Introduction
Bone tissue is a dynamic structure that presents
continuous remodeling and metabolism coupled with the action of
osteoblasts and osteoclasts (1). The
functions of osteoblasts and osteoclasts serve an important role in
maintaining the normal skeletal system function (2,3).
Dysfunction of osteoblasts and osteoclasts frequently leads to bone
disease (4). Osteoclasts are
responsible for bone resorption caused by bone microstructure
damage and bone-associated disorders (5). Currently, statistical data have
indicated that osteoporosis is becoming a serious health problem in
the aging population since it results in weakened bone structure
and fractures in the elderly (6,7).
Pathological research has indicated that osteoclast activity and
subtypes are crucial for the treatment of osteoporosis (8). The clinical consequences of
osteoporosis include fractures of the upper extremities, hips and
even spine, resulting in the loss of function and independence of
patients, impairing their quality of life, as well as increasing
morbidity and mortality (9).
Therefore, it is important to further understand the molecular
mechanism of osteoporosis and develop more efficient drugs for the
treatment of this disease. The present study investigated the
molecular mechanism of glucocorticoid-induced osteoporosis in
osteoblasts and osteoclasts in mice.
Adrenomedullin is a 52-amino acid regulatory peptide
that is coded by the gene location on mouse chromosome 7, which has
a ubiquitous distribution and various physiological functions
(10). In recent years,
adrenomedullin has been reported to serve a multifunctional role in
numerous human diseases due to its anti-inflammatory,
anti-apoptotic, anti-oxidative and anti-cancer properties (11). In addition, Hu et al (12) have identified that plasma
concentration levels of calcitonin gene-related peptide and
adrenomedullin are associated with the disease progression in
patients with primary osteoporosis. The plasma levels of
adrenomedullin receptor may affect the function of adrenomedullin
in the progression of osteoporosis. Furthermore, Kim et al
(13) have demonstrated that
adrenomedullin prevented bone loss in a mice model of
postmenopausal osteoporosis. A previous study also showed that
adrenomedullin was able to attenuate interleukin-1β-induced
inflammation and apoptosis in rat Leydig cells through inhibition
of nuclear factor-κB (NF-κB) signaling pathway (12). Notably, receptor activator of NF-κB
ligand (RANKL)-induced osteoclast differentiation through the
inhibition of c-Fos protein proteolysis has been investigated via
inhibition of IkB degradation (13,14).
Antibodies that target tumour necrosis factor (TNF)-α and RANKL may
ameliorate local inflammation and osteoporosis (15). Additionally, cyclooxygenase (COX) 2
induction is associated with osteoporosis by regulation the ratio
of osteoblast and osteoclast (16).
These previous studies have suggested that adrenomedullin shows a
beneficial effect in the treatment of osteoporosis. However, the
molecular mechanism of adrenomedullin for the
glucocorticoid-induced osteoporosis has not been investigated to
date.
In the present study, the therapeutic effects of
adrenomedullin on glucocorticoid-induced osteoporosis were
investigated. The bone mass loss, bone density, bone strength and
osteoporosis disease were examined, and the mRNA expression levels
of osteoporosis-associated factors, including NF of activated
T-cells 1 (NFATc1), tartrate-resistant acid phosphatase (TRAP),
osteoclast-associated immunoglobulin-like receptor (OSCAR) and
c-Fos, in osteocytes from experiment mice were detected (17–19).
Furthermore, the study analyzed the signaling pathway mediated by
adrenomedullin in mice with osteoporosis and researched the
efficacy of adrenomedullin treatment on RANKL-induced osteoclast
differentiation. The observed data suggested that adrenomedullin
inhibits osteoclast differentiation through suppression of
RANKL-induced NF-κB activation in glucocorticoid-induced
osteoporosis.
Materials and methods
Ethics statement
The present study was conducted in strict accordance
with the Care and Use of Laboratory Animals guidelines of the
Chinese PLA General Hospital (Beijing, China). All experimental
protocols were approved by the Ethics Committee of the Chinese PLA
General Hospital. All surgery and euthanasia procedures ensured
minimization of suffering.
Animals
A total of 42 female ICR mice (age, 6–8 weeks old;
weight, 33–38 g) were purchased from Jackson Laboratory (Bar
Harbor, ME, USA). The mice were feed under pathogen-free conditions
and maintained at a controlled environment (temperature, 23±1°C;
humidity, 50–60%) with an artificial simulation of 12 h light/dark
cycles. All mice received intravenous injection of glucocorticoid
(Sigma Aldrich; Merk KGaA; Darmstadt, Germany; 5 mg/kg) once a day
for 15 days in order to induce osteoporosis. The mandibular
cortical width was used as an indicator to determine that the
osteoporosis model was successfully established, as previously
described (20). Because
diphosphonate is an efficient drug for osteoporosis therapy,
diphosphonate was used to as a positive control group (21). ICR mice with osteoporosis were
randomly divided into three groups (n=14 per group) and received
14-day once daily intraperitoneal injection treatment as follows:
Adrenomedullin (0.5 mg/kg), diphosphonate (0.5 mg/kg) or
phosphate-buffered saline (PBS; serving as the untreated
osteoporosis model group, 0.5 mg/kg). The drug treatment procedures
were conducted according to a previous study (22). The therapeutic efficacies of
adrenomedullin were analyzed assessing bone characteristics
according to a previous study (23).
Bone resorption assays
Experimental mice were sacrificed on day 15 and long
bones were obtained. To generate osteoclasts or osteoblasts,
5×104 cells were plated per well in 24-well tissue
culture plates and treated with different doses of M-CSF (5 mg/ml)
or RANKL (5 mg/ml), respectively as described previously (24) Cells cultured in the presence of
10−6M 1,25-dihydroxyvitamin D3 and
10−6 M prostaglandin E2 (Sigma-Aldrich;
Merck, Darmstadt, Germany) for 7 days at 37°C. The total bone
resorption was measured using ImagePro Plus version 4.0 (Media
Cybernetics, Inc., Silver Spring, MD, USA) as described previously
(25).
Analysis of adrenomedullin effects on
osteoclast differentiation
Bone marrow cells (BMCs) were obtained from
adrenomedullin-, diphosphonate- and PBS-treated ICR mice with
osteoporosis on day 15 as described previously (26) and cultured in minimum essential
medium (MEM, Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (FBS, Gibco; Thermo
Fisher Scientific, Inc.) at 37°C. The suspending BMCs were
carefully collected by centrifuging at 300 × g for 10 min at room
temperature and cultured for 72 h in 10 ng/ml macrophage
colony-stimulating factor (M-CSF) at 37°C, followed by addition of
adrenomedullin (10 ng/ml), diphosphonate (10 ng/ml) or the same
volume of PBS. The TRAP activity was then measured using the TRAP
kit (cat. no. 387A-1KT; Sigma-Aldrich; Merck KGaA) in order to
identify the number of osteoclast presenting differentiation.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assays
Total RNA was extracted from osteoblasts and
osteoclasts in mouse femurs using an RNAeasy Mini Kit (Qiagen,
Gaithersburg, MD, USA). Cyclooxygenase (COX) 2, TNF-α, NFATc1,
TRAP, OSCAR, osteoclast differentiation factor (ODF) and
osteoclastogenesis inhibitory factor (OCIF) mRNA expression levels
in osteoblast and osteoclasts were analyzed using RT-qPCR, as
previously described (27). All the
forward and reverse primers were synthesized by Thermo Fisher
Scientific, Inc (28). PCR
amplification followed preliminary denaturation at 94°C for 2 min,
followed by 45 cycles of 95°C for 30 sec, annealing at 57.5°C for
30 sec, and 72°C for 10 min in a total volume of 20 µl containing
50 ng of genomic DNA, 200 µM dNTP, 2.5 units of Taq DNA polymerase,
and 200 µM of each primers. Relative mRNA expression changes were
calculated using the 2−ΔΔCq method (25). The results are expressed as the
n-fold values compared with the β-actin.
Overexpression of c-Fos
Osteoclasts or osteoblasts were cultured in MEM with
5% FBS until 90% confluence and the media was subsequently removed.
The c-Fos gene (GenBank: Y14808.1) was synthesized and cloned into
pCMVp-NEO-X system (Takara Biotechnology Co., Ltd., Dalian, China)
as described previously (29). The
recombinant vector was named pCMVp-NEO-c-Fos. Cells were
transfected by pCMVp-NEO-c-Fos (2 µg) using Lipofectamine 2000
(Sigma-Aldrich; Merck KGaA) according to the manufacturer's
protocol. After 48 h, subsequent experimentations were
performed.
Western blot assay
Osteoblasts from experimental mice with osteoporosis
treated by adrenomedullin, diphosphonate or PBS were homogenized in
lysate buffer containing protease-inhibitor and centrifuged at
8,000 × g at 4°C for 10 min. The supernatant was then used for
analysis of the protein expression. For detection of the target
protein, transmembrane protein were extracted using a Transmembrane
Protein Extraction kit (Qiagen) according to the manufacturer's
instructions. SDS-PAGE (1 µg protein) was performed as previously
described (30). Protein were
transferred to membranes following SDS-PAGE. Subsequent to blocking
in 5% skimmed milk for 1 h at 37°C, primary rabbit anti-mouse
antibodies IκBα (1:500; cat. no. SAB4502716), p65 (1:500, cat. no.
SAB4502609), IKK-β (1:1,000; cat. no. SAB4501536) and β-actin
(1:2,000, cat. no. A3854; Sigma-Aldrich; Merck KGaA) were added and
incubated for 1 h at 37°C. Samples were then incubating with
horseradish peroxidase-conjugated anti-rabbit IgG (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) were used at a 1:5,000
dilution for 24 h at 4°C. The results were visualized using a
chemiluminescence detection system (Bio-Rad Laboratories,
Inc.).
Statistical analysis
All data are represented as the mean ± standard
deviation, and statistical analysis was performed using SPSS
software (version 19.0; IBM Corp., Armonk, NY, USA). All
experiments were conducted at least three times. Unpaired data were
analyzed by Student's t test, while comparisons of data among
multiple groups were analyzed by analysis of variance. A value of
P<0.05 was considered as an indication of a statistically
significant difference.
Results
Adrenomedullin suppresses
RANKL-mediated differentiation of osteoclasts isolated from
experimental mice
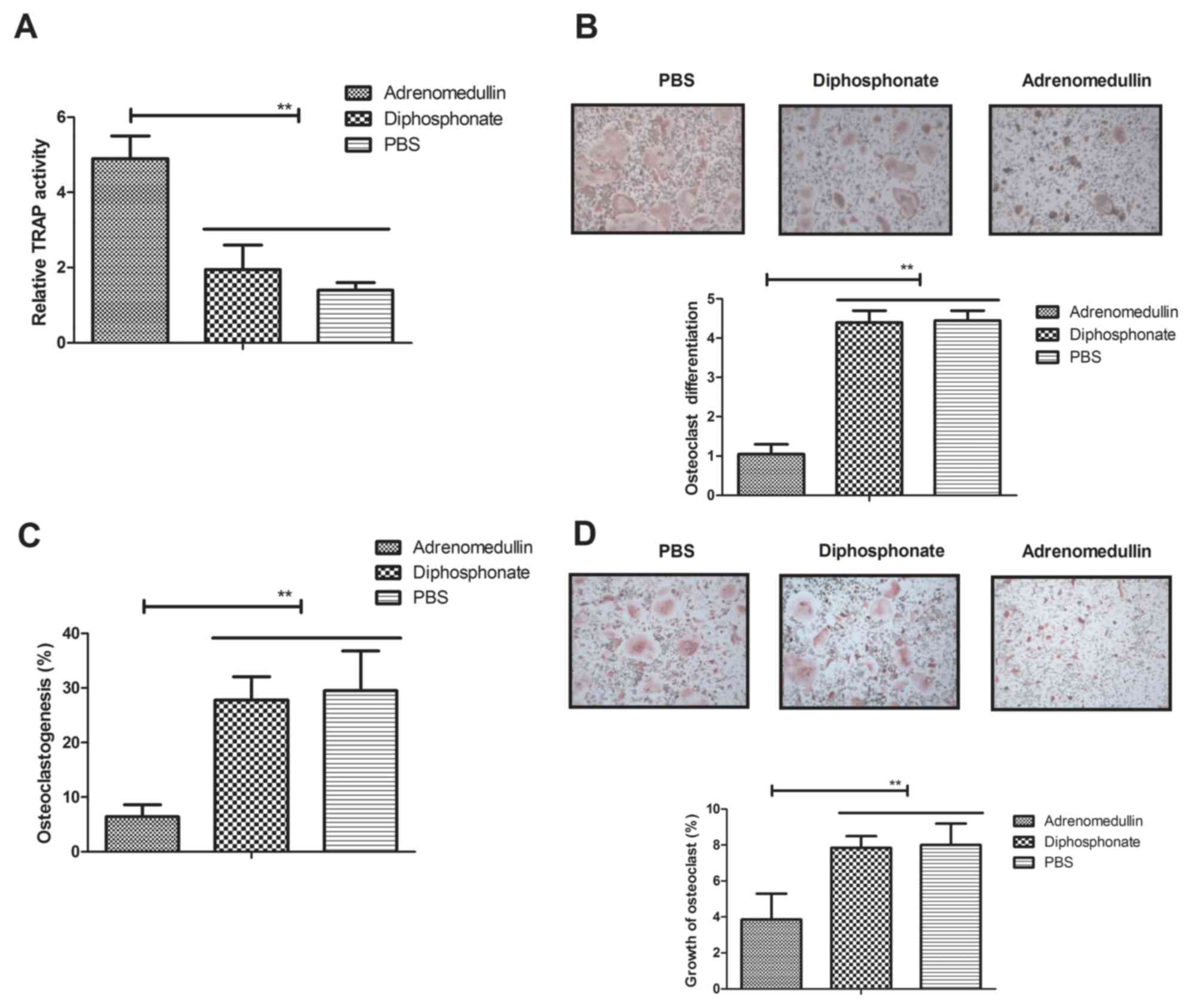
To investigate the efficacy of adrenomedullin
treatment, the osteoclastogenesis was determined in the presence of
RANKL and MCSF. The results indicated that TRAP activity was
suppressed in osteoclasts following treatment with adrenomedullin
(0.5 mg/kg) as compared with the diphosphonate-treated and
PBS-treated control groups (Fig.
1A). It was also observed that adrenomedullin significantly
suppressed the RANKL-mediated differentiation of osteoclasts
isolated from experimental mice (P<0.01; Fig. 1B). In addition, the results shown in
Fig. 1C revealed the inhibitory
effects of adrenomedullin on osteoclastogenesis after 72-h RANKL
stimulation, as compared with the osteoclastogenesis observed in
the diphosphonate and PBS control groups. Morphological analysis
also indicated that adrenomedullin suppressed the growth of
osteoclasts (Fig. 1D). Taken
together, these results suggest that adrenomedullin is able to
inhibit RANKL-mediated osteoclast differentiation and upregulate
TRAP activity in osteoclasts isolated from experimental mice.
Adrenomedullin regulates
RANKL-mediated osteoclastogenesis through inhibition of NF-κB
activation in osteocytes
A previous study has indicated that the NF-κB
signaling pathway is involved in the RANKL-mediated
osteoclastogenesis (31). Therefore,
the current study investigated NF-κB activation in osteocytes from
experimental mice subsequent to treatment with adrenomedullin. The
results demonstrated in Fig. 2A
revealed that adrenomedullin upregulated the NF-κB activation in
osteoblasts and downregulated the NF-κB activation in osteoclasts.
Subsequently, the expression levels of several molecules in the
NF-κB signaling pathway that are involved in osteoclast
differentiation were investigated. As shown in Fig. 2B-D, following treatment with
adrenomedullin, the p65, IKK-β and IκBα protein expression levels
were significantly downregulated in osteoclasts and upregulated in
osteoblasts. In addition, it was observed that the NF-κB target
genes, TNF-α and COX2, were evidently decreased in the osteoblasts,
while no significant effect was detected for osteoclasts (Fig. 2E and F). These results indicate that
adrenomedullin treatment was able to regulate RANKL-mediated
osteoclastogenesis through inhibition of NF-κB signaling pathway in
osteocytes from experimental mice.
Adrenomedullin suppresses osteoclast
differentiation through regulation of c-Fos expression
A previous study revealed that c-Fos is involved in
osteoclast differentiation (32,33).
Therefore, the present study investigated the c-Fos expression
levels in osteoblasts. The expression levels of c-Fos were
significantly inhibited by adrenomedullin in osteoclasts as
compared with the diphosphonate and PBS control groups (Fig. 3A). However, overexpression of c-Fos
activity suppressed the adrenomedullin-mediated NF-κB activity in
osteoclasts (Fig. 3B). Next, the
NFATc1, TRAP and OSCAR expression levels in osteoclasts following
treatment with adrenomedullin were analyzed. The results shown in
Fig. 3C-E revealed that NFATc1, TRAP
and OSCAR expression levels were decreased in osteoclasts following
treatment with adrenomedullin. However, no regulatory effects of
adrenomedullin on osteoblasts were observed in the present study.
Furthermore, the results illustrated in Fig. 3F-G revealed that adrenomedullin
markedly reduced the gene expression levels of ODF and OCIF in
osteoclasts. The aforementioned indicated that adrenomedullin
inhibited osteoclast differentiation through regulation of the
c-Fos-mediated NF-κB signaling pathway in osteoclasts or
osteoblasts.
 | Figure 3.Expression of c-Fos is involved in
adrenomedullin-mediated osteoclast differentiation. (A) Expression
levels of c-Fos in osteoclasts following treatment with
adrenomedullin, diphosphonate or PBS. (B) Inhibition of c-Fos
expression suppresses the adrenomedullin-mediated NF-κB activity.
(C) NFATc1, (D) TRAP and (E) OSCAR mRNA expression levels in
osteoclasts, following treatment with adrenomedullin, diphosphonate
or PBS. Gene expression levels of (F) ODF, and (G) OCIF in
osteoclast after treatment with adrenomedullin, diphosphonate or
PBS. Data are presented as the mean ± standard deviation.
*P<0.05 and **P<0.01 vs. control group. NF-κB, nuclear
factor-κB; NFATc1, NF of activated T-cells 1; TRAP,
tartrate-resistant acid phosphatase; OSCAR, osteoclast-associated
immunoglobulin-like receptor; ODF, osteoclast differentiation
factor; OCIF, osteoclastogenesis inhibitory factor. |
Adrenomedullin prevents the
progression of glucocorticoid-induced osteoporosis in vivo
Subsequent to analysis of the in vitro
effects of adrenomedullin on osteoclast differentiation, the in
vivo efficacy of adrenomedullin for the treatment of
glucocorticoid-induced osteoporosis was further examined. As
expected, adrenomedullin treatment markedly decreased the bone mass
loss of mice with glucocorticoid-induced osteoporosis as determined
by immunostaining assay (Fig. 4A).
The femur bone density of mice with glucocorticoid-induced
osteoporosis was improved following treatment with adrenomedullin
when compared with the controls (Fig.
4B). Furthermore, the maximum load, stiffness, energy to
failure, ultimate strength, elastic modulus, and post-yield
displacement were also increased in mice after treatment with
adrenomedullin (Fig. 4C-H). These
findings demonstrated that adrenomedullin exerted a beneficial
effect against glucocorticoid-induced osteoporosis in mice by
regulation of the bone mineral density and bone strength in
vivo.
Discussion
Previously, RANKL-mediated osteoclastogenesis and
osteoclast differentiation have been demonstrated to serve a
crucial role in the progression and development of osteoporosis
(34,35). In addition, studies have indicated
that adrenomedullin is a multifunctional protein that is associated
with disease progression in patients with primary osteoporosis
(36). Furthermore, the efficacy of
adrenomedullin on osteoclast differentiation has been examined in
previous studies, which suggested that adrenomedullin efficiently
inhibited the differentiation of osteoclasts (37,38). In
the current study, in order to better understand the association
between adrenomedullin treatment and osteoporosis, the molecular
mechanism of adrenomedullin-mediated osteoclastogenesis and
osteoclast differentiation was investigated in mouse osteoclasts
and in glucocorticoid-induced osteoporosis mice. The present study
also analyzed the c-Fos-induced osteoclast differentiation after
treatment with adrenomedullin. The results revealed that
adrenomedullin regulated RANKL-mediated osteoclastogenesis through
inhibition of NF-κB activation in osteocytes in vitro and
in vivo. Notably, the current findings presented that
adrenomedullin treatment not only inhibited the osteoclastogenesis
and osteoclast differentiation, but also improved the bone quality
of mice with glucocorticoid-induced osteoporosis in vivo.
These results suggest that adrenomedullin may be an efficient drug
for the treatment of glucocorticoid-induced osteoporosis.
The functions of osteoclasts include bone resorption
and bone dynamic equilibrium, while they are also involved in
bone-associated disorders (39,40).
Numerous studies have reported the regulatory effects of
osteoclasts in the progression of osteoporosis. It has been
indicated that inhibition of osteoclast differentiation improves
osteoporosis (41,42). In addition, osteoclast activity is
upregulated during osteoporosis, and regulation of osteoclast
activity may be an effective target for clinical treatment of
osteoporosis (43,44). Although several studies have
investigated the efficiency of various drugs for the
differentiation and function of osteoclasts by regulating
differentiation, the actual molecular signaling pathway has seldom
been reported (45–48). The present study investigated the
cellular mechanism underlying the adrenomedullin-mediated
osteoclast differentiation and bone resorption in a mouse model of
osteoporosis. Despite the consistency of the current results with
previous findings reported in the literature (38), the present study also suggested that
adrenomedullin regulated the activities of osteoclasts through
NF-κB activation in osteocytes in vitro and in
vivo.
To date, numerous studies have been conducted
attempting to understand the adrenomedullin-associated therapeutic
regimen via targeting specific molecules and disrupting the
dopaminergic system, leading to various syndrome symptoms of
osteoporosis (49,50). Although previous study demonstrated
the prevention of bone loss in a model of postmenopausal
osteoporosis through adrenomedullin inhibition (37), the mechanism underlying the
adrenomedullin-inhibited osteoclast differentiation has not been
elaborated. In addition, Pleguezuelos et al (51) have demonstrated that the NF-κB
signaling pathway was involved in the adrenomedullin-induced
function in multiple biological processes. Furthermore, the role of
NF-κB on the production and secretion of adrenomedullin has been
investigated in a previous study (52). Lee et al (53) have suggested that NF-κB activation is
associated with the osteoclast differentiation and that
downregulation of RANKL-induced osteoclast differentiation through
inhibition of IkB degradation. In the current study, it was assumed
that adrenomedullin regulated osteoclast activity via the NF-κB
signaling pathway. The present results confirmed this hypothesis
and indicated that adrenomedullin exerted a beneficial effect in
mice with glucocorticoid-induced osteoporosis. These findings
identified that the maximum load, stiffness, energy to failure,
ultimate strength, elastic modulus and post-yield displacement were
improved following treatment with adrenomedullin.
In conclusion, the results of the present study
revealed that adrenomedullin exerts inhibitory effects on
RANKL-induced osteoclastogenesis and differentiation of osteoclasts
via inhibition of the NF-κB signaling pathway, as well as inhibits
c-Fos degradation in osteoblasts. Therefore, it is suggested that
adrenomedullin may be identified as a potential candidate agent for
the treatment of RANKL-induced osteoclast differentiation and
glucocorticoid-induced osteoporosis.
References
|
1
|
An G, Acharya C, Feng X, Wen K, Zhong M,
Zhang L, Munshi NC, Qiu L, Tai YT and Anderson KC: Osteoclasts
promote immune suppressive microenvironment in multiple myeloma:
Therapeutic implication. Blood. 128:1590–1603. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim TH, Park EK, Huh MI, Kim HK, Kim SY
and Lee SH: Rhus javanica gall extract inhibits the differentiation
of bone marrow-derived osteoclasts and ovariectomy-induced bone
loss. Evid Based Complement Alternat Med. 2016:32847042016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jeon OH, Panicker LM, Lu Q, Chae JJ,
Feldman RA and Elisseeff JH: Human iPSC-derived osteoblasts and
osteoclasts together promote bone regeneration in 3D biomaterials.
Sci Rep. 6:267612016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paglia DN, Yang X, Kalinowski J,
Jastrzebski S, Drissi H and Lorenzo J: Runx1 regulates myeloid
precursor differentiation into osteoclasts without affecting
differentiation into antigen presenting or phagocytic cells in both
males and females. Endocrinology. 157:3058–3069. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Laperine O, Blin-Wakkach C, Guicheux J,
Beck-Cormier S and Lesclous P: Dendritic-cell-derived osteoclasts:
A new game changer in bone-resorption-associated diseases. Drug
Discov Today. 21:1345–1354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scott LJ: Denosumab: A review of its use
in postmenopausal women with osteoporosis. Drugs Aging. 31:555–576.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu S, Liu F, Cheng Z and Wang Q:
Association between osteoporosis and benign paroxysmal positional
vertigo: A systematic review. BMC Neurol. 14:1102014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Henriksen K, Bollerslev J, Everts V and
Karsdal MA: Osteoclast activity and subtypes as a function of
physiology and pathology-implications for future treatments of
osteoporosis. Endocr Rev. 32:31–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ghosh M and Majumdar SR: Antihypertensive
medications, bone mineral density and fractures: A review of old
cardiac drugs that provides new insights into osteoporosis.
Endocrine. 46:397–405. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iesato Y, Yuda K, Chong KT, Tan X, Murata
T, Shindo T and Yanagi Y: Adrenomedullin: A potential therapeutic
target for retinochoroidal disease. Prog Retin Eye Res. 52:112–129.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
DE LA, Torre-Prados MV, Garcia-DE LA,
Torre A, Enguix A, Mayor-Reyes M, Nieto-González M and
Garcia-Alcantara A: Mid-regional pro-adrenomedullin as prognostic
biomarker in septic shock. Minerva Anestesiol. 82:760–766.
2016.PubMed/NCBI
|
|
12
|
Hu W, Zhou PH, Rao T, Zhang XB, Wang W and
Zhang LJ: Adrenomedullin attenuates interleukin-1β-induced
inflammation and apoptosis in rat Leydig cells via inhibition of
NF-κB signaling pathway. Exp Cell Res. 339:220–230. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim JH, Kim K, Kim I, Seong S, Nam KI, Lee
SH, Kim KK and Kim N: Role of CrkII signaling in RANKL-induced
osteoclast differentiation and function. J Immunol. 196:1123–1131.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ito S, Ohmi A, Sakamiya A, Yano T, Okumura
K, Nishimura N and Kagontani K: Ginger hexane extract suppresses
RANKL-induced osteoclast differentiation. Biosci Biotechnol
Biochem. 80:779–785. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qian H, Yuan H, Wang J, Du Y, Zhang X, Sun
Y, Li Z and Zhao W: A monoclonal antibody ameliorates local
inflammation and osteoporosis by targeting TNF-α and RANKL. Int
Immunopharmacol. 20:370–376. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lakhan R, Baylink DJ, Lau KH, Tang X,
Sheng MH, Rundle CH and Qin X: Local administration of AAV-DJ
pseudoserotype expressing COX2 provided early onset of transgene
expression and promoted bone fracture healing in mice. Gene Ther.
22:721–728. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arrabal-Polo MA, Girón-Prieto MS,
Cano-García Mdel C, Poyatos-Andujar A, Quesada-Charneco M,
Abad-Menor F, Arias-Santiago S, Zuluaga-Gomez A and Arrabal-Martin
M: Retrospective review of serum and urinary lithogenic risk
factors in patients with osteoporosis and osteopenia. Urology.
85:782–785. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chiu CK, Kuo MC, Yu SF, Su BY and Cheng
TT: Adherence to osteoporosis regimens among men and analysis of
risk factors of poor compliance: A 2-year analytical review. BMC
Musculoskelet Disord. 14:2762013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Srikanth R, Cassidy G, Joiner C and
Teeluckdharry S: Osteoporosis in people with intellectual
disabilities: A review and a brief study of risk factors for
osteoporosis in a community sample of people with intellectual
disabilities. J Intellect Disabil Res. 55:53–62. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Papamanthos M, Varitimidis S, Dailiana Zh,
Kogia E and Malizos K: Computer-assisted evaluation of Mandibular
Cortical Width (MCW) index as an indicator of osteoporosis.
Hippokratia. 18:251–257. 2014.PubMed/NCBI
|
|
21
|
Schwarz P: Diphosphonate treatment of
osteoporosis and risk of atypical femoral fractures. Ugeskr Laeger.
174:302012.(In Danish). PubMed/NCBI
|
|
22
|
Kushwaha P, Khedgikar V, Ahmad N, Karvande
A, Gautam J, Kumar P, Maurya R and Trivedi R: A neoflavonoid
dalsissooal isolated from heartwood of Dalbergia sissoo Roxb. Has
bone forming effects in mice model for osteoporosis. Eur J
Pharmacol. 788:65–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shum LC, White NS, Nadtochiy SM, Bentley
KL, Brookes PS, Jonason JH and Eliseev RA: Cyclophilin D Knock-Out
mice show enhanced resistance to osteoporosis and to metabolic
changes observed in aging bone. PLoS One. 11:e01557092016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Penolazzi L, Lolli A, Sardelli L,
Angelozzi M, Lambertini E, Trombelli L, Ciarpella F, Vecchiatini R
and Piva R: Establishment of a 3D-dynamic osteoblasts-osteoclasts
co-culture model to simulate the jawbone microenvironment in vitro.
Life Sci. 152:82–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baltacioglu E, Kehribar MA, Yuva P, Alver
A, Atagün OS, Karabulut E and Akalın FA: Total oxidant status and
bone resorption biomarkers in serum and gingival crevicular fluid
of patients with periodontitis. J Periodontol. 85:317–326. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ishihara A, Helbig HJ, Sanchez-Hodge RB,
Wellman ML, Landrigan MD and Bertone AL: Performance of a
gravitational marrow separator, multidirectional bone marrow
aspiration needle, and repeated bone marrow collections on the
production of concentrated bone marrow and separation of
mesenchymal stem cells in horses. Am J Vet Res 74: 854–863, 2013.
Am J Vet Res. 74:854–863. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiao S, Wang J and Xiao N: MicroRNAs as
noninvasive biomarkers in bladder cancer detection: A diagnostic
meta-analysis based on qRT-PCR data. Int J Biol Markers.
31:e276–e285. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lisi S, Sisto M, Lofrumento DD and D'Amore
M: Sjögren's syndrome autoantibodies provoke changes in gene
expression profiles of inflammatory cytokines triggering a pathway
involving TACE/NF-κB. Lab Invest. 92:615–624. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shiraishi T, Fukuda K, Morotomi N, Imamura
Y, Mishima J, Imai S, Miyazawa K and Taniguchi H: Influence of
menstruation on the microbiota of healthy women's labia minora as
analyzed using a 16S rRNA gene-based clone library method. Jpn J
Infect Dis. 64:76–80. 2011.PubMed/NCBI
|
|
30
|
Wai-Hoe L, Wing-Seng L, Ismail Z and
Lay-Harn G: SDS-PAGE-based quantitative assay for screening of
kidney stone disease. Biol Proced Online. 11:145–160. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pengjam Y, Madhyastha H, Madhyastha R,
Yamaguchi Y, Nakajima Y and Maruyama M: NF-κB pathway inhibition by
anthrocyclic glycoside aloin is key event in preventing
osteoclastogenesis in RAW264.7 cells. Phytomedicine. 23:417–428.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kwak HB, Lee BK, Oh J, Yeon JT, Choi SW,
Cho HJ, Lee MS, Kim JJ, Bae JM, Kim SH and Kim HS: Inhibition of
osteoclast differentiation and bone resorption by rotenone, through
down-regulation of RANKL-induced c-Fos and NFATc1 expression. Bone.
46:724–731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee CH, Kwak SC, Kim JY, Oh HM, Rho MC,
Yoon KH, Yoo WH, Lee MS and Oh J: Genipin inhibits RANKL-induced
osteoclast differentiation through proteasome-mediated degradation
of c-Fos protein and suppression of NF-κB activation. J Pharmacol
Sci. 124:344–353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee EG, Sung MS, Yoo HG, Chae HJ, Kim HR
and Yoo WH: Increased RANKL-mediated osteoclastogenesis by
interleukin-1β and endoplasmic reticulum stress. Joint Bone Spine.
81:520–526. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee SY, Lee KS, Yi SH, Kook SH and Lee JC:
Acteoside suppresses RANKL-mediated osteoclastogenesis by
inhibiting c-Fos induction and NF-κB pathway and attenuating ROS
production. PLoS One. 8:e808732013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yun HJ, Lee EG, Lee SI, Chae HJ and Yoo
WH: Adrenomedullin inhibits MAPK pathway-dependent rheumatoid
synovial fibroblast-mediated osteoclastogenesis by IL-1 and
TNF-alpha. Rheumatol Int. 29:1161–1168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Martinez-Herrero S, Larrayoz IM,
Ochoa-Callejero L, Fernández LJ, Allueva A, Ochoa I and Martínez A:
Prevention of bone loss in a model of postmenopausal osteoporosis
through adrenomedullin inhibition. Front Physiol. 7:2802016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lin J, Lu C and Gao L: Study on the level
of plasma calcitonin gene-related peptide and adrenomedullin in
subjects with primary osteoporosis. Zhonghua Yi Xue Za Zhi.
81:841–843. 2001.(In Chinese). PubMed/NCBI
|
|
39
|
Prause M, Seeliger C, Unger M, Balmayor
Rosado E, van Griensven M and Haug AT: Pantoprazole decreases cell
viability and function of human osteoclasts in vitro. Mediators
Inflamm. 2015:4130972015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Touaitahuata H, Cres G, de Rossi S, Vives
V and Blangy A: The mineral dissolution function of osteoclasts is
dispensable for hypertrophic cartilage degradation during long bone
development and growth. Dev Biol. 393:57–70. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu C, Zhang J and Tian J: Research
progress of osteoclasts function beyond bone resorption. Zhongguo
Xiu Fu Chong Jian Wai Ke Za Zhi. 29:1038–1042. 2015.(In Chinese).
PubMed/NCBI
|
|
42
|
Yang C, Madhu V, Thomas C, Yang X, Du X,
Dighe AS and Cui Q: Inhibition of differentiation and function of
osteoclasts by dimethyl sulfoxide (DMSO). Cell Tissue Res.
362:577–585. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Takami M: Differentiation and function of
osteoclasts. Nihon Rinsho. 69:1170–1173. 2011.(In Japanese).
PubMed/NCBI
|
|
44
|
Reyes JP, Sims SM and Dixon SJ: P2
receptor expression, signaling and function in osteoclasts. Front
Biosci (Schol Ed). 3:1101–1118. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
45
|
Agrawal A, Buckley KA, Bowers K, Furber M,
Gallagher JA and Gartland A: The effects of P2X7 receptor
antagonists on the formation and function of human osteoclasts in
vitro. Purinergic Signal. 6:307–315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li H, Hong S, Qian J, Zheng Y, Yang J and
Yi Q: Cross talk between the bone and immune systems: Osteoclasts
function as antigen-presenting cells and activate CD4+ and CD8+ T
cells. Blood. 116:210–217. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Imai Y, Youn MY, Kondoh S, Nakamura T,
Kouzmenko A, Matsumoto T, Takada I, Takaoka K and Kato S: Estrogens
maintain bone mass by regulating expression of genes controlling
function and life span in mature osteoclasts. Ann N Y Acad Sci.
1173 Suppl 1:E31–E39. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Witten PE and Huysseune A: A comparative
view on mechanisms and functions of skeletal remodelling in teleost
fish, with special emphasis on osteoclasts and their function. Biol
Rev Camb Philos Soc. 84:315–346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dai X, Ma W, Jha RK and He X:
Adrenomedullin and its expression in cancers and bone. A literature
review. Front Biosci (Elite Ed). 2:1073–1080. 2010.PubMed/NCBI
|
|
50
|
Iwase T, Nagaya N, Fujii T, Itoh T,
Ishibashi-Ueda H, Yamagishi M, Miyatake K, Matsumoto T, Kitamura S
and Kangawa K: Adrenomedullin enhances angiogenic potency of bone
marrow transplantation in a rat model of hindlimb ischemia.
Circulation. 111:356–362. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pleguezuelos O, Hagi-Pavli E, Crowther G
and Kapas S: Adrenomedullin signals through NF-kappaB in epithelial
cells. FEBS Lett. 577:249–254. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang J, Wang W, Dong M, Yu X and Luo Q:
Effect of nucleoprotein factor-kB (NF-κB) in endothelial cells
during high blood flow-associated pulmonary vascular remodeling on
vasoactive substances adrenomedullin and prostacyclin. Int J Clin
Exp Med. 8:13842–13847. 2015.PubMed/NCBI
|
|
53
|
Lee CH, Jeon YT, Kim SH and Song YS:
NF-kappaB as a potential molecular target for cancer therapy.
Biofactors. 29:19–35. 2007. View Article : Google Scholar : PubMed/NCBI
|