Introduction
Cardiovascular and cerebrovascular diseases now have
become the principal diseases that threaten human health and
atherosclerosis is their principal pathogenic mechanism.
Homocysteine (Hcy) has received much attention due to its role in
the development of atherosclerosis. Numerous clinical studies and
animal experiments have shown that hyperhomocysteinemia (HHcy) is
an independent risk factor for vascular diseases, including
ischemic heart disease, stroke and peripheral vascular disease
(1,2). HHcy is a pathological condition
characterized by an increase (>15 µmol/l) in the plasma
concentration of total Hcy. Numerous studies have proposed that the
atherogenic effects of HHcy are based on the induction of oxidative
stress, endothelial dysfunction, thrombosis and activation of
proinflammatory factors (3–8). Yet, the mechanisms by which Hcy
promotes cardiovascular disease have remained to be fully
elucidated. Carotid artery wall thickness is evaluated by measuring
its intima-media thickness (IMT). Coronary artery IMT is an easily,
reproducibly and non-invasively measured parameter for estimating
the severity of carotid atherosclerosis and has been reported to be
an independent predictor of coronary heart disease. Studies have
demonstrated that allicin prevents atherosclerosis by exerting
anti-aggregatory, anti-migratory (9)
and anti-oxidant effects (10) as
well as lowing blood pressure (11)
and blood lipids (12). A previous
study by our group reported that allicin obviously decreases the
serum Hcy levels in rats with HHcy and also alleviates the injury
of Hcy to endothelial cells (13).
These results suggested that allicin may be useful as a drug for
atherosclerosis. The present study hypothesized that allicin
decreases plasma Hcy levels and exerts anti-atherosclerotic effects
in coronary heart disease. The effect of allicin treatment on
carotid artery IMT and plasma Hcy in coronary heart disease was
assessed.
Materials and methods
Study design and subjects
Sixty-two patients (42 males and 20 females) between
the age of 50 and 80 years diagnosed with coronary heart disease
and HHcy at Qilu Hospital of Shandong University (Jinan, China)
were consecutively recruited as outpatients and inpatients from
August 2006 to September 2007. Levels indicative of atherosclerotic
vascular disease were set according to the European Society of
Hypertension-European Society of Cardiology guidelines (14). HHcy was defined as the plasma
concentration of total Hcy exceeding 15 µmol/l. Exclusion criteria
included overt liver and renal dysfunction, hematological system
disease, major psychiatric illness, hypersensitivity constitution
and hypersensitivity to the drug, pregnancy or lactation. All
subjects were divided into two groups by simple randomization and
were treated for 12 weeks by enteric-coated aspirin (100 mg/day),
simvastatin (20 mg/day), isosorbide mononitrate tablets (20 mg
twice daily) and metoprolol tartrate (from 12.5 mg twice daily
gradually increased to the maximum tolerable dose), with the heart
rate not lower than 55 beats/minute in the resting phase. One group
was also administered allicin (40 mg thrice daily for 12 weeks;
allicin group; n=32) and the other group did not receive any
allicin (control group; n=30). The clinical characteristics of the
subjects are shown in Table I.
Conditions at baseline and at the end of the study were recorded
and measurements of Hcy and lipids in blood samples as well as
carotid artery IMT were undertaken. During the present study, all
subjects maintained their normal living habits and did not change
their medication. The purpose and potential risks of the study were
explained to all patients and their voluntary written consent for
participation in the study was obtained prior to enrolment. The
present study was approved by the Committee on the Ethics of
Clinical Experiments (Qilu Hospital of Shandong University, Jinan,
China).
 | Table I.Clinical background of the
subjects. |
Table I.
Clinical background of the
subjects.
| Characteristics | Allicin group
(n=32) | Control group
(n=30) |
|---|
| Sex (% male) | 68.9 | 66.7 |
| Age (years) | 61.1±12.0 | 60.4±13.4 |
| Education
(years) | 10.5±2.5 | 10.8±2.8 |
| Time since infarction
(years) | 6.4±3.1 | 6.5±2.8 |
| CAD type |
|
|
| Angina
pectoris | 24 (75.0) | 20 (66.7) |
|
Myocardial infarction | 8 (25.0) | 10 (33.3) |
| Fasting blood glucose
(mmol/l) | 5.79±1.72 | 5.72±1.61 |
| Serum triglycerides
(mmol/l) | 3.03±1.76 | 3.09±1.60 |
| Serum cholesterol
(mmol/l) | 5.60±1.20 | 5.55±1.28 |
| Serum homocysteine
(mmol/l) | 19.92±2.11 | 20.05±2.15 |
| Major medical
problems |
|
|
|
Hypertension | 21 (65.6) | 17 (56.7) |
|
Hyperlipemia | 11 (34.4) | 13 (43.3) |
|
Hypercholesterolemia | 8 (25.0) | 7 (23.3) |
|
Diabetes mellitus | 7 (21.9) | 9 (30.0) |
| Drugs for blood
glucose | 7 (21.9) | 9 (30.0) |
| Drugs for blood
pressure | 21 (65.6) | 17 (56.7) |
Blood sampling
Blood samples were collected after overnight
fasting. Serum was separated within 1 h and used for measuring of
total cholesterol (TC) and triglyceride (TG). Hcy levels were
determined in EDTA-treated plasma.
Plasma Hcy assay
In accordance to a previously published method
(15), plasma Hcy was assayed at an
excitation wavelength of 335 nm and an emission wavelength of 455
nm with a Waters 2695 high efficiency liquid chromatograph and a
Waters 2475 fluorescence detector and a Millennium 32
chromatographic workstation (Waters Co., Milford, MA, USA).
Standard Hcy and o-phthalaldehyde were obtained from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). All of the above parameters were
detected at the Drug Testing Center of Qilu Hospital of Shandong
University (Jinan, China).
Serum biochemical indices assay
Serum biochemical indices, including TC and TG, were
determined with a Bayer 1650 supermatic biochemistry analyzer
(Bayer AG, Leverkusen, Germany) at the Biochemistry Department of
Shandong University (Jinan, China).
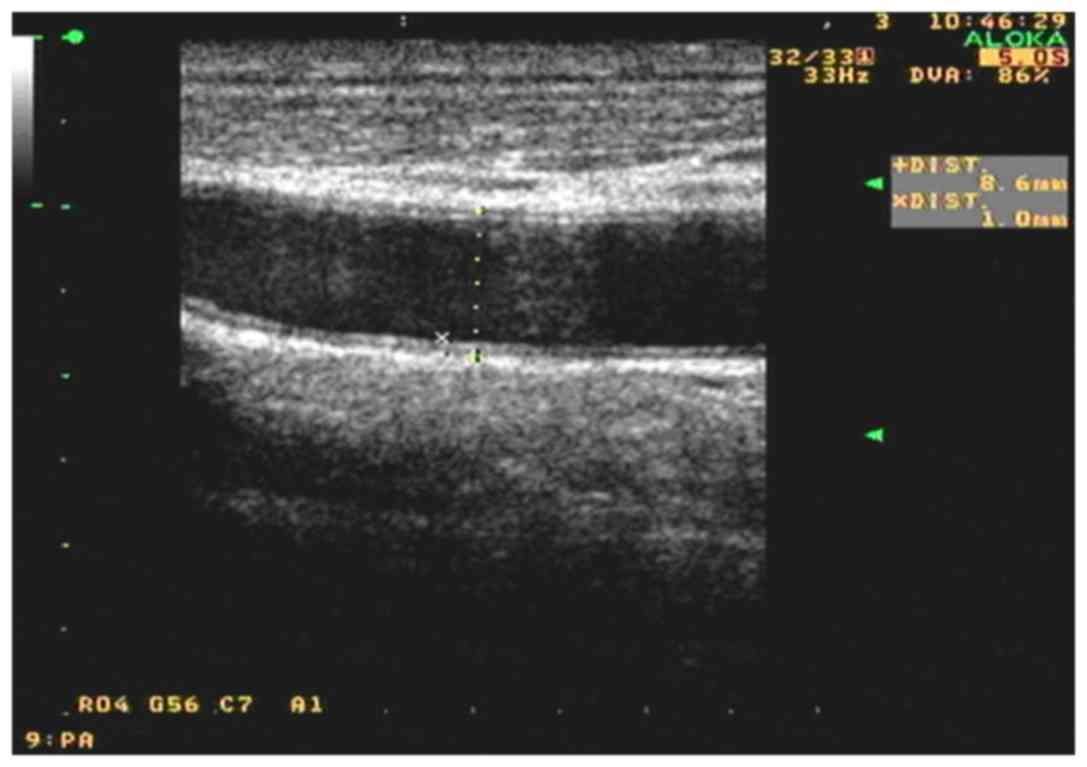
Ultrasound examination and IMT
quantification
All measurements of carotid artery IMT were
performed as part of a routine examination by the same technician,
who was blinded to the subjects' clinical data. High-resolution
B-mode carotid ultrasonography was performed using an 8–12 MHz
transducer and an Aloka SSD-5500 ultrasound scanner (Aloka,
Andover, Japan). The patients lied in the supine position with the
head slightly tilted to either side at examination. Wall changes
were carefully identified in the carotid arteries from different
longitudinal and transverse views. The images were focused on the
far wall of the artery. In all subjects, the common carotid artery,
the internal and external carotid arteries and the carotid bulb
were examined. Subsequent to identification of a region ~1.0 cm
proximal to the carotid bifurcation, the IMT of the far wall was
evaluated as the distance between the luminal-intimal interface and
the medial-adventitial interface, measured thrice, and the average
IMT value was used. Based on the data in healthy Chinese subjects,
abnormal IMT was defined as an IMT of >1.0 mm.
Statistical analysis
Values are expressed as the mean ± standard
deviation and data were subjected to a test of normality.
Statistical analyses were performed by using the SPSS statistical
package, version 12.0 (SPSS Inc., Chicago, IL, USA). Student's
t-test and the paired t-test were performed for statistical
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical characteristics
Characteristics of the study population are shown in
Table I. No differences were found
between the two groups with regard to age, sex, TC, TG, blood
glucose, mean blood pressure, plasma Hcy, carotid artery IMT and
morbidity duration of coronary heart disease. There were also no
differences in the ratio of subjects using drugs for lowering blood
glucose or blood pressure between the two groups.
Plasma Hcy levels
The Hcy levels are shown in Table II. The basal plasma Hcy
concentration did not differ between the two groups. A significant
decrease of Hcy was observed in the allicin group after 12 weeks
(P<0.01) and the decrease in the allicin group was significantly
larger than that in the control group (13.18±2.88 vs. 17.91±2.09
µmol/l; P<0.05).
 | Table II.Effect of allicin on plasma Hcy and
IMT. |
Table II.
Effect of allicin on plasma Hcy and
IMT.
|
|
| Hcy (µmol/l) | IMT (mm) |
|---|
|
|
|
|
|
|---|
| Group | n | Before
treatment | After
treatment | Before
treatment | After
treatment |
|---|
| Allicin group | 32 | 19.92±2.11 |
13.18±2.88a,b | 1.28±0.13 |
1.13±0.10a,b |
| Control group | 30 | 20.05±2.15 |
17.91±2.09c | 1.28±0.08 |
1.23±0.08c |
Carotid artery IMT
The IMTs are shown in Table II. After 12 weeks of allicin
administration, the carotid artery IMT in the allicin group was
significantly decreased compared with that in the control group
(1.13±0.10 vs. 1.23±0.08 µmol/l; P<0.05). Compared with the
baseline value, the IMT was significantly decreased after allicin
treatment (P<0.01). The IMT was also significantly decreased in
the control group (P<0.05), but that the decrease was greater in
the allicin group (P<0.01). As shown in Figs. 1 and 2, after allicin treatment, the IMT was
decreased from 1.4 to 1.0 mm.
Serum biochemical index levels
The levels of serum biochemical indices are shown in
Table III. At baseline, the
biochemical indices did not differ between the two groups. However,
after 12 weeks of allicin treatment, significant decreases in TC
and TG were observed (P<0.01). Furthermore, the decreases in the
allicin group were significant larger than those in the control
group (P<0.05).
 | Table III.Levels of serum biochemical indices
(mmol/l). |
Table III.
Levels of serum biochemical indices
(mmol/l).
|
| Allicin group | Control group |
|---|
|
|
|
|
|---|
| Index | Before
treatment | After
treatment | Before
treatment | After
treatment |
|---|
| TC | 5.60±1.20 |
4.81±1.00a,b | 5.55±1.28 |
5.33±0.78c |
| TG | 3.53±1.76 |
1.93±0.91a,b | 3.09±1.60 |
2.54±0.79c |
Discussion
Atherosclerosis, initiated at sites of endothelial
cell injury, is the pathological basis of cardiovascular and
cerebrovascular diseases, such as coronary heart disease, cerebral
apoplexy and post-angioplasty restenosis. Characteristic features
of atherosclerotic lesions include infiltration of monocytic cells,
cholesterol deposition, elaboration of extracellular matrix as well
as proliferation and migration of vascular smooth muscle cells.
HHcy is an independent risk factor of arteriosclerosis (16,17) and
has been associated with an increased relative risk of
cardiovascular events (18). In 587
coronary disease patients followed up for four years, a mortality
rate of 3.8% was observed among those with homocysteinemia (Hcy,
<9 µmol/l), and a mortality rate of 24.7% among those with Hcy
levels of >15 µmol/l (19). The
rate of HHcy among patients with atherosclerotic diseases is up to
40%. In the clinic, HHcy is classified into three categories
according to the plasma Hcy concentration: Mild (15–30 µmol/l),
moderate (30–100 µmol/l) and severe (>100 µmol/l). The present
study identified that coronary heart diseases were closely
associated with mild HHcy. Few of the patients with coronary heart
diseases had moderate HHcy, but none of them had severe HHcy. In
the allicin group, a significant decrease of plasma Hcy levels was
observed after 12 weeks of treatment, which was significantly
larger than the decrease in the control group, suggesting that the
use of allicin may decrease Hcy levels in patients with coronary
heart disease. Hcy, a sulfur-containing amino acid, is an
intermediate product of methionine metabolism and causes multifold
effects through the reactivity of its sulfhydryl group. Laboratory
studies have suggested a role of Hcy in activating pathways
attributed to atherogenesis and thrombosis (20), including endothelial dysfunction,
inflammation, oxidative stress, lipid peroxidation (21), endoplasmic reticulum stress (22), stimulation of vascular smooth muscle
cell proliferation and apoptosis (23). Garlic consumption has been inversely
correlated with multiple risk factors associated with
cardiovascular diseases, including increased platelet aggregation
(9,24), reactive oxygen species (25), high blood pressure (11), high cholesterol (12,26),
proliferation and migration of vascular smooth muscle cells
(27,28), and apoptosis of heart cell (29). Allicin, diallyl trisulfide
(CH2=CH-CH2-S-S-S-CH2-CH=CH2),
is an organosulfur compound considered responsible for most of the
pharmacological activities of garlic. Therefore, it is hypothesized
that allicin has certain lowering effects on Hcy. A previous study
by our group reported that allicin obviously decreases the serum
Hcy levels in rats with HHcy and also alleviates Hcy-induced injury
to endothelial cells (30). Another
study suggested that garlic and garlic-derived organic polysulfides
induce H2S production in a thiol-dependent manner
(31). H2S is an
endogenous cardioprotective vascular cell-signaling molecule. It
can not only activate KATP channels, but also react with
S-nitrosothiol species to release NO as a reducing agent and
nucleophile, causing vascular smooth muscle cell relaxation.
Whether H2S generation from allicin is able to decrease
Hcy in HHcy patients requires further investigation. The decrease
of Hcy levels in coronary heart disease patients with HHcy after
treatment with allicin was confirmed in the present study.
Lipid dysbolism is involved in the development of
atherosclerosis. However, evidence linking allicin to blood lipids
remains inconclusive. In the present study, significant decreases
in TC and TG were observed in the allicin group compared with those
in the control group. The possible mechanisms of allicin in
reducing blood lipids are as follows: A displacement reaction of
allicin with an enzyme containing an SH moiety may take place,
which interferes the synthesis of lipids. Allicin may increase
lipoidase activity and accelerate the hydrolysis of triglycerides.
In the metabolic processes, allicin requires NADPH, which is also
the sine qua non substrate in the biosynthesis of
cholesterol and triglycerides. Allicin reacts with oxidized
glutathione, causing a deficiency of glutathione, which affects the
metabolism of insulin in the liver and indirectly affects the
metabolism of lipids.
Due to its simplicity, reproducibility,
cost-effectiveness and non-invasiveness, IMT measurement of carotid
arteries has been widely adopted as a method for assessing systemic
atherosclerosis and cardiovascular risk in adults. IMT has been
reported to be an independent predictor of coronary heart disease
(32–34). In carotid atherosclerosis patients,
an increase in the IMT by 0.1 mm is associated with an 11% increase
in the risk of acute myocardial infarction (35). The increased thickness of the carotid
artery IMT is a signal of the total body vascular intima. Several
biological mechanisms of the increased thickness of the vascular
intima associated with HHcy include impaired endothelial function,
dysregulation of cholesterol and triglyceride biosynthesis and
stimulation of vascular smooth muscle cell proliferation. The
oxidant stress hypothesis is frequently used to explain the
damaging effects of Hcy on vascular cells. Due to the reactive
sulfhydryl group (−SH), Hcy, as most thiols (RSH), can undergo
oxidation to the disulfide (RSSR) at physiological pH in the
presence of O2: 2RSH + O2 → RSSR +
[O2−] + 2H → H2O2. By
inducing oxidative stress, the free radicals can injure the
vascular endothelium. Allicin is able to quench oxygen and hydroxyl
radicals to alleviate oxidative stress in a dose-effect manner.
Nutritional deficiencies in B vitamin cofactors, including folic
acid, vitamin B6 (pyridoxal phosphate) and/or
B12 (methylcobalamin) are required for Hcy metabolism.
Of note, dietary enrichment in B vitamins can decrease the levels
of Hcy but does not attenuate these adverse effects of HHcy.
Therefore, only decreasing the Hcy levels is insufficient. The
present findings indicated that the carotid artery IMT in the
allicin group was significantly decreased compared with that in the
control group, which showed the beneficial effect of allicin in
atherosclerosis. It is therefore indicated that the beneficial
effects may be associated with the anti-oxidative effects of
allicin.
In conclusion, the present study indicated that
allicin may be useful for the prevention of atherosclerosis, at
least in part by decreasing Hcy levels, regulating lipid metabolism
and decreasing the carotid artery IMT. Medication prescribed for
patients with coronary heart disease may also modify Hcy levels.
However, certain studies have indicated that vitamin therapy, such
as folic acid and B vitamins, lowered Hcy levels but failed to
rescue cardiovascular or neurovascular disease outcomes (36–39).
Therefore, allicin may be a prospective drug for the prevention of
cardiovascular and cerebrovascular diseases.
Acknowledgements
The present study was supported by Shandong
University and by the Tackle Key Problems in Science and Technology
Article of Shandong, China (grant no. Y2006C55). The authors would
like to thank the Ultrasound and Central Laboratory departments of
Qilu Hospital of Shandong University (Jinan, China) for technical
assistance.
References
|
1
|
Fallon UB, Ben-Shlomo Y, Elwood P, Ubbink
JB and Smith GD: Homocysteine and coronary heart disease in the
caerphilly cohort: A 10 year follow up. Heart. 85:153–158. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Suhara T, Fukuo K, Yasuda O, Tsubakimoto
M, Takemura Y, Kawamoto H, Yokoi T, Mogi M, Kaimoto T and Ogihara
T: Homocysteine enhances endothelial apoptosis via upregulation of
Fas-mediated pathways. Hypertension. 43:1208–1213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lauricella AM, Quintana IL and Kordich LC:
Effects of homocysteine thiol group on fibrin networks: Another
possible mechanism of harm. Thromb Res. 107:75–79. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dardik R, Varon D, Tamarin I, Zivelin A,
Salomon O, Shenkman B and Savion N: Homocysteine and oxidized low
density lipoprotein enhance platelet adhesion to endothelial cells
under flow conditions: Distinct mechanisms of thrombogenic
modulation. Thromb Haemost. 83:338–344. 2000.PubMed/NCBI
|
|
5
|
Maron BA and Loscalzo J: The treatment of
hyperhomocysteinemia. Annu Rev Med. 60:39–54. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eberhardt RT, Forgione MA, Cap A, Leopold
A, Rudd MA, Trolliet M, Heydrick S, Stark R, Klings ES, Moldovan
NI, et al: Endothelial dysfunction in a murine model of mild
hyperhomocyst(e)inemia. J Clin Invest. 106:483–491. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heydrick SJ, Weiss N, Thomas SR, Cap AP,
Pimentel DR, Loscalzo J and Keaney JF Jr: L-Homocysteine and
L-homocystine stereospecifically induce endothelial nitric oxide
synthase-dependent lipid peroxidation in endothelial cells. Free
Radic Biol Med. 36:632–640. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dayal S and Lentz SR: Role of redox
reactions in the vascular phenotype of hyperhomocysteinemic
animals. Antioxid Redox Signal. 9:1899–1909. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patumraj S, Tewit S, Amatyakul S,
Jariyapongskul A, Maneesri S, Kasantikul V and Shepro D:
Comparative effects of garlic and aspirin on diabetic
cardiovascular complications. Drug Deliv. 7:91–96. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kanth V Rajani, Uma Maheswara Reddy P and
Raju TN: Attenuation of streptozotocin- induced oxidative stress in
hepatic and intestinal tissues of Wistar rat by methanolic-garlic
extract. Acta Diabetol. 45:243–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Varmaghany S, Torshizi MA Karimi, Rahimi
S, Lotfollahian H and Hassanzadeh M: The effects of increasing
levels of dietary garlic bulb on growth performance, systolic blood
pressure, hematology, and ascites syndrome in broiler chickens.
Poult Sci. 94:1812–1820. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ashraf R, Aamir K, Shaikh AR and Ahmed T:
Effects of garlic on dyslipidemia in patients with type 2 diabetes
mellitus. J Ayub Med Coll Abbottabad. 17:60–64. 2005.PubMed/NCBI
|
|
13
|
Liu DS, Gao W, Liang ES, Wang SL, Lin WW,
Zhang WD, Jia Q, Guo RC and Zhang JD: Effects of allicin on
hyperhomocysteinemia-induced experimental vascular endothelial
dysfunction. Eur J Pharmacol. 714:163–169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
European Society of Hypertension-European
Society of Cardiology Guidelines Committee, . 2003 European society
of hypertension-European society of cardiology guidelines for the
management of arterial hypertension. J Hypertens. 21:1011–1053.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo J, Xiao F and Tang ZY: Determination
of homocysteine in plasma by high performance liquid
chromatography. Chin J Lab Med. 217–219. 2000.(In Chinese).
|
|
16
|
Wald DS, Law M and Morris JK: Homocysteine
and cardiovascular disease: Evidence on causality from a
meta-analysis. BMJ. 325:12022002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Homocysteine Studies Collaboration:
Homocysteine and risk of ischemic heart disease and stroke: A
meta-analysis. JAMA. 288:2015–2022. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mudd SH, Skovby F, Levy HL, Pettigrew KD,
Wilcken B, Pyeritz RE, Andria G, Boers GH, Bromberg IL, Cerone R,
et al: The natural history of homocystinuria due to cystathionine
beta-synthase deficiency. Am J Hum Genet. 37:1–31. 1985.PubMed/NCBI
|
|
19
|
Nygård O, Nordrehaug JE, Refsum H, Ueland
PM, Farstad M and Vollset SE: Plasma homocysteine levels and
mortality in patients with coronary artery disease. N Eng J Med.
337:230–236. 1997. View Article : Google Scholar
|
|
20
|
de Koning AB Lawrence, Werstuck GH, Zhou J
and Austin RC: Hyperhomocysteinemia and its role in the development
of atherosclerosis. Clin Biochem. 36:431–441. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blom HJ, Kleinveld HA, Boers GH, Demacker
PN, Har-Lemmers HL, Te Poele-Pothoff MT and Trijbels JM: Lipid
peroxidation and susceptibility of low-density lipoprotein to in
vitro oxidation in hyperhomocysteinaemia. Eur J Clin Invest.
25:149–154. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou J and Austin RC: Contributions of
hyperhomocysteinemia to atherosclerosis: Causal relationship and
potential mechanisms. Biofactors. 35:120–129. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Skurk C and Walsh K: Death receptor
induced apoptosis: A new mechanism of homocysteine-mediated
endothelial cell cytotoxicity. Hypertension. 43:1168–1170. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ohaeri OC and Adoga GI: Anticoagulant
modulation of blood cells and platelet reactivity by garlic oil in
experimental diabetes mellitus. Biosci Rep. 26:1–6. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kanth V Rajani, Uma Maheswara Reddy P and
Raju TN: Attenuation of streptozotocin-induced oxidative stress in
hepatic and intestinal tissues of Wistar rat by methanolic-garlic
extract. Acta Diabetol. 45:243–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu MW and Tang SL: Curative effects of
allicin on 59 hyperlipidemia patients. Chin General Practice.
496–497. 2002.(In Chinese).
|
|
27
|
Tan B, Hu YS, Hang H, Yu ZL and Li YJ:
Protecting function of Thera-Garlicin on injury of blood vessel
endothelium in rats with diabetes mellitus. Modern J Integrated
Traditional Chin Western Med. 3335–3336. 2006.(In Chinese).
|
|
28
|
Baluchnejadmojarad T, Roghani M,
Homayounfar H and Hosseini M: Beneficial effect of aqueous garlic
extract on the vascular reactivity of streptozotocin-diabetic rats.
J Ethnopharmacol. 85:139–144. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin YL and Yang SF: Effect of allicin on
apoptosis and MAPK of heart cell in diabetic rats. J Lialaoning
Univ TCM. 13:56–58. 2011.
|
|
30
|
Wang SL, Liu DS, Guo RC and Zhang WD:
Effect of garlicin on the serum homocysteine level in rats. J
Shandong Univ. 389–391. 2006.(In Chinese).
|
|
31
|
Benavides GA, Squadrito GL, Mills RW,
Patel HD, Isbell ST, Patel RP, Darley-Usmar VM, Doeller JE and
Kraus DW: Hydrogen sulfide mediates the vasoactivity of garlic.
Proc Natl Acad Sci USA. 104:pp. 17977–17982. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Adams MR, Nakagomi A, Keech A, Robinson J,
McCredie R, Bailey BP, Freedman BS and Celermajer DS: Carotid
intima-media thickness is only weakly correlated with the extent
and severity of coronary artery disease. Circulation. 92:2127–2134.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chambless LE, Heiss G, Folsom AR, Rosamond
W, Szklo M, Sharrett AR and Clegg LX: Association of coronary heart
disease incidence with carotid arterial wall thickness and major
risk factors: The Atherosclerosis Risk in Communities (ARIC) Study,
1987–1993. Am J Epidemiol. 146:483–494. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Burke GL, Evans GW, Riley WA, Sharrett RA,
Howard G, Barnes RW, Rosamond W, Crow RS, Rautaharju PM and Heiss
G: Arterial wall thickness is associated with prevalent
cardiovascular disease in middle-aged adults. The Atherosclerosis
Risk in Communities (ARIC) Study. Stroke. 26:386–391. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Karpe F, Boquist S, Tang R, Bond GM, de
Faire U and Hamsten A: Remnant lipoproteins are relatedto
intima-media thickness of the carotid artery independently of LDL
cholesterol and plasma triglycerides. J Lipid Res. 42:17–21.
2001.PubMed/NCBI
|
|
36
|
Albert CM, Cook NR, Gaziano JM, Zaharris
E, MacFadyen J, Danielson E, Buring JE and Manson JE: Effect of
folic acid and B vitamins on risk of cardiovascular events and
total mortality among women at high risk for cardiovascular
disease: A randomized trial. JAMA. 299:2027–2036. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ebbing M, Bleie Ø, Ueland PM, Nordrehaug
JE, Nilsen DW, Vollset SE, Refsum H, Pedersen EK and Nygård O:
Mortality and cardiovascular events in patients treated with
homocysteine-lowering B vitamins after coronary angiography: A
randomized controlled trial. JAMA. 300:795–804. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lonn E, Yusuf S, Arnold MJ, Sheridan P,
Pogue J, Micks M, McQueen MJ, Probstfield J, Fodor G, Held C, et
al: Homocysteine lowering with folic acid and B vitamins in
vascular disease. N Engl J Med. 354:1567–1577. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Toole JF, Malinow MR, Chambless LE, Spence
JD, Pettigrew LC, Howard VJ, Sides EG, Wang CH and Stampfer M:
Lowering homocysteine in patients with ischemic stroke to prevent
recurrent stroke, myocardial infarction, and death: The vitamin
intervention for stroke prevention (VISP) randomized controlled
trial. JAMA. 291:565–575. 2004. View Article : Google Scholar : PubMed/NCBI
|