Introduction
Diabetic nephropathy (DN) remains the leading cause
of end-stage renal disease, indicated by albuminuria and reduced
glomerular filtration rate (GFR) as the predictors for prognosis
(1,2). The early identification and monitoring
of DN is one of the major research areas in diabetes, apart from
the control of glycemia, hypertension and dyslipidemia. The
National Kidney Foundation's Kidney Disease Outcomes Quality
Initiative (KDOQI) guidelines (1)
recommend using urinary albumin-creatinine ratio (UCAR) and
estimated GFR (eGFR) as the screening method for DN during the
annual examination for patients with type 2 diabetic mellitus
(T2DM), which has facilitated earlier recognition of DN and formed
the basis for clinical staging (1,3).
Systematic reporting of eGFR using different equations, including
modification of diet in renal disease (MDRD) or chronic kidney
disease epidemiology collaboration (CKD-EPI) equations, are based
on the demographic and laboratory variables, including age and
serum creatinine (SCR) level. However, the testing of SCR in an
everyday clinical setting may be invasive and costly (3–7).
SUDOSCAN (Impeto Medical SAS, Paris, France) is a
non-invasive device for the assessment of sudomotor function
through evaluation of sweat gland secretory function as an early
reflection of sympathetic nerve impairment (8–10). An
electrical current (typically <4 V) is applied to the patients
automatically by the device, which allows the electrochemical skin
conductance (ESC) of the hands and feet to be evaluated. The device
may be used to predict diabetic kidney disease with built-in
algorithms, by evaluating early deficits in sudomotor function. In
a previous cross-sectional study, after adjusting for age, sex, BMI
and HbA1c, hands and feet ESC have been demonstrated to be
associated with eGFR [<60 ml/min/1.73 m2
(P<0.01)], UACR [>30 mg/g (P<0.01)] and UACR [>300 mg/g
(P<0.01)] in populations of European Americans and African
Americans with T2DM (11). In a
recent study, Luk et al (12)
evaluated the clinical utility of SUDOSCAN in detecting CKD and
determined the cut-off point for DN score at 53 for detecting
patients at risk of CKD by using eGFR as the golden standard.
Furthermore, the area under the receiver operating characteristic
curve of SUDOSCAN for CKD was 0.75 (95% confidence interval:
0.72–0.79). However, it has been indicated in other studies that
eGFR has ~90% chance of being within 30% of the measured GFR at
best (4,5,13).
Based on all previously performed studies on
SUDOSCAN and its diagnostic value in kidney dysfunction by using
eGFR as the golden standard for comparison, the present study
decided to use a more direct method to determine kidney function in
T2DM patients by using 99mTc-pentetic acid (DTPA) renal dynamic
imaging method as the confirmatory golden standard (14,15).
The current study aimed to evaluate the diagnostic
value of SUDOSCAN in detecting renal dysfunction of patients with
T2DM in comparison with eGFR results calculated by MDRD and EPI by
using 99mTc-pentetic acid (DTPA) renal dynamic imaging
method as the confirmatory golden standard to provide a more
comprehensive view into the use of SUDOSCAN in screening CKD in
patients with T2DM.
Patients and methods
Subjects
The present study was conducted in Huashan Hospital,
Fudan University (Shanghai, China) from September 2014 to September
2015. The Ethics Committee of Huashan Hospital approved the study.
A total of 176 patients (Male: Female 113:63) diagnosed with T2DM,
aged 18–80 years, with or without symptoms of nephropathy were
continuously enrolled. Written consent was obtained from all
patients enrolled in the study. Exclusion criteria were as follows:
Undiagnosed hyperglycemia, T1DM diagnosis, treatment with drugs
that may have an effect on the sympathetic system such as
beta-blockers and antineoplastic drugs, implantation of electrical
implantable devices, history of seizures or epilepsy, lumbar
sciatic nerve lesion, severe varices of the lower limbs, and other
metabolic diseases including thyroid disease or vitamin B12
deficiency.
Physical examination
One trained nurse examined all patients and recorded
the results. Basic physical characteristics (height, weight, waist
and hip circumference) were measured using standard methods and
body mass index (BMI) and waist-hip ratio (WHR) were calculated.
Blood pressure was recorded in the supine position following 5 min
of rest. Medical history (diabetes, hypertension, dyslipidemia,
cardiovascular disease, cerebrovascular disease and other diseases)
was recorded for each patient. Cardiovascular disease was defined
as history of coronary heart disease. Cerebrovascular disease was
defined as history of stroke.
Laboratory examination
Patients underwent comprehensive metabolic
assessments. Blood and urine samples were collected for fasting
plasma glucose (FPG), glycated hemoglobin (HbA1c) (1–3),
glycated albumin, total cholesterol, low density
lipoprotein-cholesterol (LDL-C), high-density
lipoprotein-cholesterol (HDL-C), triglyceride (1–3)
(16,17), renal function test including serum
creatinine, blood urea nitrogen and uric acid and UACR, after ≥8 h
of fasting (16). HbA1c and glycated
albumin were determined by high-pressure liquid chromatography and
liquid enzymatic assay, respectively (14). FPG, total cholesterol, triglyceride,
LDL-C, HDL-C and SCR were analyzed using an automatic analyzer
(AU640; Olympus Corporation, Tokyo, Japan) (18). Urinary creatinine levels were
determined using the alkaline picrate method according to previous
studies (12). UACR was calculated
as a mean average of albumin (mg)/creatinine (g) from three
repeats. Microalbuminuria was defined as urine ACR 2.5–25.0 mg/mmol
in males and 3.5–25 in females, and macroalbuminuria defined as
urine ACR 25.0 mg/mmol in both, as previously described (12) GFR was measured using the
99mTc-DTPA renal dynamic imaging method (4). eGFR was calculated using two different
equations: MDRD recalibrated for Chinese patients and CKD-EPI
(4,19,20).
MDRD equation was as follows: eGFR (ml/min/1.73
m2)=186x(SCRx0.011)−1.154x(age)−0.203x(0.742
if female/1 if male)x1.233, where SCR was in µmol/l and 1.233 was
the adjusting coefficient for Chinese patients (12). CKD-EPI equation was as follows: eGFR
(ml/min/1.73 m2)=141 × min(SCR/k, 1a) ×
max(SCR/k, 1−1.209)x(0.993age)x(1.018 if
female/1 if male), where k is 0.7 for females and 0.9 for males, a
is −0.329 for females and −0.411 for males (19,20).
Peripheral neuropathy and vascular
examination
Peripheral sensory polyneuropathy was diagnosed by
MNSI B score, which consists of two parts: The appearance of the
feet (deformity, dry skin, callus, infection or fissures) and
examination of foot ulceration, ankle reflex and vibration
perception with a 128 Hz tuning fork. Evaluation of each parameter
was made at both sides with a maximum score of 8 points. The
diagnostic criterion of DPN was a MNSI examination score of ≥2, as
previously described (21). All
assessments were performed by trained nurses and the analysis of
results was undertaken by specialists. The ankle-brachial index was
detected by ultrasonic Pocket Doppler-Edan-Sonotrax-Basic
(Edan-Instruments Inc., Shenzhen, China) Doppler technique, with an
8 MHz probe and mercury sphygmomanometer Riester
diplomat-presameter (Rudolf Riester GmbH, Jungingen, Germany) with
an adult cuff (arm circumference 24–32 cm). ABI measurements were
performed according to previous studies (22,23).
SUDOSCAN test procedure
The SUDOSCAN device is composed of two sets of
electrodes for the feet and hands, both of which are connected to a
computer for recording and data analysis. The test is non-invasive
and no special preparation is required. Patients place the palms of
their hands and the soles of their feet on the electrodes for 2–3
min and a low-voltage (<4 V) electrical current stimulus will be
applied by the device automatically. The device is able to measure
ESC values expressed in micro-Siemens (µS) for the hands and the
feet (both right and left sides). The mean of left and right ESC
values was used for statistical analysis. The machine also has
built-in algorithms which integrate ESC with age height, weight and
HbA1c level to produce a score that estimates current risk of
kidney dysfunction (SUDOSCAN-DN score). The SUDOSCAN procedure was
completed by all subjects without any complaints of discomfort and
no adverse effects were reported.
Diagnostic criteria
CKD was defined as eGFR <60 ml/min/1.73
m2. Microalbuminuria was defined as UACR >30 and
<300 and macroalbuminuria was defined as UACR >300, according
to the criteria of the National Kidney Foundation (24). In the diagnosis of diabetic
peripheral sensory polyneuropathy, a cut-off point of ≥2 was used
as the diagnosis standard of MNSI B score, based on previous
studies (18,25,26).
Peripheral vascular disease was defined as non-traumatic lower
extremity amputation and/or ankle-brachial ratio <0.9 by Doppler
ultrasound scan (27).
Statistical analysis
All data are expressed as the mean ± standard
deviation, median (inter-quartile range) or percentages according
to different data types. Analysis of variance was used to compare
mean differences of clinical factors between two groups and
χ2 analyses were used to assess differences between
categorical variables. Correlation was determined using Spearman's
rho rank tests. Association between ESC value and biological
determinants (such as age, gender, BMI, WHR and duration of T2DM)
was tested using multiple linear regression analysis.
Receiver-operator characteristic (ROC) curves were constructed to
evaluate the sensitivity and specificity of SUDOSCAN-DN score in
detecting CKD in T2DM patients. The GFR result was used as the gold
standard measurement of the degree of neuropathy, based on the
cutoff value of 60 ml/min/1.73 m2. The area under the
ROC curve was calculated and the optimal cut-off point was the peak
of the curve where the sum of sensitivity and specificity was
greatest. SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA) was
used for all statistical analyses. P<0.05 was considered to
indicate a statistically significant difference.
Results
Enrolled patients
A total of 176 patients with T2DM (113 males and 63
females) were eligible for the present study. Amongst these
patients (mean age, 56.0±10.2 years; median duration of T2DM, 7
years, interquartile range 3–12 years), 15.3±1.0% of the subjects
had CKD, 19.3±2.5% had microalbuminuria and 5.0±0.7% had
macroalbuminuria.
Clinical and biochemical
characteristics
Clinical and biochemical characteristics of the 176
subjects are presented in Table I.
Patients with CKD had significantly higher age (P<0.01), longer
duration of T2DM (P<0.01), higher serum creatinine level
(P<0.01), higher BUN (P<0.05) and higher UCAR level
(P<0.05) compared with patients without CKD. The mean GFR value
was significantly lower in the CKD group compared with the non-CKD
group (48.13±7.91 vs. 85.83±15.4 ml/min/1.73 m2;
P<0.001). Mean SUDOSCAN-DN score was significantly lower in the
CKD group compared with the non-CKD group (44.69±11.9 vs.
63.16±16.5; P<0.001). A significantly higher incidence of
macroalbuminuria (27.8% in CKD vs. 1% in non-CKD; P<0.001),
diabetic peripheral neuropathy (57.9% in CKD vs. 29.3% in non-CKD;
P<0.01) and peripheral vascular disease (10.5% in CKD vs. 1% in
non-CKD; P<0.05) was observed in CKD patients compared with
non-CKD patients. There was no significant difference in the
incidence of coronary heart disease or stroke between the two
groups. There was also no significant difference in the use of
medications between the two groups, including metformin, insulin
and anti-hypertension drugs.
 | Table I.Clinical characteristics of patients
with T2DM classified by the presence of CKD with normal reference
values. |
Table I.
Clinical characteristics of patients
with T2DM classified by the presence of CKD with normal reference
values.
| Variable | Patients with
CKDa (n=27) | Patients without
CKDb (n=149) | P-value |
|---|
| Sex, n (M/F) | 14/13 | 79/70 | 0.62 |
| Age, years |
67.75±9.48c | 53.01±11.87 | <0.001 |
| Duration of T2DM,
years | 14 (8,
23)c | 7 (3, 11) | 0.001 |
| Smoking, % | 15.8 | 31.4 | 0.17 |
| Family history of
T2DM, % | 26.3 | 46.2 | 0.11 |
| Body mass index,
kg/m2 |
|
Male | 24.42±3.55 | 24.52±4.96 | 0.87 |
|
Female | 25.6±3.1 | 24.3±4.5 | 0.38 |
| Waist-hip
ratio |
|
Male | 1.03±0.18 | 0.95±0.06 | 0.14 |
|
Female | 0.95±0.1 | 0.93±0.08 | 0.59 |
| Systolic BP,
mmHg | 135.25±15.8 | 128.5±15.2 | 0.091 |
| Diastolic BP,
mmHg | 79.63±11.94 | 80.57±9.66 | 0.79 |
| Glycated
hemoglobin, % (normal range, <6.5) | 8.8±2.3 | 8.7±2.1 | 0.93 |
| Glycated albumin, %
(normal range, 5–9) | 20.3±7.1 | 21.0±7.8 | 0.91 |
| Fasting blood
glucose, mmol/l (normal range: 3.9–6.1) | 8.6±2.8 | 8.0±2.6 | 0.16 |
| Low-density
lipoprotein cholesterol, mmol/l (normal range, <3.36) | 2.21 (1.81,
2.66) | 2.47 (1.88,
2.96) | 0.18 |
| High-density
lipoprotein cholesterol, mmol/l (normal range, 0.9–2.1) | 1 (0.91, 1.23) | 0.97 (0.83,
1.2) | 0.54 |
| Triglyceride,
mmol/l (normal range, 0.6–1.5) | 1.15 (0.99,
2.2) | 1.5 (0.96,
2.28) | 0.62 |
| Cholesterol, mmol/l
(normal range, 3.1–5.7 mmol/l) | 4.22 (3.36,
4.78) | 4.3 (3.5,
4.92) | 0.66 |
| Serum creatinine,
µmol/l (normal range, 35–71) |
89.3±34.6c | 59.6±14.7 | <0.001 |
| Blood urea
nitrogen, mmol/l (normal range, 2.9–7.1) | 8 (4.2,
10.3)d | 5.4 (4.8, 6.6) | 0.03 |
| Uric acid, mg/dl
(normal range, 0.15–0.42 mg/dl) | 0.36 (0.27,
0.47) | 0.29 (0.25,
0.36) | 0.17 |
| Mean urinary
albumin-creatinine ratio | 221.5 (10.6,
441.9)d | 12.6 (6.0,
24.9) | 0.012 |
| Glomerular
filtration rate, ml/min/1.73 m2 |
48.13±7.91c | 85.83±15.4 | <0.001 |
| Diabetic
complications, % |
|
Microalbuminuria | 16.7 | 19.4 | 0.26 |
|
Macroalbuminuria | 27.8c | 1.0 | <0.001 |
|
Coronary heart disease | 2.1 | 1.6 | 0.58 |
|
Stroke | 7.1 | 13.1 | 0.1 |
|
Diabetic peripheral
neuropathy | 57.9c | 24.3 | 0.003 |
|
Peripheral vascular
disease | 10.5d | 1.0 | 0.01 |
| SUDOSCAN results,
µS |
| Hands
ESC value | 56.74±20.5 | 59.34±18.65 | 0.06 |
| Feet
ESC value | 49.11±23.13 | 59.58±21.84 | 0.66 |
|
Diabetic nephropathy
value |
44.69±11.9c | 63.16±16.5 | <0.001 |
| Medication use,
% |
|
Metformin | 10.5 | 24.8 | 0.172 |
|
Insulin | 42.1 | 41.9 | 0.98 |
|
Statins | 8.3 | 39.4 | 0.83 |
| Angiotensin
converting enzyme inhibitor or angiotensin II receptor blocker,
% | 68.4 | 44.8 | 0.06 |
Correlation analysis
Spearman correlation analysis (Table II) demonstrated a significant
negative correlation between GFR and age (r=−0.48; P<0.01),
duration of diabetes (r=−0.22; P<0.05), WHR (r=−0.25;
P<0.01), SCR (r=−0.47; P<0.01), BUN level (r=−0.306,
P<0.01) and uric acid (r=−0.307; P<0.01). A significant
positive correlation was demonstrated between GFR and SUDOSCAN-DN
score (r=0.52; P<0.01), LDL-C (r=0.2; P<0.05) and blood
hemoglobin (r=0.22; P<0.05).
 | Table II.Spearman correlation analysis between
glomerular filtration rate and clinical characteristics. |
Table II.
Spearman correlation analysis between
glomerular filtration rate and clinical characteristics.
| Variable | R | P-value |
|---|
| Age | −0.48a | <0.001 |
| Duration of
diabetes | −0.22b | 0.015 |
| Body mass
index | −0.11 | 0.24 |
| Waist-hip
ratio | −0.25a | 0.006 |
| Systolic BP | −0.18 | 0.052 |
| Diastolic BP |
0.013 | 0.89 |
| Glycated
hemoglobin | 0.15 | 0.11 |
| Glycated
albumin | 0.07 | 0.47 |
| Fasting blood
glucose | 0.15 | 0.16 |
| Total
cholesterol | 0.15 | 0.1 |
| Triglycerides | 0.08 | 0.37 |
| High-density
lipoprotein cholesterol | 0.01 | 0.9 |
| Low-density
lipoprotein cholesterol | 0.2b | 0.03 |
| Serum
creatinine | −0.47a | <0.001 |
| Blood urea
nitrogen | −0.31a | 0.001 |
| Uric acid | −0.31a | 0.001 |
| Mean urinary
albumin-creatinine ratio | −0.16 | 0.08 |
| Hands ESC
value | 0.13 | 0.14 |
| Feet ESC value | 0.23b | 0.01 |
| SUDOSCAN-DN
value | 0.52a | <0.001 |
Multiple linear regression (Table III) indicated that low GFR was
significantly associated with low SUDOSCAN-DN score
(β-coefficient=0.42; P<0.001), as well as with older age
(β-coefficient=−0.368; P<0.001), longer disease duration
(β-coefficient=−0.227; P<0.01) and higher WHR
(β-coefficient=−0.24; P<0.01).
 | Table III.Multiple linear regression analysis
between glomerular filtration rate and clinical characteristics in
Chinese patients with type 2 diabetes. |
Table III.
Multiple linear regression analysis
between glomerular filtration rate and clinical characteristics in
Chinese patients with type 2 diabetes.
| Clinical
factors | Standard
β-coefficient | P-value |
|---|
| Age | −0.368a | <0.001 |
| Duration of
diabetes | −0.227a | 0.008 |
| Glycated
hemoglobin | 0.11 | 0.21 |
| Body mass
index | −0.48 | 0.24 |
| Waist-hip
ratio | −0.24b | 0.007 |
| Low-density
lipoprotein cholesterol | 0.016 | 0.85 |
| SUDOSCAN-DN
score | 0.42a | <0.001 |
ROC curve
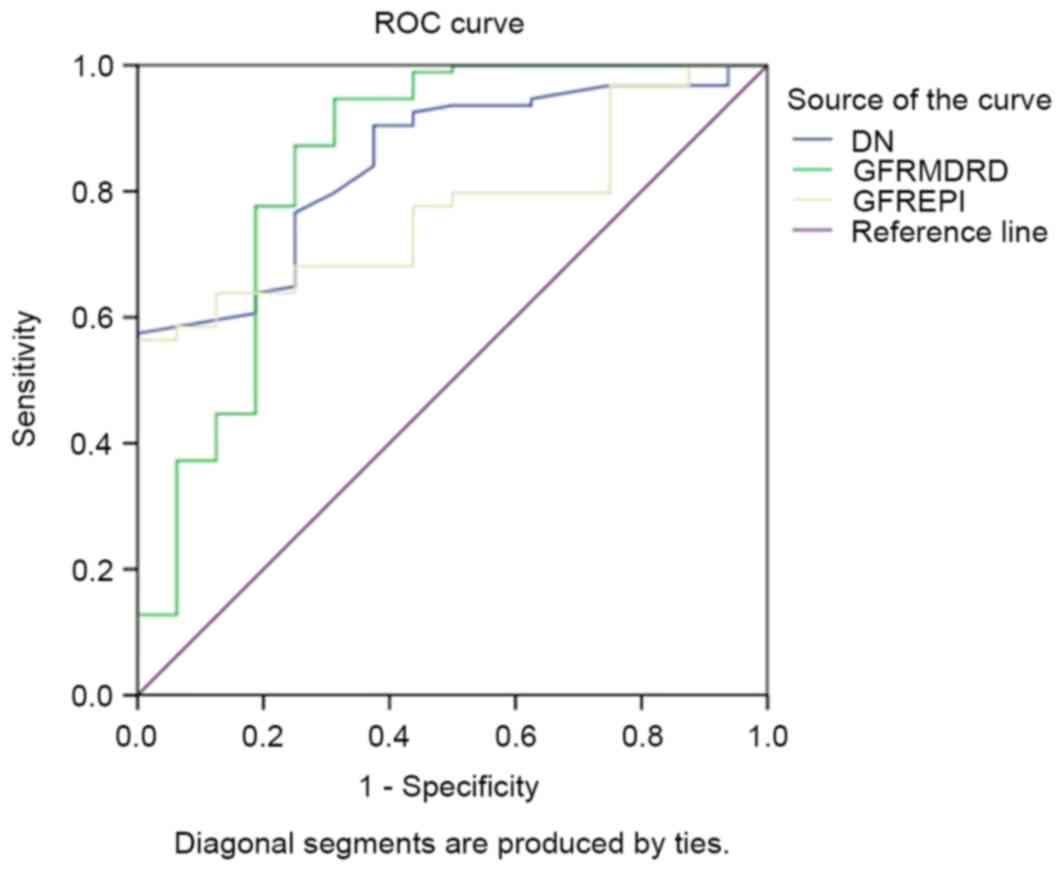
The area under the ROC curve of SUDOSCAN-DN score to
predict CKD was 0.85 [95% confidence interval (CI), 0.76–0.93;
Fig. 1] compared with 0.84 for
GFRMDRD (95% CI, 0.71–0.98) and 0.77 for
GFREPI (95% CI, 0.68–0.87). The sensitivity and
specificity to detect CKD with SUDOSCAN-DN score was 57.6 and 100%,
at a cut-off of 59.5.
Patient comparison
The clinical characteristics of the subjects were
further analyzed when patients were divided into two groups by
SUDOSCAN-DN score at the cut-off point of 59.5 (Table IV). Patients with DN score <59.5
had a significantly higher age, longer duration of T2DM, lower
blood hemoglobin and lower GFR level compared patients with score
≥59.5 (all P<0.001). A significantly increased rate of stroke
(13.2 vs. 3.1%; P<0.01), diabetic peripheral neuropathy (41.9
vs. 15.4%; P<0.001) and peripheral vascular disease (4.9 vs.
1.0%; P<0.05) was observed in the group of T2DM patients with DN
level <59.5. A significant decrease in the ESC level of hands
and feet (P<0.001) in the group of T2DM patients with DN level
<59.5 was also detected in the study, as presented in Table IV. ESC values of hands in DN the
≥59.5 group was 65.1±17.1 vs. 56.7±20.5 in the DN<59.5 group and
ESC of feet in DN≥59.5 group was 66.4±19.5 vs. 51.2±21.7 in the
DN<59.5 group. Both of these differences are significant
(P<0.001).
 | Table IV.Clinical characteristics of patients
with or without CKD by SUDOSCAN-DN score. |
Table IV.
Clinical characteristics of patients
with or without CKD by SUDOSCAN-DN score.
| Variable | DN score
<59.5a (n=79) | DN score
≥59.5b (n=97) | P-value |
|---|
| Sex, n (M/F) | 45/34 | 48/49 | 0.052 |
| Age, years |
64.7±9.9c | 46.7±9.9 | <0.001 |
| Duration of T2DM,
years | 10 (5,
15)c | 6.5 (1, 9.5) | <0.001 |
| Smoking, % | 20.9 | 36.7 | 0.13 |
| Family history of
T2DM, % | 39.5 | 49.4 | 0.20 |
| Body mass index,
kg/m2 |
|
|
|
|
Male | 24 (21,26) | 25 (23, 28) | 0.09 |
|
Female | 25 (22, 26) | 22 (20, 26.3) | 0.24 |
| Waist-hip
ratio |
|
|
|
|
Male | 0.96 (0.91,
1.02) | 0.96 (0.91,
0.99) | 0.55 |
|
Female | 0.92 (0.86,
0.98) | 0.93 (0.86,
0.98) | 0.77 |
| Systolic BP,
mmHg | 132.9±14.2 | 126.9±15.2 | 0.006 |
| Diastolic BP,
mmHg | 80.2±10.2 | 80.4±9.4 | 0.84 |
| Glycated
hemoglobin, % | 8.2±2 | 8.8±2.1 | 0.06 |
| Low-density
lipoprotein cholesterol, mmol/l | 2.21 (1.81,
2.66) | 2.47 (1.88,
2.96) | 0.48 |
| High-density
lipoprotein cholesterol, mmol/l | 1.03 (0.9,
1.3) | 0.95 (0.82,
1.2) | 0.07 |
| Triglyceride,
mmol/l | 1.2 (0.9,
1.9)c | 1.8 (1.2, 2.6) | 0.003 |
| Cholesterol,
mmol/l | 4.4 (3.5, 5.2) | 4.4 (3.7, 4.8) | 0.39 |
| Serum creatinine,
µmol/l | 66.9±27.6 | 60.0±13.8 | 0.36 |
| Blood urea
nitrogen, mmol/l | 5.5 (4.6, 7.3) | 5.2 (4.5, 6.2) | 0.054 |
| Uric acid,
mg/dl | 0.29 (0.25,
0.40) | 0.31 (0.26,
0.38) | 0.70 |
| Mean urinary
albumin-creatinine ratio | 290 (10.6,
441.9) | 16 (6.0, 24.9) | 0.16 |
| Glomerular
filtration rate, ml/min/1.73 m2 |
72.5±19.7c | 89.9±16 | <0.001 |
| Diabetic
complications, % |
|
|
|
|
Microalbuminuria | 14.1 | 20.5 | 0.12 |
|
Macroalbuminuria | 0.9 | 1.4 | 0.08 |
|
Coronary heart disease | 2.3 | 1.5 | 0.32 |
|
Stroke | 13.2c | 3.1 | 0.005 |
|
Diabetic peripheral
neuropathy | 41.9c | 15.4 | <0.001 |
|
Peripheral vascular
disease | 4.9d | 1.0 | 0.048 |
| SUDOSCAN results,
µS |
|
|
|
| Hands
ESC value |
56.7±20.5c | 65.1±17.1 | <0.001 |
| Feet
ESC value |
51.2±21.7c | 66.4±19.5 | <0.001 |
| DN
value |
46.8±10.6c | 73.8±11.6 | <0.001 |
| Medication use,
% |
|
|
|
|
Metformin | 23.3 | 27.8 | 0.5 |
|
Insulin | 38.4 | 50.6 | 0.11 |
|
Statins | 24.1 | 24.1 | 0.57 |
|
Angiotensin converting enzyme
inhibitor or angiotensin II receptor blocker, % | 52.1c | 47.9 | 0.003 |
Discussion
Sudomotor function is a subtype of autonomic
function reflecting the integrity of sympathetic C fibers
innervating the sweat glands, which can be highly susceptible to
damage by metabolic processes, including longstanding diabetes
(9,28–31).
Processes downstream to sustained hyperglycemia, including
activation of protein kinase C, activation of the polyol pathway
and formation of advanced glycosylation end products, which are
known to drive diabetic renal changes, have been implicated in
causing reduction of endoneurial blood flow as well as causing
direct nerve injury (1,3,32).
Therefore, a previous study proposed that sudomotor dysfunction may
have similar pathogenic mechanisms to diabetic kidney disease and
SUDOSCAN may be used to perform early detection of CKD in diabetic
patients (33). This proposal has
been supported by previous studies that used SUDOSCAN to detect CKD
in diabetic patients based on the premise that patients with CKD
are likely to have vascular and nerve dysfunction (9). In a previous study, SUDOSCAN, as the
modified and improved generation of EZSCAN with different built in
algorithms, was reported to be effective in detecting CKD in a
large cross-sectional sample of Chinese patients with T2DM
(5). Statistics in that study showed
the area under ROC curve of SUDOSCAN-DN score for CKD was 0.75 (95%
CI, 0.72–0.79), which indicated SUDOSCAN may be useful in detecting
patients at risk of having CKD. In 2011, Gin et al (30) first reported EZSCAN as the new
screening tool for kidney disease in Chinese patients with T2DM.
Freedman et al (11) studied
390 African and European American patients with T2DM and 166
controls, and found an independent association between ESC and GFR
in African Americans.
In the present cross-sectional study, GFR was used
instead of eGFR as the diagnostic standard for patients with or
without CKD. The diagnostic value of SUDOSCAN in the detection of
CKD in T2DM patients was evaluated using ROC curve analysis. The
area under ROC curve was 0.85 (95% CI, 0.76–0.93) with a cut-off
point of 59.5 for DN score. This cut-off point had 57.6%
sensitivity and 100% specificity in detecting CKD. Compared with
those without CKD, patients with CKD were older, had longer
duration of disease, lower blood hemoglobin and more diabetic
complications including peripheral neuropathy and peripheral
vascular disease. By multiple linear regression analysis, the
associated risk factors with GFR were found to be SUDOSCAN-DN
score, disease duration, age, waist-hip ratio and hemoglobin level.
Clinical characteristics were also compared in two groups divided
by cut-off point of DN drawn from ROC analysis, and a lower GFR
level was observed in patients with DN score <59.5.
The natural progression of kidney dysfunction in
T2DM involves the gradual progress from albuminuria to declined
GFR. Microalbuminuria is traditionally viewed as an early indicator
of diabetic renal involvement, but its predictive value for renal
dysfunction is challenged by poor sensitivity and specificity as
well as many impact factors including pathological or physiological
processes unrelated to diabetes such as posture, exercise, obesity
and infection (2). This may explain
the insignificant correlation between UCAR and DN score that was
observed in the current study.
The current study had some limitations. The sample
size of this cross-sectional cohort was not large enough to analyze
the correlation between kidney function with all associated
clinical characteristics. The possibility of selection bias could
not be excluded in drawing the conclusion of high specificity of
SUDOSCAN-DN score in detecting CKD. Further studies with larger
sample sizes are needed to confirm the clinical use of SUDOSCAN for
diagnosing risk of CKD.
The majority of guidelines recommend regular
screening for complications and risk factors in patients with
diabetes, including eye, foot, blood and urine examinations
(1,3,28,34,35).
The results of the current study suggest that SUDOSCAN may be
considered as a useful screening tool in an outpatient service or
low resource setting, as part of a CKD screening program, due to
its low invasiveness and convenience.
In conclusion, the current results suggested that
the assessment of sudomotor function using SUDOSCAN may provide an
effective screening method for the detection of kidney dysfunction
in Chinese patients with T2DM.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 81370884, to
Professor Bin Lu), the Shanghai New Excellent Youth Program (grant
no. XYQ2013120, to Professor Bin Lu), Pudong Municipal Commission
of Health and Family Planning (PW2014D-2, to Professor Bin Lu; and
PW2013D-2, to Professor HongLi Shi) and the Shanghai Science and
Technology Committee Program (grant no. 14411962200, to Professor
Yiming Li).
Glossary
Abbreviations
Abbreviations:
|
T2DM
|
type 2 diabetes mellitus
|
|
BMI
|
body mass index
|
|
DN
|
diabetic nephropathy
|
|
WHR
|
waist-hip ratio
|
|
HbA1c
|
glycated hemoglobin
|
|
LDL-C
|
low-density lipoprotein
cholesterol
|
|
HDL-C
|
high-density lipoprotein
cholesterol
|
|
BUN
|
blood urea nitrogen
|
|
UACR
|
urinary albumin-creatinine ratio
|
|
GFR
|
glomerular filtrate rate
|
|
ESC
|
electrochemical skin conductance
|
|
MDRD
|
modification of diet in renal
disease
|
|
EPI
|
epidemiology collaboration
|
|
CKD
|
chronic kidney disease
|
|
KDOQI
|
National Kidney Foundation's Kidney
Disease Outcomes Quality Initiative
|
References
|
1
|
Hass VM: Updated management of chronic
kidney disease in patients with diabetes. JAAPA. 27:17–22.
2014.PubMed/NCBI
|
|
2
|
MacIsaac RJ, Ekinci EI and Jerums G:
‘Progressive diabetic nephropathy. How useful is microalbuminuria?:
Contra'. Kidney Int. 86:50–57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tuttle KR, Bakris GL, Bilous RW, Chiang
JL, de Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K,
Narva AS, Navaneethan SD, et al: Diabetic kidney disease: A report
from an ADA Consensus Conference. Am J Kidney Dis. 64:510–533.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schold JD, Navaneethan SD, Jolly SE,
Poggio ED, Arrigain S, Saupe W, Jain A, Sharp JW, Simon JF,
Schreiber MJ Jr and Nally JV Jr: Implications of the CKD-EPI GFR
estimation equation in clinical practice. Clin J Am Soc Nephrol.
6:497–504. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Levey AS, Stevens LA, Schmid CH, Zhang YL,
Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene
T, et al: A new equation to estimate glomerular filtration rate.
Ann Intern Med. 150:604–612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Korhonen PE, Kivelä SL, Aarnio PT,
Kautiainen H, Järvenpää S and Kantola IM: Estimating glomerular
filtration rate in hypertensive subjects: Comparison of the chronic
kidney disease epidemiology collaboration (CKD-EPI) and
modification of diet in renal disease (MDRD) study equations. Ann
Med. 44:487–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Teo BW, Koh YY, Toh QC, Li J, Sinha AK,
Shuter B, Sethi S and Lee EJ: Performance of the CKD-EPI
creatinine-cystatin C glomerular filtration rate estimation
equations in a multiethnic Asian population. Singapore Med J.
55:656–659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eranki VG, Santosh R, Rajitha K, Pillai A,
Sowmya P, Dupin J and Calvet JH: Sudomotor function assessment as a
screening tool for microvascular complications in type 2 diabetes.
Diabetes Res Clin Pract. 101:e11–e13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ramachandran A, Moses A, Shetty S,
Thirupurasundari CJ, Seeli AC, Snehalatha C, Singvi S and Deslypere
JP: A new non-invasive technology to screen for dysglycaemia
including diabetes. Diabetes Res Clin Pract. 88:302–306. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Casellini CM, Parson HK, Richardson MS,
Nevoret ML and Vinik AI: Sudoscan, a noninvasive tool for detecting
diabetic small fiber neuropathy and autonomic dysfunction. Diabetes
Technol Ther. 15:948–953. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Freedman BI, Bowden DW, Smith SC, Xu J and
Divers J: Relationships between electrochemical skin conductance
and kidney disease in Type 2 diabetes. J Diabetes Complications.
28:56–60. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luk AO, Fu WC, Li X, Ozaki R, Chung HH,
Wong RY, So WY, Chow FC and Chan JC: The clinical utility of
SUDOSCAN in chronic kidney disease in Chinese patients with type 2
diabetes. PLoS One. 10:e01349812015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
van den Brand JA, van Boekel GA, Willems
HL, Kiemeney LA, den Heijer M and Wetzels JF: Introduction of the
CKD-EPI equation to estimate glomerular filtration rate in a
Caucasian population. Nephrol Dialysis Transplantat. 26:3176–3181.
2011. View Article : Google Scholar
|
|
14
|
Yuan X, Zhang J, Tang K, Quan C, Tian Y,
Li H, Ao G and Qiu L: Determination of glomerular filtration rate
with CT measurement of renal clearance of iodinated contrast
material versus 99mTc-DTPA dynamic imaging ‘Gates’ Method: A
Validation Study in Asymmetrical Renal Disease. Radiology.
282:552–560. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma YC, Zuo L, Zhang CL, Wang M, Wang RF
and Wang HY: Comparison of 99mTc-DTPA renal dynamic imaging with
modified MDRD equation for glomerular filtration rate estimation in
Chinese patients in different stages of chronic kidney disease.
Nephrol Dial Transplant. 22:417–423. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qiao X, Zhang S, Zhao W, Ye H, Yang Y,
Zhang Z, Miao Q, Hu R, Li Y and Lu B: Serum phosphorylated
neurofilament-heavy chain, a potential biomarker, is associated
with peripheral neuropathy in patients with type 2 diabetes.
Medicine (Baltimore). 94:e19082015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fox CS, Golden SH, Anderson C, Bray GA,
Burke LE, de Boer IH, Deedwania P, Eckel RH, Ershow AG, Fradkin J,
et al: Update on prevention of cardiovascular disease in adults
with type 2 diabetes mellitus in light of recent evidence: A
scientific statement from the American Heart Association and the
American Diabetes Association. Circulation. 132:691–718. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Joint Committee for Developing Chinese
guidelines on Prevention and Treatment of Dyslipidemia in Adults:
Chinese guidelines on prevention and treatment of dyslipidemia in
adults. Zhonghua Xin Xue Guan Bing Za Zhi. 35:390–419. 2007.(In
Chinese). PubMed/NCBI
|
|
19
|
Sudchada P and Laehn S: Comparisons of GFR
estimation using the CKD Epidemiology Collaboration (CKD-EPI)
equation and other creatinine-based equations in Asian population:
A systematic review. Int Urol Nephrol. 48:1511–1517. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu X, Gan X, Chen J, Lv L, Li M and Lou
T: A new modified CKD-EPI equation for Chinese patients with type 2
diabetes. PLoS One. 9:e1097432014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiong Q, Lu B, Ye H, Wu X, Zhang T and Li
Y: The diagnostic value of neuropathy symptom and change score,
neuropathy impairment score and michigan neuropathy screening
instrument for diabetic peripheral neuropathy. Eur Neurol.
74:323–327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Herráiz-Adillo Á, Martínez-Vizcaíno V,
Cavero-Redondo I, Álvarez-Bueno C, Garrido-Miguel M and
Notario-Pacheco B: Diagnostic accuracy study of an oscillometric
ankle-brachial index in peripheral arterial disease: The influence
of oscillometric errors and calcified legs. PLoS One.
11:e01674082016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aboyans V, Criqui MH, Abraham P, Allison
MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jönsson B, Lacroix P,
et al: Measurement and interpretation of the ankle-brachial index:
A scientific statement from the American Heart Association.
Circulation. 126:2890–2909. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eknoyan G, Hostetter T, Bakris GL, Hebert
L, Levey AS, Parving HH, Steffes MW and Toto R: Proteinuria and
other markers of chronic kidney disease: A position statement of
the national kidney foundation (NKF) and the national institute of
diabetes and digestive and kidney diseases (NIDDK). Am J Kidney
Dis. 42:617–622. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yajnik CS, Kantikar VV, Pande AJ and
Deslypere JP: Quick and simple evaluation of sudomotor function for
screening of diabetic neuropathy. ISRN Endocrinol. 2012:1037142012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee CC, Perkins BA, Kayaniyil S, Harris
SB, Retnakaran R, Gerstein HC, Zinman B and Hanley AJ: Peripheral
neuropathy and nerve dysfunction in individuals at high risk for
type 2 diabetes: The PROMISE cohort. Diabetes Care. 38:793–800.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen SC, Hsiao PJ, Huang JC, Lin KD, Hsu
WH, Lee YL, Lee MY, Chang JM and Shin SJ: Abnormally low or high
ankle-brachial index is associated with proliferative diabetic
retinopathy in type 2 diabetic mellitus patients. PLoS One.
10:e01347182015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vinik AI, Nevoret ML and Casellini C: The
new age of sudomotor function testing: A sensitive and specific
biomarker for diagnosis, estimation of severity, monitoring
progression and regression in response to intervention. Front
Endocrinol (Lausanne). 6:942015.PubMed/NCBI
|
|
29
|
Calvet JH, Dupin J, Winiecki H and Schwarz
PE: Assessment of small fiber neuropathy through a quick, simple
and non invasive method in a German diabetes outpatient clinic. Exp
Clin Endocrinol Diabetes. 121:80–83. 2013.PubMed/NCBI
|
|
30
|
Gin H, Baudoin R, Raffaitin CH, Rigalleau
V and Gonzalez C: Non-invasive and quantitative assessment of
sudomotor function for peripheral diabetic neuropathy evaluation.
Diabetes Metab. 37:527–532. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen L, Chen X, Ding R, Shi Q Jr and Hu D:
Evaluation of EZSCAN as a screening tool for impaired glucose
metabolism. Diabetes Res Clin Pract. 100:210–214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Garasto S, Fusco S, Corica F, Rosignuolo
M, Marino A, Montesanto A, De Rango F, Maggio M, Mari V, Corsonello
A and Lattanzio F: Estimating glomerular filtration rate in older
people. Biomed Res Int. 2014:9165422014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ozaki R, Cheung KK, Wu E, Kong A, Yang X,
Lau E, Brunswick P, Calvet JH, Deslypere JP and Chan JC: A new tool
to detect kidney disease in Chinese type 2 diabetes patients:
Comparison of EZSCAN with standard screening methods. Diabetes
Technol Ther. 13:937–943. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chamberlain JJ, Rhinehart AS, Shaefer CF
Jr and Neuman A: Diagnosis and management of diabetes: Synopsis of
the 2016 American diabetes association standards of medical care in
diabetes. Ann Intern Med. 164:542–552. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tesfaye S, Boulton AJ, Dyck PJ, Freeman R,
Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, et
al: Diabetic neuropathies: Update on definitions, diagnostic
criteria, estimation of severity, and treatments. Diabetes Care.
33:2285–2293. 2010. View Article : Google Scholar : PubMed/NCBI
|