Introduction
Myocardial infarction (MI) is the most common cause
of mortality for patients with cardiovascular diseases (1). Elevation of left ventricular (LV)
filling pressures following MI may lead to secondary pulmonary
hypertension (PH) and ventricular remodeling, and even heart
failure (HF) (2). LV failure causes
PH and increased right ventricular (RV) afterload, leading to RV
remodeling and dysfunction (3). PH
due to left heart disease (LHD) mainly arises from left heart
systolic or diastolic dysfunction, or valvular heart disease, and
is associated with poor clinical outcome (4). Cardiac remodeling following MI involves
several molecular and cellular mechanisms in the ischemic and
non-ischemic myocardium (5). The
persistently elevated pulmonary pressures may deteriorate
endothelial dysfunction, decrease nitric oxide (NO) availability
and increase endothelin expression (2,6). PH
associated with LHD may be reversible in the early stages, while
long-standing PH may be irreversible due to cardiac remodeling
(7).
The guidelines for PH generally discourage the use
of drugs approved for PH reduction to treat PH due to LHD as
evidence to support their use is lacking (8). However, the guidelines give little
advice on the management of excessive vasoconstriction or
remodeling of the pulmonary vasculature in reactive PH. Therefore,
it is important to develop and evaluate novel therapies directly
affecting the pulmonary vascular component.
NO is a small gaseous molecule produced by
endogenous NO synthases that is involved in the regulation of
vascular tone and blood pressure, in angiogenesis and in
endothelial integrity (9,10). Reduced NO bioavailability is
associated with a number of cardiovascular diseases (11–13),
including PH. Therefore, the use of exogenous NO is important in
the treatment of PH following LHD (14,15). NO
donors such as organic nitrates (for example, nitroglycerin) have
been used for over a century; however, the development of tolerance
is a major problem with these drugs (16).
Inhalation of NO during myocardial ischemia may
alleviate secondary pulmonary hemodynamic dysfunction, reduce
infarct size and improve cardiac function (14). In the last decade, studies have shown
that inhalation of nebulized nitrates is able to alleviate PH,
whereas the effects of NO on ventricular remodeling and cardiac
function are poorly understood (17,18).
Isosorbide dinitrate (ISDN) is an NO donor that prevents LV
remodeling and degradation of cardiac function following MI
(19,20).
However, there is a lack of supportive management of
excessive vasoconstriction or remodeling of the pulmonary artery in
PH following MI. Therefore, the present study investigated the
benefits of intratracheal ISDN instillation in the amelioration of
pulmonary pressure and ventricular remodeling in a rat model of HF
following MI.
Materials and methods
Study design
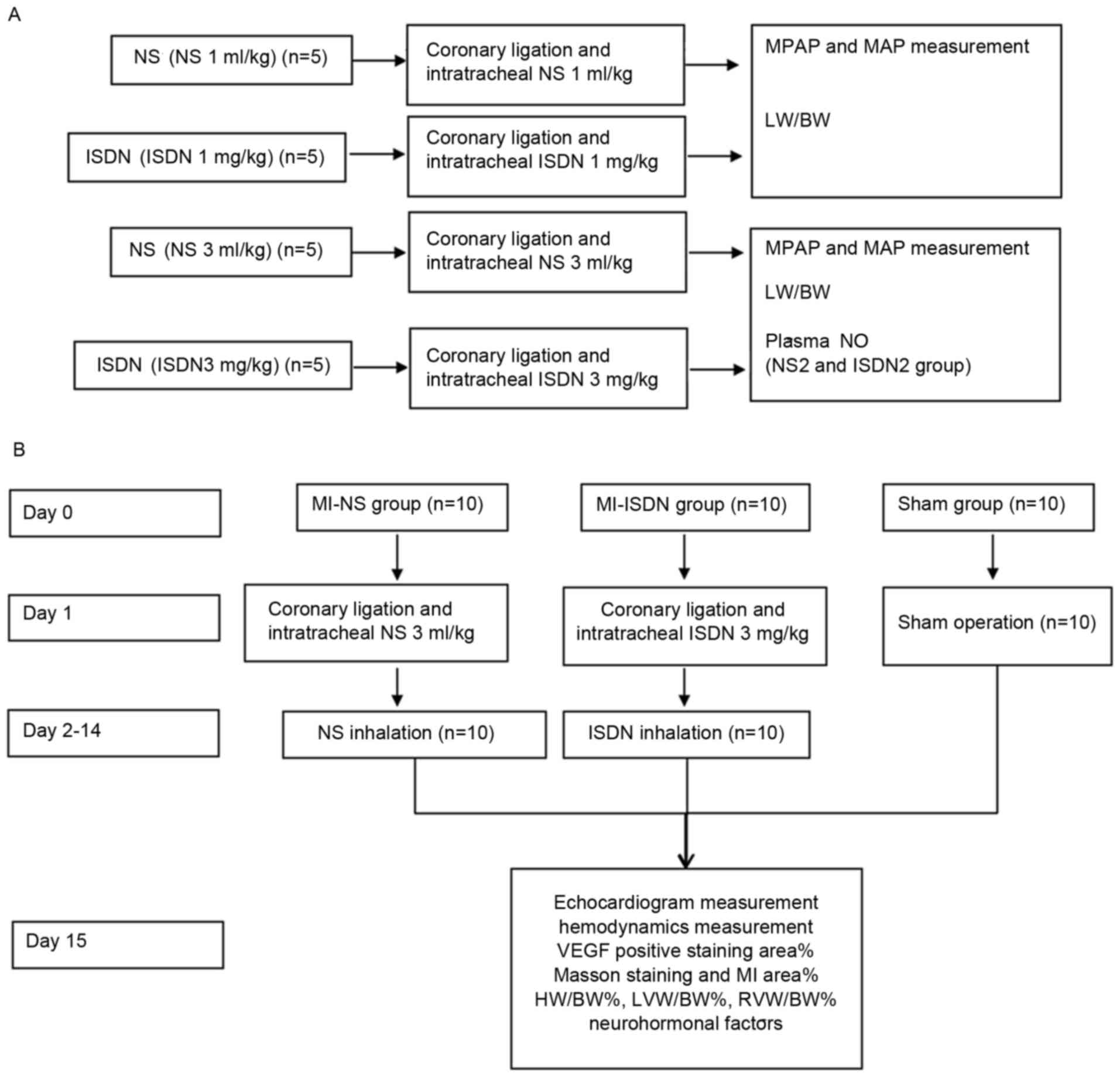
The study design and the protocol are presented in
Fig. 1. The study comprised two
parts. The first part examined the effect of ISDN instillation on
pulmonary pressure, and the second part examined the effect of ISDN
instillation on ventricular remodeling.
Animals
The study was conducted on 50 male juvenile
Sprague-Dawley (SD) rats (5–6 weeks old), with a body weight (BW)
of 200–250 g, which were purchased from the Shanghai Laboratory
Animal Centre (CAS; Shanghai, China). A standard pellet diet and
water were provided ad libitum. The animals were housed in a
temperature-(21–23°C) and humidity-controlled (40–70%) room and
maintained on a 12-h light/dark cycle. The study protocol was
approved by the Animal Research Ethics Committee of Huadong
Hospital Affiliated to Fudan University (Shanghai, China). All
animals received care in accordance with the Guidance for the Care
and Use of Laboratory Animals (21).
MI rat model
The MI model has been described previously (22). In brief, rats were anesthetized with
chloral hydrate [0.3 g/kg, intraperitoneally (i.p.)] and placed in
a supine position. The chest wall was shaved. A left thoracotomy
was performed at the fourth intercostal space. Respiration was
maintained by mechanical ventilation following orotracheal
intubation. Following exposure of the heart, the middle left
ascending coronary artery was ligated, ~4 mm below the
anterior-inferior edge of the left atrium. The local myocardium
became white immediately following the ligation due to MI. The
thorax was closed and the rats recovered from anesthesia.
Electrocardiograms were recorded prior to and following surgery.
Successful establishment of this model was defined by a significant
ST segment elevation. The rats received 400,000 U/kg penicillin
intramuscularly immediately following the surgery and daily for 3
days.
Mean pulmonary arterial pressure
(MPAP) and mean arterial pressure (MAP) measurements subsequent to
ISDN or normal saline (NS) instillation in the trachea
The pharmacological effects of intratracheal ISDN
instillation were observed. Twenty successful rat models were
randomized in the following four groups (each n=5): NS (1 ml/kg)
group, ISDN (1 ml/kg) group, NS (3 ml/kg) group and ISDN (3 ml/kg)
group. Polyvinyl catheters (internal diameter, 0.7 mm) were
directly inserted into the left carotid artery and right jugular
vein. When hemodynamic data were stable for 15 min after ligation,
NS or ISDN was instilled into the trachea of the MI model rats
(23). For rats in the NS (1 ml/kg)
or ISDN (1 ml/kg) groups, 1 ml/kg NS or 1 mg/kg ISDN (batch no.
555600; UCB Pharma GmbH, Monheim am Rhein, Germany) was instilled
into the trachea. In the NS (3 ml/kg) group, 1 ml/kg NS was
instilled thrice at 5-min intervals to provide a total dose of 3
ml/kg. In the ISDN (3 ml/kg) group, ISDN was administered using the
protocol described for the NS (3 ml/kg) group. The dosage regimen
and methodology were based on a pilot study demonstrating that the
instillation of three 1 mg/kg doses of ISDN at 5-min intervals
achieved the desired effects without causing adverse effects such
as hypotension or sudden death (data not shown). MPAP and MAP were
measured using a specialized system (MFLab3.01; Shanghai Jia Long
Educational Instrument Factory, Shanghai, China) (24). Changes in MPAP (ΔMPAP%) and in MAP
(ΔMAP%) were calculated using the following formulae and compared
among the groups: ΔMPAP%x min = (MPAPx min -
MPAP0 min)/MPAP0 min × 100; ΔMAP%x
min = (MAPx min - MAP0 min)/MAP0
min × 100.
Lung weight/BW ratio and plasma NO
measurement
Following the measurement of MPAP and MAP, the lungs
of rats in the NS (3 ml/kg) and ISDN (3 ml/kg) groups were
dissected and weighed. The ratios of wet lung weight to BW, dry
lung weight to BW, and dry to wet lung weight were calculated.
Blood samples were taken from rats in the NS (3
ml/kg) and ISDN (3 ml/kg) groups immediately following the final NS
or ISDN instillation. Blood samples were centrifuged 3,000 × g for
10 min at 4°C and the supernatant was used to assess plasma NO
levels using an NO assay kit (cat. no. A012; Nanjing Jiancheng
Bioengineering Institute, Nanjing, China). In order to determine
the immediate effects of ISDN, NO was measured from blood samples
taken immediately following ISDN/NS instillation.
ISDN/NS inhalation in rats following
MI
In the second part of the study, on day 0, 30 SD
rats were randomized into three groups (each n=10): MI-NS group,
MI-ISDN group and sham group. On day 1, coronary ligation was
performed on the rats in the MI-NS and MI-ISDN groups, as
previously described (22). When
hemodynamic stability was achieved 15 min after ligation, 1 ml/kg
NS was instilled intratracheally every 5 min thrice to provide a
total dose of 3 ml/kg in the MI-NS group. In the MI-ISDN group, 3
mg/kg ISDN was instilled intratracheally using the same protocol.
The thoracic cavity of rats in the sham group was opened without
coronary ligation.
For the following 13 days (days 2–14), ISDN or NS
inhalation was performed in the MI-NS and MI-ISDN groups,
respectively. ISDN (3 mg/kg) or NS (3 ml/kg) were nebulized using
an ultrasonic nebulizer (NE105; Guangying Electronics Co., Ltd.,
Foshan, China) and inhaled by the rats. Inhalation was conducted
for 15 min daily. Rats in the sham group did not receive any
inhalation treatment.
Echocardiogram and hemodynamic
measurements
On day 15, all rats from each group were
anesthetized with urethane (1 mg/kg, i.p.). Echocardiogram
measurements were obtained. LV internal diameter at end-diastole
(LVIDd) and systole (LVIDs), LV volume at end-diastole (LV Vol d)
and systole (LV Vol s), LV post wall diameters at end-diastole
(LVPWd) and systole (LVPWs), LV anterior wall diameters at
end-diastole (LVAWd) and systole (LVAWs), LV ejection fraction
(LVEF) and fraction shortening (FS) were evaluated.
Catheters were inserted into the left carotid artery
and right jugular vein to measure RV ventricular end-diastolic
pressure (RVEDP), central venous pressure (CVP), LV systolic
pressure, LV end-diastolic pressure (LVEDP) and the maximum rising
and dropping rates of LV or RV pressure (± dp/dtmax)
were evaluated and compared among the groups. All values were
recorded and analyzed using an MFLab 3.01 system.
Levels of neurohormonal factors
On day 15, blood was sampled from the abdominal
aorta (~10 ml/rat). Plasma levels of B-type natriuretic peptide
(BNP), epinephrine, norepinephrine and angiotensin II were assessed
using ELISA kits; BNP ELISA kit (cat. no. CK-E30445R), epinephrine
ELISA kit (cat. no. CK-E30233R), norepinephrine ELISA kit (cat. no.
CK-E30189R), and an angiotensin II ELISA kit (cat. no. CK-E30668R;
all from Biocalvin, Suzhou, China).
Heart weight (HW)/BW%, LV weight/BW%
and RV weight/BW% evaluation
On day 15, the hearts were harvested and weighed.
The HW/BW%, LV weight/BW% and RV RVW/BW% values were
calculated.
Immunohistochemical staining, and
estimation of MI and VEGF-positive area percentages
On day 15, hearts were sliced transversely into
several pieces from the basal to apex plane. Sections with
thickness of 4 µm were fixed in 4% paraformaldehyde at 37°C for 10
min and stained using Masson staining methods (Masson stain kit;
HL70013; Nanjing Jiancheng Bioengineering Institute, Nanjing,
China) according to the manufacturer's protocol and the MI area %
was calculated using the IMS Imaging system (version 2.1.1;
Shanghai ShenTeng Information Technology Co., Ltd., Shanghai,
China). The infarct size was determined as the mean percentage of
the epicardial and endocardial circumference occupied by scar
tissue, as observed on the stained sections using a light
microscope (25).
The myocardium at the border of the MI area was
immunohistochemically stained using rabbit anti-rat vascular
endothelial growth factor (VEGF) antibody (cat. no. 9698; Cell
Signaling Technology, Inc., Danvers, MA, USA). Tissues were
dehydrated in a graded series of ethanol and fixed at 37°C in 4%
paraformaldehyde for 24 h and embedded in paraffin.
Paraffin-embedded tissues were sectioned at 4 µm and
deparaffinized. They were subjected to epitope retrieval by
immersion in 0.01 mol/l sodium citrate buffer (Sinopharm Chemical
Reagent Co., Ltd., Shanghai, China) with pH 6.0, heated in a
microwave (98°C for 10 min) and allowed to cool for ~20 min.
Endogenous peroxidase was inactivated with 3%
H2O2. Samples were incubated for 120 min with
1:200 rabbit anti-rat VEGF antibody at 37°C, washed and
subsequently incubated with peroxidase AffiniPure Goat Anti-Rabbit
immunoglobulin G (1:200; cat. no. 111-035-003; Jackson
ImmunoResearch Laboratories, Inc., West Grove, PA, USA) conjugated
to biotin for 30 min at 37°C. The sections were rinsed with PBS,
counterstained with hematoxylin at 37°C for 1 min, rinsed again and
mounted. Microscopic analysis was performed using a light
microscope under high-power magnification (×200). The VEGF-positive
area (%) was evaluated as previously described (26) using the IMS Imaging system.
Statistical analysis
All values are presented as the mean ± standard
deviation. Values were compared using one-way analysis of variance
(ANOVA) for intergroup data. The least significant difference test
or Tamhane's T2 test was performed as a post hoc test following
ANOVA. Data were analyzed using SPSS 17.0 software (SPSS, Inc.,
Chicago, Il, USA). Two-sided P-values <0.05 were considered to
indicate a statistically significant result.
Results
Acute effects of ISDN intratreacheal
administration on hemodynamics in MI rats
Following the intratracheal instillation of 1 mg/kg
ISDN, MPAP and MAP were decreased at 1 min and returned gradually
to near the baseline within 5 min, as shown by ΔMPAP% and ΔMAP%
being approximately-24 and −34%, respectively, at 1 min, and
gradually tending to return to 0% (Fig.
2A and B). Therefore, the dosage of 3 mg/kg of ISDN
administered in a divided form (1 mg/kg every 5 min) achieved the
desired effects without significant adverse events. ΔMPAP% and
ΔMAP% were significantly greater in the ISDN (1 ml/kg) group
compared with the NS (1 ml/kg) group at all time points, with the
exception of 5 min (P<0.05; Fig. 2A
and B). Similar results were observed in the ISDN (3 ml/kg) and
NS (3 ml/kg) groups (Fig. 2C and D),
where the 5-min cycles were clearly observed.
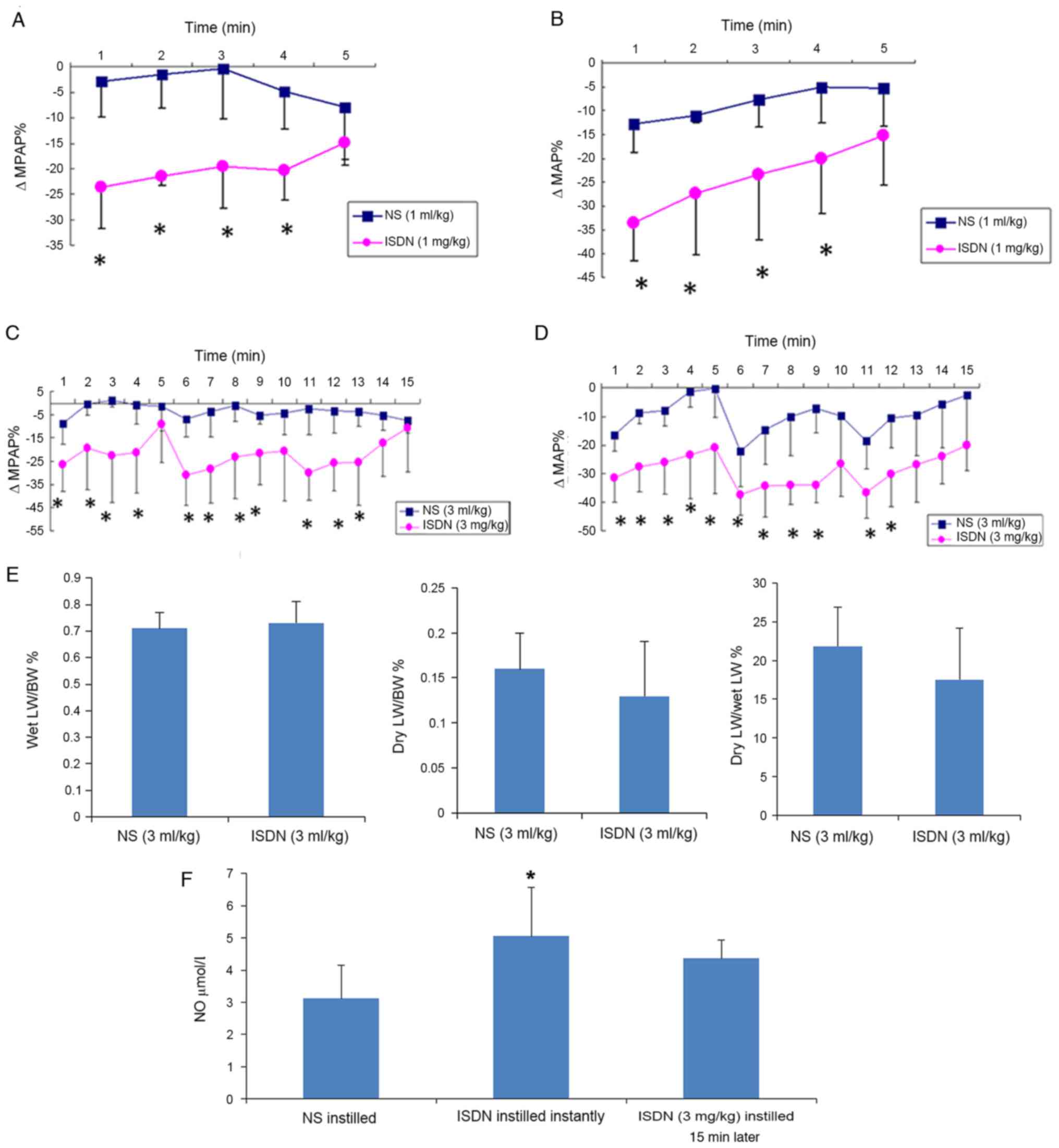 | Figure 2.ΔMPAP, ΔMAP, lung weight and NO in
the NS and ISDN groups. Differences in (A) ΔMPAP% and (B) ΔMAP%
were detected following the intratracheal instillation of 1 mg/kg
ISDN vs. 1 ml/kg NS in MI rats. Changes in (C) ΔMPAP% and (D) ΔMAP%
were detected following the intratracheal instillation of 3 mg/kg
ISDN vs. 3 ml/kg NS in MI rats. *P<0.05 vs. the NS group;
n=5/group. (E) Wet LW/BW%, dry LW/BW% and dry LW/BW% following the
intratracheal instillation of 3 ml/kg NS or 3 mg/kg ISDN. (F)
Plasma NO levels following NS/ISDN instillation. *P<0.05 vs. the
NS group; n=5/group. ΔMPAP%, percentage change in mean pulmonary
arterial pressure; ΔMAP%, percentage change in mean arterial
pressure; NO, nitric oxide; NS, normal saline; ISDN, isosorbide
dinitrate; MI, myocardial infarction; LW, lung weight; BW, body
weight. |
Lung weight/BW ratio calculation
following NS/ISDN instillation
No significant differences between the NS (3 ml/kg)
and ISDN (3 ml/kg) groups were observed for the following
parameters: Wet lung weight to BW (0.71±0.06 vs. 0.73±0.08%), dry
lung weight to BW (0.16±0.04 vs. 0.13±0.06%) and dry to wet lung
weight (21.82±5.10 vs. 17.52±6.62%; Fig.
2E).
Plasma NO concentration assessment
following NS/ISDN intratracheal instillation
Fig. 2F shows that
ISDN intratracheal instillation increased plasma NO levels.
Following ISDN instillation, plasma NO levels were rapidly and
significantly increased (P=0.017), as shown by analysis of the
blood sample taken immediately after instillation. The
aforementioned results demonstrated that the changes in MPAP and
MAP induced by 3 mg/kg ISDN lasted for the 15 min of
administration. However, plasma NO levels in the ISDN (3 mg/kg)
group were higher than those in NS rats even following 15 min of
ISDN instillation (Fig. 2F). The
onset of action of ISDN after inhalation was very rapid (<1 min)
but lasted for 5 min.
Echocardiogram and hemodynamic
measurement in rats 14 days after MI
LV enlargement was attenuated in the MI-ISDN group
on day 15 since LVIDd and LV Vol d were markedly reduced compared
with those in the MI-NS group. LV systolic function was also
improved in the MI-ISDN group as LVIDs and LV Vol s were decreased
in comparison with those in the MI-NS group, and LVEF% and FS% were
significantly increased. LVAWd and LVAWs in rats of the MI-ISDN
group were thicker compared with those in the MI-NS group, which
may be associated with a reduction in the MI area. Moreover, LVPWd
and LVPWs were thinner in rats treated with ISDN inhalation
compared with those receiving NS inhalation, suggesting that
myocardium hypertrophy occurred in the LV posterior wall and was
improved by ISDN inhalation (Table
I).
 | Table I.Echocardiogram measurements. |
Table I.
Echocardiogram measurements.
| Variables | MI-NS | MI-ISDN | Sham |
|---|
| LVIDd (mm) |
7.63±1.03a,b |
6.07±1.29b | 5.03±0.65 |
| LVIDs (mm) |
6.26±1.38a,b |
3.75±1.99b | 1.76±1.01 |
| LV Vol d (µl) |
317.03±91.00a,b |
195.24±93.68b | 122.64±37.58 |
| LV Vol s (µl) |
209.74±102.15a,b | 83.54±94.22 | 14.67±25.18 |
| LVPWd (mm) |
1.92±0.34a,b |
1.56±0.22b | 1.32±0.18 |
| LVPWs (mm) | 2.50±0.20 | 2.34±0.28 | 2.32±0.34 |
| LVAWd (mm) |
1.00±0.21a,b | 1.45±0.45 | 1.55±0.14 |
| LVAWs (mm) |
1.14±0.36a,b |
2.01±0.83b | 2.94±0.35 |
| LVEF% |
36.76±15.14a,b |
66.35±24.73b | 90.73±12.93 |
| FS% |
18.79±8.55a,b |
41.09±20.21b | 66.32±14.25 |
Hemodynamic variables (Table II) further suggested that LV
systolic function was improved by ISDN inhalation, since
LV+dp/dtmax was significantly higher in the MI-ISDN
group compared with the MI-NS group. LV diastolic function was
improved in the MI-ISDN group, as shown by the LVEDP and
LV-dp/dtmax measurements. The diastolic function
improvement may be associated with attenuation of LV hypertrophy,
as shown by the echocardiogram data.
 | Table II.Hemodynamic variables. |
Table II.
Hemodynamic variables.
| Variables | MI-NS | MI-ISDN | Sham |
|---|
| MAP (mmHg) | 146.54±24.06 | 134.06±28.96 | 149.70±29.45 |
| HR (bpm) | 426±65a | 397±41 | 358±64 |
| LVSP (mmHg) | 171.35±24.50 | 157.75±35.47 | 166.70±20.81 |
| LVEDP (mmHg) |
11.06±5.10a,b | 5.85±3.50 | 3.82±0.70 |
|
LV+dp/dtmax (mmHg/sec) |
5,284.52±621.76a,b |
7,998.46±2,761.47 |
6,837.68±424.74 |
|
LV-dp/dtmax (mmHg/sec) |
3,558.97±842.38a,b |
5,322.14±1,325.26 |
4,837.96±868.72 |
| RVSP (mmHg) |
47.72±5.05a,b | 30.76±16.94 | 23.58±6.18 |
| RVEDP (mmHg) |
7.63±1.44a,b | 2.09±4.38 | 0.26±3.51 |
|
RV+dp/dtmax (mmHg/sec) |
2,902.55±485.22a,b |
2,375.61±421.41 |
2,432.13±407.53 |
|
RV-dp/dtmax (mmHg/sec) |
2,173.92±343.32a,b |
1,621.45±301.87 |
1,789.64±444.28 |
| CVP
(cmH2O) |
9.39±1.63a,b | 6.91±2.51 | 5.44±1.25 |
Systolic pulmonary pressure, as estimated by RV
systolic pressure (RVSP), was elevated in the MI-NS and MI-ISDN
groups compared with the sham group. ISDN inhalation reduced RSVP
and improved RV systolic and diastolic functions, as characterized
by improvements of RV±dp/dtmax, RVEDP and CVP, in
comparison with NS inhalation. MAP was lower in the MI-ISDN group
compared with the MI-NS and sham groups, but the difference was not
statistically significant. The HR in the MI-NS group was faster
than those in the MI-ISDN and sham groups (Table II).
HW/BW%, LV/BW% and RV/BW%
evaluations
The calculated values of HW/BW%, LV/BW% and RV/BW%
indicated that LV and RV hypertrophy occurred in rats following MI
(P<0.05 MI-NS vs. sham) and was improved by ISDN inhalation
(P<0.05 MI-ISDN vs. MI-NS; Fig.
3A).
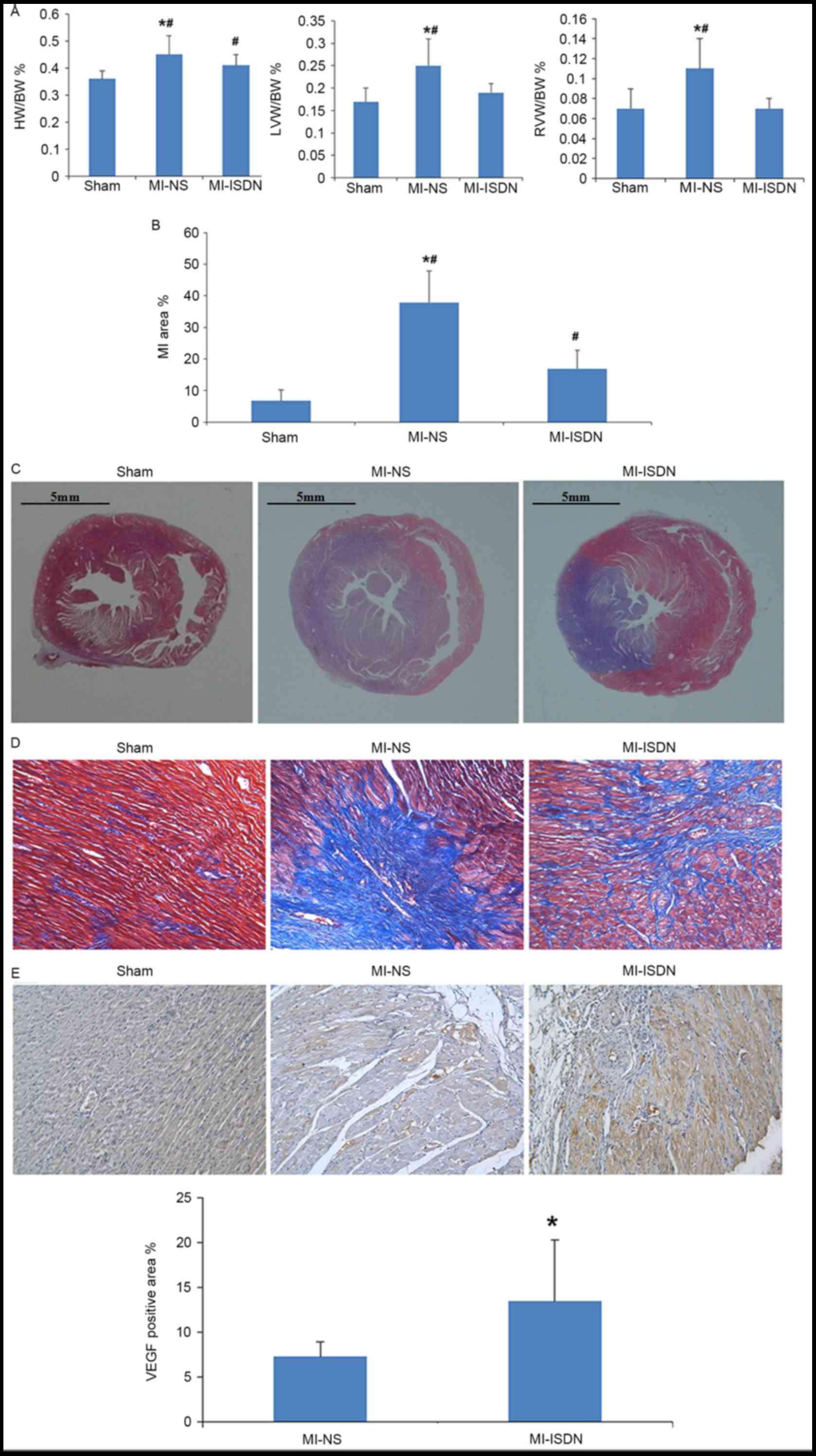 | Figure 3.Parameters of heart damage among the
three groups. (A) HW/BW%, LVW/BW% and RVW/BW% of rats on day 15.
*P<0.05 vs. MI-ISDN; #P<0.05 vs. sham; n=10/group.
(B) MI area % in rats on day 15. *P<0.05 vs. MI-ISDN;
#P<0.05 vs. sham; n=10/group. (C) Myocardium tissues
with Masson staining. The blue area indicates collagen
proliferation following myocardial necrosis. Scale bar, 5 mm. (D)
Myocardium tissues with Masson staining. The blue area indicates
collagen proliferation following myocardial necrosis
(magnification, ×200). (E) VEGF positive area in each group. The
brown area indicates areas of VEGF expression on the border of the
MI region (magnification, ×200). *P<0.05 vs. MI-NS; n=10/group.
HW, heart weight; BW, body weight; LVW, left ventricular weight;
RVW, right ventricular weight; MI, myocardial infarction; ISDN,
isosorbide dinitrate; NS, normal saline; VEGF, vascular endothelial
growth factor. |
MI area % and VEGF-positive area %
estimation
The MI area %, as estimated by Masson staining, was
observed to be decreased by ISDN inhalation in rats following MI,
although not to as low a level as that in the sham group (P<0.05
among the three groups; Fig. 3B-D).
In addition, the VEGF-positive area % at the border of the MI
region was significantly increased (P=0.012) by ISDN inhalation, as
shown in Fig. 3E.
Levels of neurohormonal factors
Plasma levels of epinephrine and norepinephrine, as
shown in Fig. 4A, were reduced by
inhaled ISDN in rats following MI. The differences in
norepinephrine levels among the groups were in accordance with the
differences of HR among the groups.
Plasma levels of BNP were significantly elevated in
the MI-NS group compared with the sham group (786.78±161.10 vs.
644.02±102.10 pg/ml, P=0.015), and ISDN inhalation decreased the
BNP levels in the MI rats (670.65±93.30 vs. 786.78±161.10 pg/ml,
P=0.044). These findings suggest that ISDN inhalation ameliorated
LV remodeling and cardiac dysfunction (Fig. 4B).
Inhaled ISDN, however, only had slight effect on the
plasma concentration of angiotensin II (35.81±3.67 vs. 42.41±12.00
pg/ml in the MI-ISDN and MI-NS groups, respectively, P=0.331;
Fig. 4C).
Discussion
PH due to LHD is associated with poor outcome and
there is a lack of supportive management for excessive
vasoconstriction or remodeling of the pulmonary artery. Available
guidelines do not recommend treatment for the direct reduction of
pulmonary pressure due to LHD, since this kind of intervention
could potentially dilate the pulmonary vessels and induce pulmonary
edema (4). Nevertheless, benefits
from sildenafil, a phosphodiesterase-5 inhibitor have been reported
in such patients (27). These
findings suggest that dilation of the pulmonary artery as well as
systemic vessels with sildenafil may lead to unloading of the left
and right ventricles and decreased pulmonary congestion (6). Therefore, the present study
investigated the benefits of ISDN inhalation on pulmonary pressure
and ventricular remodeling in a rat model of HF following MI.
Results demonstrated that intratracheal ISDN led to significantly
greater ΔMPAP% and ΔMAP% than NS, without pulmonary edema. These
changes were associated with increased plasma NO levels. ISDN
inhalation for 14 days reduced MI size and alleviated LV and RV
remodeling following MI. These hemodynamic and morphological
improvements were associated with decreased plasma levels of
epinephrine, norepinephrine and BNP, and an increased VEGF positive
area at the border of the MI region.
Experimental studies conducting morphometric
analysis ~4 weeks after coronary ligation are not rare; however,
significant hemodynamic and structural changes can be detected only
2 weeks after MI (28). Hence, the
present study assessed hemodynamics ≤15 days after coronary
ligation. The LVW/BW% was elevated in the MI-NS group, which
indicated that LV hypertrophy occurred in rats following MI. In
addition, LV hypertrophy was mainly present in the posterior wall,
remote from the infarcted area, according to echocardiogram
measurements. Thus, LV diastolic dysfunction in rats following MI,
assessed by increased LVEDP and decreased LV-dp/dtmax,
may be a consequence of LV hypertrophy, or LV eccentric remodeling
with LV enlargement. In the present study, an ISDN dose restricted
to 3 mg/kg inhaled for 15 min every day for 14 days was
demonstrated to be effective in improving ventricular parameters.
In addition, echocardiogram and hemodynamic measurements indicated
that ISDN inhalation improved LV and RV remodeling and function.
LV/BW% and LVPWd were similar between the MI-ISDN and sham groups.
Thus, amelioration of LV hypertrophy by ISDN inhalation resulted in
improvements in LV diastolic function, which was reflected by
decreased LVEDP and increased LV-dp/dtmax.
RV dysfunction is known to be a complication of MI
with or without PH in experimental and clinical studies (3,29). In
the present study, RV hypertrophy with systolic and diastolic
dysfunction was detected in rats of the MI-NS group. Increased RVSP
may be a response to the increased afterload of RV due to elevated
LVEDP and potentially increased pulmonary arterial resistance.
Therefore, direct pulmonary pressure reduction with ISDN inhalation
could lead to RV unloading and prove beneficial to RV remodeling.
In addition, improvement of LV function, such as decline of LVEDP,
may be another mechanism of RV remodeling reversion observed in the
present study. The precise mechanism of RV remodeling following LV
MI remains unclear. However, the results of the present study
suggest that ISDN inhalation could be a promising therapy for RV
dysfunction following MI.
Excess pressure or volume load, neurohormonal
activation and progressive myocardial remodeling with LV wall
stress may be detrimental to cardiac cycles and result in
ventricular dilation and cardiac dysfunction following MI (30). Pure volume and pressure unloading,
achieved by implantation of an LV assist device (LVAD), have been
demonstrated to be efficient in reversing ventricular remodeling in
patients with HF (31). This
remodeling improvement has been indicated to be associated with a
normalization of circulating neurohormones, including epinephrine
and norepinephrine (32). In view of
these findings, LV volume and pressure unloading with ISDN
inhalation may directly disrupt detrimental cycles and alleviate
ventricular remodeling. Sympathetic efferent neuronal activity is
increased in patients with HF (33).
Excessive exposure of the myocardium to norepinephrine has been
shown to result in worsening HF with downregulation of α and β
receptors, increased oxygen consumption, and loss of contractile
reserve (34). In addition, it has
been reported that elevated sympathetic activity causes enlargement
of the MI area and exacerbates myocardial remodeling (25). Thus, a reduction in the plasma levels
of epinephrine and norepinephrine by ISDN inhalation in rats
following MI is likely to be beneficial to LV remodeling.
Therefore, LV and RV unloading by ISDN inhalation may establish a
beneficial cycle associated with reduced neurohormonal activation,
finally improving LV and RV remodeling.
Reduction of the area of MI with ISDN inhalation may
provide a great contribution to LV morphological alterations and
systolic function improvements. The present study suggests some
possible mechanisms. First, an increase in the VEGF-positive area
at the border of the MI region following ISDN inhalation may reduce
the MI area. VEGF potentially induces angiogenesis under ischemic
conditions and plays a key role in repair of the myocardium
following MI (35). Moreover, it has
been reported that NO regulates VEGF expression and mediates
VEGF-induced endothelial cell proliferation and migration (36). Therefore, regulation of VEGF
distribution in the ischemic area by ISDN, a NO donor, may promote
myocardium viability following MI. However, it remains to be
elucidated whether the effects on VEGF expression are caused only
by NO released from ISDN or by the NO-dependent actions of ISDN or
its derivatives (37).
Secondly, hemodynamic improvement may itself
minimize MI size. Sun et al (38) observed that the reversion of unstable
hemodynamics supported by LVAD decreased LV volume and wall stress,
relieved LV remodeling, and preserved LV function due to
minimization of the MI size in a swine model of acute MI. Similar
findings were noted in the present study since LV and RV unloading
by ISDN inhalation improved the hemodynamics in the systematic and
pulmonary circulations. This type of hemodynamic support may
directly contribute to a reduction in MI size.
Third, increased plasma NO levels following the
intratracheal instillation of ISDN indicated that the
pharmacological effects of ISDN were not limited to the pulmonary
circulation. In addition, relatively persistent increased NO levels
in the peripheral blood further indicated that the protective
effects of ISDN may go beyond hemodynamic improvements. It may be
assumed that reduction of the MI area is regulated by the
NO-soluble guanylyl cyclase-cyclic guanosine monophosphate axis, as
previously indicated (14). However,
additional studies are necessary to address the mechanisms
properly.
There are some limitations to this study. Firstly,
it was not clear whether high doses of ISDN inhalation lasting for
a longer time could produce greater effects on ventricle remodeling
and cardiac function. In addition, the mechanism by which MI size
is reduced by ISDN inhalation requires further investigation.
In conclusion, intratracheal instillation of ISDN
has been demonstrated to improve the hemodynamics of the pulmonary
and systemic circulation in MI rats without inducing pulmonary
edema. These benefits were associated with increased plasma NO
levels. ISDN instillation/inhalation for 14 days decreased MI area
and alleviated LV and RV remodeling in rats following MI. The
hemodynamic and morphological improvements were associated with
decreased plasma levels of epinephrine, norepinephrine and BNP, and
an increased VEGF positive area at the border of the MI region.
Acknowledgements
The authors would like to thank Mr. Hao Wang and Mr.
Zhonghua Wu for their contribution to the establishment of the rat
MI model and immunohistochemical measurements. This study was
supported by the Discovery Fund from the Chinese Medical Doctor
Association (DFCMDA201309).
References
|
1
|
Kumar A and Cannon CP: Acute coronary
syndromes: Diagnosis and management, part I. Mayo Clin Proc. 84:pp.
917–938. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barnett CF and De Marco T: Pulmonary
hypertension associated with left-sided heart disease. Heart Fail
Clin. 8:447–459. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Toldo S, Bogaard HJ, Van Tassell BW,
Mezzaroma E, Seropian IM, Robati R, Salloum FN, Voelkel NF and
Abbate A: Right ventricular dysfunction following acute myocardial
infarction in the absence of pulmonary hypertension in the mouse.
PLoS One. 6:e181022011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galiè N, Hoeper MM, Humbert M, Torbicki A,
Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS,
et al: Guidelines for the diagnosis and treatment of pulmonary
hypertension: The task force for the diagnosis and treatment of
pulmonary hypertension of the european society of cardiology (ESC)
and the european respiratory society (ERS), endorsed by the
international society of heart and lung transplantation (ISHLT).
Eur Heart J. 30:2493–2537. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gajarsa JJ and Kloner RA: Left ventricular
remodeling in the post-infarction heart: A review of cellular,
molecular mechanisms, and therapeutic modalities. Heart Fail Rev.
16:13–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lundgren J and Rådegran G: Pathophysiology
and potential treatments of pulmonary hypertension due to systolic
left heart failure. Acta Physiol (Oxf). 211:314–333. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vachiéry JL, Adir Y, Barberà JA, Champion
H, Coghlan JG, Cottin V, De Marco T, Galiè N, Ghio S, Gibbs JS, et
al: Pulmonary hypertension due to left heart diseases. J Am Coll
Cardiol. 62 25 Suppl:D100–D108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galie N, Humbert M, Vachiery JL, Gibbs S,
Lang I, Torbicki A, Simonneau G, Peacock A, Noordegraaf A Vonk,
Beghetti M, et al: 2015 ESC/ERS Guidelines for the diagnosis and
treatment of pulmonary hypertension. The Joint Task Force for the
Diagnosis and Treatment of Pulmonary Hypertension of the European
Society of Cardiology (ESC) and the European Respiratory Society
(ERS). Eur Respir J. 46:903–975. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ignarro LJ: Nitric oxide as a unique
signaling molecule in the vascular system: A historical overview. J
Physiol Pharmacol. 53:503–514. 2002.PubMed/NCBI
|
|
10
|
Ignarro LJ, Buga GM, Wood KS, Byrns RE and
Chaudhuri G: Endothelium-derived relaxing factor produced and
released from artery and vein is nitric oxide. Proc Natl Acad Sci
USA. 84:pp. 9265–9269. 1987; View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Napoli C and Ignarro LJ: Nitric oxide and
pathogenic mechanisms involved in the development of vascular
diseases. Arch Pharm Res. 32:1103–1108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blum M, Yachnin T, Wollman Y, Chernihovsky
T, Peer G, Grosskopf I, Kaplan E, Silverberg D, Cabili S and Iaina
A: Low nitric oxide production in patients with chronic renal
failure. Nephron. 79:265–268. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schmidt RJ and Baylis C: Total nitric
oxide production is low in patients with chronic renal disease.
Kidney Int. 58:1261–1266. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Neye N, Enigk F, Shiva S, Habazettl H,
Plesnila N, Kuppe H, Gladwin MT and Kuebler WM: Inhalation of NO
during myocardial ischemia reduces infarct size and improves
cardiac function. Intensive Care Med. 38:1381–1391. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghofrani HA, Galiè N, Grimminger F, Grünig
E, Humbert M, Jing ZC, Keogh AM, Langleben D, Kilama MO, Fritsch A,
et al: Riociguat for the treatment of pulmonary arterial
hypertension. N Engl J Med. 369:330–340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dejam A, Hunter CJ, Tremonti C, Pluta RM,
Hon YY, Grimes G, Partovi K, Pelletier MM, Oldfield EH, Cannon RO
III, et al: Nitrite infusion in humans and nonhuman primates:
Endocrine effects, pharmacokinetics, and tolerance formation.
Circulation. 116:1821–1831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Puikuan K, Chunyu Z, Jin F, Chaoshu T and
Junbao D: Inhalation of nebulized nitroglycerin, a nitric oxide
donor, for the treatment of pulmonary hypertension induced by high
pulmonary blood flow. Heart Vessels. 21:169–179. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xia HP, Huang GY, Zhu JX and Sun B: Effect
of inhalation of nebulized NO donor substance on acute hypoxic lung
injury in newborn piglets. Chin Med J (Engl). 121:1622–1626.
2008.PubMed/NCBI
|
|
19
|
Horinaka S, Kobayashi N, Yagi H, Mori Y
and Matsuoka H: Nicorandil but not ISDN upregulates endothelial
nitric oxide synthase expression, preventing left ventricular
remodeling and degradation of cardiac function in Dahl
salt-sensitive hypertensive rats with congestive heart failure. J
Cardiovasc Pharmacol. 47:629–635. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cohn JN, Tam SW, Anand IS, Taylor AL,
Sabolinski ML and Worcel M: A-HeFT Investigators: Isosorbide
dinitrate and hydralazine in a fixed-dose combination produces
further regression of left ventricular remodeling in a well-treated
black population with heart failure: Results from A-HeFT. J Card
Fail. 13:331–339. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
National Research Council (US), . Guide
for the Care and Use of Laboratory Animals. 8th. National Academies
Press (US); Washington, DC: 2011, PubMed/NCBI
|
|
22
|
Zornoff LA, Paiva SA, Minicucci MF and
Spadaro J: Experimental myocardium infarction in rats: Analysis of
the model. Arq Bras Cardiol. 93:434–440, 426-432. 2009.(In English,
Portuguese, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Horie M, Yoshiura Y, Izumi H, Oyabu T,
Tomonaga T, Okada T, Lee BW, Myojo T, Kubo M, Shimada M and
Morimoto Y: Comparison of the pulmonary oxidative stress caused by
intratracheal instillation and inhalation of NiO nanoparticles when
equivalent amounts of NiO are retained in the lung. Antioxidants
(Basel). 5(pii): E42016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stefanon I, Valero-Muñoz M, Fernandes AA,
Ribeiro RF Jr, Rodríguez C, Miana M, Martínez-González J, Spalenza
JS, Lahera V, Vassallo PF and Cachofeiro V: Left and right
ventricle late remodeling following myocardial infarction in rats.
PLoS One. 8:e649862013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi S, Liang J, Liu T, Yuan X, Ruan B, Sun
L, Tang Y, Yang B, Hu D and Huang C: Depression increases
sympathetic activity and exacerbates myocardial remodeling after
myocardial infarction: Evidence from an animal experiment. PLoS
One. 9:e1017342014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao Q, Sun C, Xu X, Zhou J, Wu Y, Tian Y,
Yuan Z and Liu Z: CD34+ cell mobilization and upregulation of
myocardial cytokines in a rabbit model of myocardial ischemia. Int
J Cardiol. 152:18–23. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reichenbach A, Al-Hiti H, Malek I, Pirk J,
Goncalvesova E, Kautzner J and Melenovsky V: The effects of
phosphodiesterase 5 inhibition on hemodynamics, functional status
and survival in advanced heart failure and pulmonary hypertension:
A case-control study. Int J Cardiol. 168:60–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jasmin JF, Mercier I, Hnasko R, Cheung MW,
Tanowitz HB, Dupuis J and Lisanti MP: Lung remodeling and pulmonary
hypertension after myocardial infarction: Pathogenic role of
reduced caveolin expression. Cardiovasc Res. 63:747–755. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Van Tassell BW, Bhardwaj HL, Grizzard JD,
Kontos MC, Bogaard H, Gomez-Arroyo J, Toldo S, Mezzaroma E, Voelkel
NF and Abbate A: Right ventricular systolic dysfunction in patients
with reperfused ST-segment elevation acute myocardial infarction.
Int J Cardiol. 155:314–316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abd-Elmoniem KZ, Tomas MS, Sasano T,
Soleimanifard S, Vonken EJ, Youssef A, Agarwal H, Dimaano VL,
Calkins H, Stuber M, et al: Assessment of distribution and
evolution of mechanical dyssynchrony in a porcine model of
myocardial infarction by cardiovascular magnetic resonance. J
Cardiovasc Magn Reson. 14:12012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Drakos SG, Kfoury AG, Selzman CH, Verma
DR, Nanas JN, Li DY and Stehlik J: Left ventricular assist device
unloading effects on myocardial structure and function: Current
status of the field and call for action. Curr Opin Cardiol.
26:245–255. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
George RS, Birks EJ, Cheetham A, Webb C,
Smolenski RT, Khaghani A, Yacoub MH and Kelion A: The effect of
long-term left ventricular assist device support on myocardial
sympathetic activity in patients with non-ischaemic dilated
cardiomyopath. Eur J Heart Fail. 15:1035–1043. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Samson R, Baydoun H, Jaiswal A and Le
Jemtel TH: Cardiac adrenergic nervous system and left ventricular
remodeling. Am J Med Sci. 350:321–326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Haider N, Baliga RR, Chandrashekhar Y and
Narula J: Adrenergic excess, hNET1 down-regulation, and compromised
mIBG uptake in heart failure poverty in the presence of plenty.
JACC Cardiovasc Imaging. 3:71–75. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cai M, Ren L, Yin X, Guo Z, Li Y, He T,
Tang Y, Long T, Liu Y, Liu G, et al: PET monitoring angiogenesis of
infarcted myocardium after treatment with vascular endothelial
growth factor and bone marrow mesenchymal stem cells. Amino Acids.
48:811–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang R, Wang L, Zhang L, Chen J, Zhu Z,
Zhang Z and Chopp M: Nitric oxide enhances angiogenesis via the
synthesis of vascular endothelial growth factor and cGMP after
stroke in the rat. Circ Res. 92:308–313. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rammos C, Luedike P, Hendgen-Cotta U and
Rassaf T: Potential of dietary nitrate in angiogenesis. World J
Cardiol. 7:652–657. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun X, Li J, Zhao W, Lu S, Guo C, Lai H
and Wang C: Early assistance with left ventricular assist device
limits left ventricular remodeling after acute myocardial
infarction in a swine model. Artif Organs. 40:243–251. 2016.
View Article : Google Scholar : PubMed/NCBI
|