Some older women are administered long-term estrogen to protect
against osteoporosis (34,35). Currently, estrogen replacement
therapy is an effective method for treating PM-OP. However,
estrogen is not suitable for all postmenopausal women and long-term
application of estrogen may result in increased risks of high blood
coagulation status, high blood pressure, edema, breast cancer and
endometrial cancer (36). Studies
have demonstrated that the use of HRT for >5 years can
significantly increase the mortality rate of breast cancer
(37). Some patients with breast
cancer who receive aromatase inhibitor therapy are more vulnerable
to osteoporosis symptoms; therefore, the use of hormones for
treating osteoporosis in such patients should be avoided (38). As a result, HRT administration should
be limited to the shortest possible duration and alternatives to
HRT should be used to treat menopausal syndromes (39). Consequently, the identification of
natural agents with minimal side effects is a novel and exciting
field for the treatment of PM-OP.
The imbalance of bone formation and resorption is an
important reason for the occurrence of PM-OP that is caused by
decreased estrogen levels. This results in a decrease in TGF-β1 in
postmenopausal women, which may predispose these women to
osteoporosis and to delayed wound repair (40). TGF-β1 has crucial roles in osteoblast
proliferation and differentiation, as well as in matrix production
(41). Previously, TGF-β1 was
injected into rat periosteum and was indicated to improve the
formation of new bone (42). The
uncoupling of bone resorption and formation caused by decreased
estrogen levels is associated with alterations in TGF-β1 and its
signaling pathway in bone tissue (43,44). The
CAGA box is essential and sufficient for TGF-β signal induction and
CAGA elements are widely used motifs in TGF-β-regulated
transcription (45). The present
study demonstrated that specific concentrations of isopsoralen
significantly increased TGF-β1 reporter gene luciferase activity.
The mechanism by which isopsoralen stimulates TGF-β1 cell signaling
has not been investigated; however, its mechanism may be attributed
to the similarity of the structure of phytoestrogens to that of
mammalian estrogen and to the estrogenic activities of
phytoestrogens (46). The ability of
estrogen to induce an increase TGF-β1 expression has been reported
previously (40,47).
Phytoestrogens are a group of plant-derived
substances that are structurally and functionally similar to
estradiol and are able to bind to the estrogen receptor and elicit
estrogenic activities (48,49). Considering these factors, it is
possible that phytoestrogens may be an alternative to
postmenopausal HRT that may ameliorate discomfort and increased
risk of disease. Isopsoralen is a type of phytoestrogen and may
therefore function by binding to the estrogen receptor, thus
promoting TGF-β1 formulation and stimulating the TGF-β1 signaling
pathway. However, few studies have examined the effect of
isopsoralen on the TGF-β1 signaling pathway and collagen secretion
in osteoblasts. Smad7 is an important inhibitory factor in the
downstream transmission path of TGF-β signaling pathways in
osteoblasts (50). Smad7 binds with
the activated TβRI receptor and induces biological inhibition
(51), which is an important
mechanism for various drug treatments of osteoporosis that function
by altering the expression of related factors in the TGF-β
signaling pathway in bone tissue (52,53).
Previous studies have demonstrated that estrogen has important
effects on TGF-β1 expression in bone tissue and promotes a series
of changes in osteoblast proliferation at the molecular biological
level. Increased bone collagen secretion in addition to other
changes maintain the relative stability of the bone mass (40,47). The
present study showed that at specific concentrations, isopsoralen
significantly increased osteoblast viability, decreased Smad7
expression, which promoted TGF-β signaling and increased collagen
synthesis. Thus, isopsoralen may be a potential agent for improving
imbalances of collagen metabolism in PM-OP. In conclusion, the
present study demonstrated that phytoestrogen isopsoralen promotes
TGF-β signaling pathway transduction by inhibiting Smad7 protein
expression and increasing type I collagen secretion (Fig. 5). Therefore, isopsoralen may be
useful in the treatment of PM-OP. The present study provides the
scientific basis of the molecular mechanisms by which isopsoralen
acts as a type of phytoestrogen. The present findings indicate that
isopsoralen may be used to treat PM-OP and may contribute to the
research and development of novel agents for the treatment of
PM-OP.
Acknowledgements
The present study was supported by the Fund of
Beijing Municipal Administration of Hospitals Incubating Program
(grant no. PZ, 2016013), the Cooperation Fund of Basic and Clinical
Research of Capital Medical University (grant no. 16JL24), the Fund
for Beijing Science and Technology Development of TCM (grant no.
QN2016-24) and the Fund of Beijing Hospital of Traditional Chinese
Medicine (grant no. YJ-201722).
References
|
1
|
Maeda SS and Lazaretti-Castro M: An
overview on the treatment of postmenopausal osteoporosis. Arq Bras
Endocrinol Metabol. 58:162–171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Janiszewska M, Kulik TB, Dziedzic MA and
Żołnierczuk-Kieliszek D: The contemporary look at the problem of
recognizing and diagnosing postmenopausal osteoporosis and
eliminating the risk of a fall. Prz Menopauzalny. 13:42–47.
2014.PubMed/NCBI
|
|
3
|
Jaha H, Husein D, Ohyama Y, Xu D, Suzuki
S, Huang GT and Mochida Y: N-terminal Dentin Sialoprotein fragment
induces type I collagen production and upregulates dentinogenesis
marker expression in osteoblasts. Biochem Biophys Rep. 6:190–196.
2016.PubMed/NCBI
|
|
4
|
Rydziel S, Varghese S and Canalis E:
Transforming growth factor beta1 inhibits collagenase 3 expression
by transcriptional and post-transcriptional mechanisms in
osteoblast cultures. J Cell Physiol. 70:145–152. 1997. View Article : Google Scholar
|
|
5
|
Katz LH, Li Y, Chen JS, Muñoz NM, Majumdar
A, Chen J and Mishra L: Targeting TGF-beta signaling in cancer.
Expert Opin Ther Targets. 17:743–760. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
López-Casillas F, Wrana JL and Massagué J:
Betaglycan presents ligand to the TGF beta signaling receptor.
Cell. 73:1435–1444. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wrana JL, Attisano L, Wieser R, Ventura F
and Massagué J: Mechanism of activation of the TGF-beta receptor.
Nature. 370:341–347. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heldin CH, Miyazono K and ten Dijke P:
TGF-beta signalling from cell membrane to nucleus through SMAD
proteins. Nature. 390:465–471. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Derynck R, Zhang Y and Feng XH: Smads:
Transcriptional activators of TGF-beta responses. Cell. 95:737–740.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu M, Chen G and Li YP: TGF-β and BMP
signaling in osteoblast, skeletal development, and bone formation,
homeostasis and disease. Bone Res. 4:160092016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steiner RD, Adsit J and Basel D:
COL1A1/2-related osteogenesis imperfectaGeneReviews. Pagon RA, Adam
MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD,
Ledbetter N, Mefford HC, Smith RJH and Stephens K: University of
Washington; Seattle, WA: 2005
|
|
12
|
Saito M and Marumo K: Collagen cross-links
as a determinant of bone quality: A possible explanation for bone
fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos
Int. 21:195–214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gelse K, Pöschl E and Avinger T:
Collagens-structure, function, and biosynthesis. Adv Drug Deliv
Rev. 55:1531–1546. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen G, Deng C and Li YP: TGF-β and BMP
signaling in osteoblast differentiation and bone formation. Int J
Biol Sci. 8:272–288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mukasa C, Nomura M, Tanaka T, Tanaka K,
Nishi Y, Okabe T, Goto K, Yanase T and Nawata H: Activin signaling
through type IB activin receptor stimulates aromatase activity in
the ovarian granulosa cell-like human granulose (KGN) cells.
Endocrinology. 144:1603–1611. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arjmandi BH: The role of phytoestrogens in
the prevention and treatment of osteoporosis in ovarian hormone
deficiency. J Am Coll Nutr. 20 5 Suppl:398S–402S, 417S-420S. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Komm BS, Terpening CM, Benz DJ, Graeme KA,
Gallegos A, Korc M, Greene GL, O'Malley BW and Haussler MR:
Estrogen binding, receptor mRNA, and biologic response in
osteoblast-like osteosarcoma cells. Science. 241:81–84. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Majeska RJ, Ryaby JT and Einhorn TA:
Direct modulation of osteoblastic activity with estrogen. J Bone
Joint Surg Am. 76:713–721. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Benz DJ, Haussler MR and Komm BS: Estrogen
binding and estrogenic responses in normal human osteoblast-like
cells. J Bone Miner Res. 6:531–541. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Virk-Baker MK, Nagy TR and Barnes S: Role
of phytoestrogens in cancer therapy. Planta Med. 76:1132–1142.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamaguchi M and Sugimoto E: Stimulatory
effect of genistein and daidzein on protein synthesis in
osteoblastic MC3T3-E1 cells: Activation of aminoacyl-tRNA
synthetase. Mol Cell Biochem. 214:97–102. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu J, Wang XX, Takasaki M, Ohta A, Higuchi
M and Ishimi Y: Cooperative effects of exercise training and
genistein administration on bone mass in ovariectomized mice. J
Bone Miner Res. 16:1829–1836. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao LH, Wu MH and Xiang BR: Analysis of
Psoralea corylifolia L. fruits in different regions. Chem Pharm
Bull (Tokyo). 53:1054–1057. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen X, Kong L, Su X, Pan C, Ye M and Zou
H: Integration of ion-exchange chromatography fractionation with
reversed-phase liquid chromatography-atmospheric pressure chemical
ionization mass spectrometer and matrix-assisted laser
desorption/ionization time-of-flight mass spectrometry for
isolation and identification of compounds in Psoralea corylifolia.
J Chromatogr A. 1089:87–100. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang D, Yang G, Engelhardt H and Zhang H:
Micellar electrokinetic capillary chromatography of psoralen and
isopsoralen. Electrophoresis. 20:1895–1899. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xin D, Wang H, Yang J, Su YF, Fan GW, Wang
YF, Zhu Y and Gao XM: Phytoestrogens from Psoralea corylifolia
reveal estrogen receptor-subtype selectivity. Phytomedicine.
17:126–131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ming LG, Cheng KM, Ge BF, Ma HP and Zai
YK: Effect of isopsoralen on the proliferation and differentiate of
osteoblasts in vitro. Zhong Yao Cai. 34:404–408. 2011.(In Chinese).
PubMed/NCBI
|
|
28
|
He Z, Feng L, Zhang X, Geng Y, Parodi DA,
Suarez-Quian C and Dym M: Expression of Col1a1, Col1a2 and
procollagen I in germ cells of immature and adult mouse testis.
Reproduction. 130:333–341. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lanyon L, Armstrong V, Ong D, Zaman G and
Price J: Is estrogen receptor alpha key to controlling bones'
resistance to fracture? J Endocrinol. 182:183–191. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
László A: Postmenopausal osteoporosis. Orv
Hetil. 145:3–13. 2004.(In Hungarian). PubMed/NCBI
|
|
31
|
Gallagher JC: Moderation of the daily dose
of HRT: Prevention of osteoporosis. Maturitas. 33 Suppl 1:S57–S63.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pardini D: Menopausal hormone therapy. Arq
Bras Endocrinol Metabol. 51:938–942. 2007.(In Portuguese).
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Prelevic GM, Kocjan T and Markou A:
Hormone replacement therapy in postmenopausal women. Minerva
Endocrinol. 30:27–36. 2005.PubMed/NCBI
|
|
34
|
Gonnelli S, Cepollaro C, Pondrelli C,
Martini S, Monaco R and Gennari C: The usefulness of bone turnover
in predicting the response to transdermal estrogen therapy in
postmenopausal osteoporosis. J Bone Miner Res. 12:624–631. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fitzpatrick LA: Estrogen therapy for
postmenopausal osteoporosis. Arq Bras Endocrinol Metabol.
50:705–719. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dören M and Samsioe G: Prevention of
postmenopausal osteoporosis with oestrogen replacement therapy and
associated compounds: Update on clinical trials since 1995. Hum
Reprod Update. 6:419–426. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pentti K, Honkanen R, Tuppurainen MT,
Sandini L, Kröger H and Saarikoski S: Hormone replacement therapy
and mortality in 52- to 70-year-old women: The kuopio osteoporosis
risk factor and prevention study. Eur J Endocrinol. 154:101–107.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nikander E, Metsä-Heikkilä M, Ylikorkala O
and Tiitinen A: Effects of phytoestrogens on bone turnover in
postmenopausal women with a history of breast cancer. J Clin
Endocrnol Metab. 89:1207–1212. 2004. View Article : Google Scholar
|
|
39
|
Deady J: Clinical monograph: Hormone
replacement therapy. J Manag Care Pharm. 10:33–47. 2004.PubMed/NCBI
|
|
40
|
Ashcroft GS, Dodsworth J, van Boxtel E,
Tarnuzzer RW, Horan MA, Schultz GS and Ferguson MW: Estrogen
accelerates cutaneous wound healing associated with an increase in
TGF-beta1 levels. Nat Med. 3:1209–1215. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bonewald LF and Mundy GR: Role of
transforming growth factor-beta in bone remodeling. Clin Orthop
Relat Res. 1–276. 1990.
|
|
42
|
Noda M and Camilliere JJ: In vivo
stimulation of bone formation by transforming growth factor-beta.
Endocrinology. 124:2991–2994. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Marie P: Growth factors and bone formation
in osteoporosis: Roles for IGF-I and TGF-beta. Rev Rhum Engl Ed.
64:44–53. 1997.PubMed/NCBI
|
|
44
|
Yamada Y, Miyauchi A, Goto J, Takagi Y,
Okuizumi H, Kanematsu M, Hase M, Takai H, Harada A and Ikeda K:
Association of a polymorphism of the transforming growth
factor-beta1 gene with genetic susceptibility to osteoporosis in
postmenopausal Japanese wome. J Bone Miner Res. 13:1569–1576. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dennler S, Itoh S, Vivien D, ten Dijke P,
Huet S and Gauthier JM: Direct binding of Smad3 and Smad4 to
critical TGF beta-inducible elements in the promoter of human
plasminogen activator inhibitor-type 1 gene. EMBO J. 17:3091–3100.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Taxvig C, Elleby A, Sonne-Hansen K,
Bonefeld-Jørgensen EC, Vinggaard AM, Lykkesfeldt AE and Nellemann
C: Effects of nutrition relevant mixtures of phytoestrogens on
steroidogenesis, aromatase, estrogen, and androgen activity. Nutr
Cancer. 62:122–131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Estai MA, Suhaimi F, Das S, Shuid AN,
Mohamed Z and Soelaiman IN: Expression of TGF-β1 in the blood
during fracture repair in an estrogen-deficient rat model. Clinics
(Sao Paulo). 66:2113–2119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Limer JL and Speirs V: Phyto-oestrogens
and breast cancer chemoprevention. Breast Cancer Res. 6:119–127.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
49
|
Knight DC and Eden JA: A review of the
clinical effects of phytoestrogens. Obstet Gynecol. 87:897–904.
1996.PubMed/NCBI
|
|
50
|
Kavsak P, Rasmussen RK, Causing CG, Bonni
S, Zhu H, Thomsen GH and Wrana JL: Smad7 binds to Smurf2 to form an
E3 ubiquitin ligase that targets the TGF beta receptor for
degradation. Mol Cell. 6:1365–1375. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Souchelnytskyi S, Nakayama T, Nakao A,
Morén A, Heldin CH, Christian JL and ten Dijke P: Physical and
functional interaction of murine and Xenopus Smad7 with bone
morphogenetic protein receptors and transforming growth factor-beta
receptors. J Biol Chem. 273:25364–25370. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Peleg S, Uskokovic M, Ahene A, Vickery B
and Avnur Z: Cellular and molecular events associated with the
bone-protecting activity of the noncalcemic vitamin D analog
Ro-26-9228 in osteopenic rats. Endocrinology. 143:1625–1636. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bord S, Beavan S, Ireland D, Horner A and
Compston JE: Mechanisms by which high-dose estrogen therapy
produces anabolic skeletal effects in postmenopausal women: Role of
locally produced growth factors. Bone. 29:216–222. 2001. View Article : Google Scholar : PubMed/NCBI
|