Introduction
Periodontitis is a common infectious and
inflammatory disease characterized by irreversible destruction of
tooth supporting tissues, including alveolar bone, periodontal
ligament and cementum (1).
Periodontitis is the predominant cause of tooth loss in adults and
has been linked to many systemic diseases, such as diabetes,
significantly impairing patients' quality of life and escalating
the healthcare burden worldwide (2,3). The
ultimate aim of periodontal treatment is to regenerate the
defective tissues and restore the function of the periodontium.
However, periodontal regeneration has been an elusive endeavor.
Current therapeutic approaches, including bone grafting, guided
tissue regeneration and use of biological factors, have had limited
success in achieving this therapeutic aim (4,5).
With the development of stem cell biology and tissue
engineering, recent insights have been focused on cell-based
strategies that have a favorable effect on periodontal regeneration
(6). The widely studied mesenchymal
stem cells (MSCs) hold great promise for tissue regeneration, owing
to their multilineage differentiation ability. MSCs from different
tissue sources, such as bone marrow mesenchymal stem cells (BMSCs),
adipose-derived stem cells and periodontal ligament stem cells,
have demonstrated the capacity to promote periodontal regeneration
to various degrees in animal studies (7–9).
Nevertheless, the utility of MSCs has been partially restricted by
limited accessibility, insufficient quantity and aging (5,10). Thus,
it is imperative to identify alternative sources of MSCs for
effective periodontal regeneration. The generation of induced
pluripotent stem cells (iPSCs) by reprogramming somatic cells has
provided a practical approach for acquisition of patient-specific
stem cells. Currently, iPSCs may be efficiently generated from
various types of easily accessible tissues. Of note, iPSCs have
been successfully differentiated into MSC-like cells, which display
comparable surface phenotype and differentiation capability to the
traditional MSCs (11). Furthermore,
iPSC-derived MSCs (iPSC-MSCs) exhibit increased proliferation
capacity, avoiding the senescence-related issues in the application
of adult MSCs (12,13). Therefore, iPSC-MSCs offer a promising
cell source for regenerative therapy, including periodontal
regeneration (14).
To enhance the in vivo efficacy of stem
cells, the concomitant use of suitable cell carriers and biological
factors is essential for creating a favorable environment to
support cell attachment, proliferation and differentiation
(4). Hyaluronic acid (HA) is a
linear, non-sulfated glycosaminoglycan and a primary component of
the extracellular matrix. HA hydrogels have been widely engineered
for biomedical use due to their biocompatibility and their ability
to incorporate and release drugs (15). In addition, HA hydrogels provide a
three-dimensional scaffold that allows spatial distribution of stem
cells, mimics the native microenvironment and maintains space for
mechanical stability (16).
Growth/differentiation factor-5 (GDF-5) is a member of the bone
morphogenetic protein family and the transforming growth factor-β
superfamily. GDF-5 has been recognized as a key regulator for MSC
differentiation and development of bone, cartilage and
tendon/ligament (17). Notably,
GDF-5 expression is associated with periodontal tissue formation
and insertion of periodontal ligament fibers in alveolar bone and
cementum during tooth root development, suggesting a regulatory
role in the establishment of the periodontium (18). Emerging preclinical and clinical
evidence demonstrates that GDF-5 may serve as a promising
therapeutic agent for periodontal wound healing/regeneration
(17,19). A recent study has identified that
GDF-5 significantly enhances periodontal specific differentiation
of iPSCs (20). However, it remains
unclear how and to what extent GDF-5 mediates the cellular
differentiation and function of iPSC-MSCs in the scenario of
periodontal regeneration.
The present study investigated the effects of
recombinant human GDF-5 (rhGDF-5) on periodontal specific
differentiation of iPSC-MSCs and BMSCs in vitro, and
characterized a HA-based delivery system of iPSC-MSCs/rhGDF-5 in
vivo to offer a potential approach for periodontal
regeneration.
Materials and methods
Ethics statement
Approval for the animal experiments in the present
study was granted by the Biomedical Ethics Committee of Peking
University (Beijing, China).
Human iPSC culture and derivation of
iPSC-MSCs
Human iPSCs derived from peripheral blood
mononuclear cells were obtained from Frankel Cardiovascular Center,
The University of Michigan (Ann Arbor, MI, USA) (21). The iPSCs were cultured on
Matrigel-coated 60-mm dishes in iPSCs culture medium containing
basal DMEM/F-12, 20% knockout serum replacement, 1 mM GlutaMAX-I
supplement, 4 ng/ml basic fibroblast growth factor (bFGF), 1%
nonessential amino acids (all from Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and 0.1 mM β-mercaptoethanol
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The
differentiation of human iPSCs toward MSC-like cells was performed
as described previously (22). In
brief, the cell colonies were manually dissected into small clumps
and incubated at 37°C in iPSCs culture medium without bFGF
supplement to generate floating embryoid bodies (EBs). The medium
was changed every other day. After 10 days of culture, ~10 EBs were
inoculated into 6-well plates coated with 0.1% gelatin and cultured
at 37°C in MSC medium comprising basal α-minimum essential medium
(Gibco; Thermo Fisher Scientific, Inc.), 20% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.), 1 mM glutaMAX-I
supplement, 1 ng/ml bFGF, 1% nonessential amino acids and 1%
penicillin-streptomycin. Cells migrated out from the EBs gradually
and MSC-like cells emerged. After 2 weeks of culture, the cells
were digested by TrypLE Express (Thermo Fisher Scientific, Inc.)
and cultured on 0.1% gelatin-coated 25-cm2 flasks to
establish passage 1 culture. The cells were then serially passaged
with trypsin up to passage 4.
Induced differentiation of iPSC-MSCs
in vitro
Human BMSCs were obtained from the School of
Dentistry, The University of Michigan (Ann Arbor, MI, USA). The
iPSC-MSCs and human BMSCs (5×106 cells) at passage 4
were cultured at 37°C in MSC medium with or without 200 ng/ml
rhGDF-5 (Pepro Tech, Inc., Rocky Hill, NJ, USA) and the media were
replenished every other day. The concentration of rhGDF-5 was
selected based on a previous study (20). After 2 weeks of incubation,
immunofluorescence staining was performed to analyze the protein
expression of osteocalcin (OCN), periostin and cementum attachment
protein (CAP). Cells were rinsed with phosphate-buffered saline
(PBS) and fixed by incubation with 4% paraformaldehyde for 30 min
at room temperature, followed by three 5-min washes with PBS.
Subsequently, the samples were blocked with blocking buffer (3%
bovine serum albumin) (Sigma-Aldrich; Merck KGaA) for 1 h at 37°C.
The cells were then incubated with primary antibodies against OCN
(ab13418), periostin (ab14041) (1:100; both from Abcam, Cambridge,
UK) and CAP (sc-53947; 1:100; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) overnight at room temperature. Following this, the
samples were incubated with Alexa Fluor 488 (A32723) or Alexa Fluor
594 (R37117)-conjugated secondary antibodies (1:100; Invitrogen;
Thermo Fisher Scientific, Inc.) for 1 h at 37°C. Nuclei were
counterstained using DAPI Fluoromount-G (SouthernBiotech,
Birmingham, AL, USA). Images were captured using a confocal laser
scanning microscope (CLSM; Nikon Eclipse C1 Plus Confocal
Workstation; Nikon Corporation., Tokyo, Japan).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Gene expression levels of OCN, periostin and CAP in
the iPSC-MSCs and BMSCs treated with rhGDF-5 were evaluated by
RT-qPCR. Total RNA was isolated from iPSC-MSCs and BMSCs using an
RNeasy mini kit (Qiagen, Inc., Valencia, CA, USA) and treated with
DNase (Qiagen, Inc.) according to the manufacturer's protocols.
Then, cDNA was synthesized with TaqMan reverse transcription
reagents (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Following this, RT-qPCR was performed on a StepOnePlus Real-Time
PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.)
using a TaqMan Universal PCR master mix (Applied Biosystems; Thermo
Fisher Scientific, Inc.) containing AmpliTaq Gold DNA Polymerase.
The TaqMan primers and probes specific for OCN (Hs01587813_g1),
periostin (Hs01566734_m1), CAP (Hs00171965_m1) and GAPDH
(Hs99999905_m1) (all from Applied Biosystems; Thermo Fisher
Scientific, Inc.) were adopted. The reactions were performed in
triplicate with a final volume of 30 µl containing 15 µl of TaqMan
2X Universal PCR Master mix, 2 µl of cDNA and 1.5 µl of TaqMan
primers and probes. The thermal cycling conditions were as follows:
50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for
15 sec and 60°C for 1 min. Relative gene expression levels were
normalized against GAPDH and analyzed with the 2−ΔΔCq
method (23).
Alizarin Red S staining
After 4 weeks of culture in the presence of rhGDF-5,
the iPSC-MSCs and BMSCs were rinsed with PBS and fixed in 10%
formalin for 30 min at room temperature. Cells were then washed
with PBS three times, each for 5 min, and stained with 40 mM
Alizarin Red S (pH 4.2) for 20 min at room temperature. Excess dye
was removed by washing the cells three times with PBS. The
deposited mineralized matrix was stained in red by Alizarin Red
S.
Hydrogel preparation and cell
encapsulation
The commercially available HyStem-C hydrogels (ESI
BIO, Alameda, CA, USA) were prepared according to the
manufacturer's instructions. Briefly, degassed, deionized water was
used to dissolve Glycosil (thiol-modified hyaluronan), Gelin-S
(thiol-modified collagen) and Extralink [thiol-reactive
Polyethylene (glycol) Diacrylate crosslinker] in individual vials.
The iPSC-MSCs were labeled with PKH67 Green Fluorescent Cell Linker
(Sigma-Aldrich; Merck KGaA). Subsequently, equal volumes of
Glycosil and Gelin-S were mixed prior to addition of the labeled
cells (5×105 ells/ml) and 200 ng/ml rhGDF-5. To form the
hydrogel, Extralink was added to the mixture in a 1:4 volume
ratio.
Surgical procedure
Three intervention groups were created: i) iPSC-MSCs
+ rhGDF-5 + hydrogel; ii) iPSC-MSCs + hydrogel; iii) rhGDF-5 +
hydrogel. The hydrogel constructs (n=4 for each group) were
subcutaneously implanted into the dorsal surface of 6–8-week old
male athymic nude mice (weight, 18–22 g; Charles River
Laboratories, Wilmington, MA, USA) under general inhalation
anesthesia using 2% isoflurane. Each mouse received 2 implants at
random. The mice were maintained under standard conditions (12-h
light/dark cycle, 22°C, 60% humidity) with free access to chow and
water. After 6 weeks, the mice were sacrificed and the implants
were harvested for further analysis.
Immunohistochemical and
immunofluorescence staining
For immunohistochemical staining, the Cell and
Tissue Staining kit [horseradish peroxidase-amino ethylcarbazole
(HRP-AEC) System; R&D Systems, Inc., Minneapolis, MN, USA] was
used, according to the manufacturer's instructions. The harvested
specimens were incubated in 10% formalin overnight at room
temperature and embedded in paraffin. Sections (5 µm thickness)
were deparaffinized, rehydrated and then immersed in 3%
H2O2 for 10 min to quench the endogenous
peroxidase activity. After blocking at room temperature, sections
were incubated with the aforementioned primary antibodies against
OCN, periostin and CAP overnight at room temperature followed by
incubation with biotinylated secondary antibodies for 1 h at room
temperature. Staining was visualized by HRP-AEC reaction under an
Olympus IX71 microscope (Olympus Corp., Tokyo, Japan).
For immunofluorescence staining, the harvested
specimens were fixed with 4% paraformaldehyde for 15 min at room
temperature and cryosectioned at a thickness of 5 mm. After
blocking with 5% bovine serum albumin for 30 min at 37°C, sections
were incubated with the aforementioned primary antibodies against
OCN, periostin and CAP at 4°C overnight followed by incubation with
Alexa Fluor 594-conjugated secondary antibody (1:100) for 1 h at
37°C. Nuclei were counterstained using DAPI Fluoromount-G. Images
were captured using a CLSM. The percentage of OCN-, periostin- and
CAP-positive cells in fluorescence-labeled donor cell populations
were calculated for four implants.
Statistical analysis
At least three samples were used for each
quantitative experiment. All quantitative data were expressed as
the mean ± standard deviation. Statistical analysis was performed
using SPSS 20.0 software (IBM Corp., Armonk, NY, USA). Student's
t-test was applied to analyze differences between the groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of rhGDF-5 on periodontal
specific differentiation of iPSC-MSCs in vitro
To investigate the differentiation capacity of the
iPSC-MSCs and BMSCs in response to rhGDF-5, the expression of
marker genes associated with periodontal tissue formation,
including OCN, periostin and CAP, were examined by RT-qPCR and
immunofluorescence staining. The iPSC-MSCs presented a
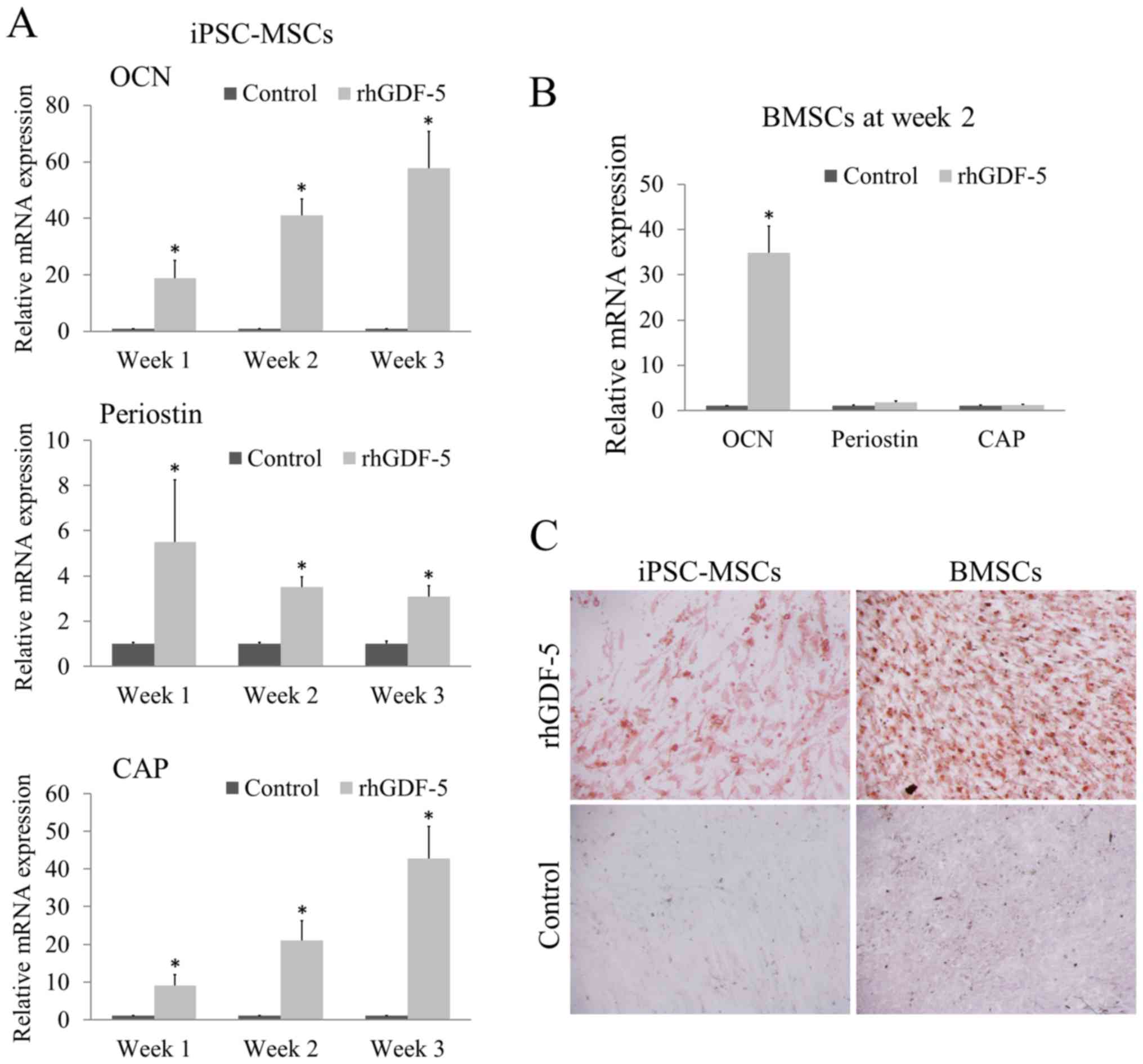
fibroblastic-like morphology. The rhGDF-5 treatment (200 ng/ml)
significantly enhanced the mRNA expression levels of OCN, periostin
and CAP in the iPSC-MSCs at weeks 1, 2 and 3 (P<0.05; Fig. 1A) compared with the corresponding
untreated control groups. As the incubation continued from week 1
to 3, the inductive effect of rhGDF-5 on OCN and CAP expression
gradually increased. With reference to the marked overexpression of
OCN and CAP, the enhancement of periostin expression was relatively
weak (Fig. 1A). For BMSCs, the cells
were cultured with rhGDF-5 for 2 weeks. BMSCs treated with rhGDF-5
exhibited significantly higher mRNA expression levels of OCN
(P<0.05), comparable to that in the iPSC-MSCs, compared with the
untreated control; however, no significant difference was observed
between the periostin and CAP expression levels of the control and
treatment groups (Fig. 1B).
The formation of mineralized matrix deposits was
assessed by Alizarin Red S staining. After 4 weeks of culture in
the presence of rhGDF-5, both the iPSC-MSCs and BMSCs demonstrated
marked formation of mineralized nodules compared with the control
groups. No deposits were observed in the untreated controls
(Fig. 1C).
Immunofluorescence analysis, after 2 weeks, revealed
intense expression of OCN, periostin and CAP in the rhGDF-5-treated
iPSC-MSCs (Fig. 2A-C). The BMSCs
treated with rhGDF-5 for 2 weeks displayed strong OCN staining and
weak periostin staining, whereas CAP expression was not detected
(Fig. 2D-F). The staining of the
markers was negative in the untreated controls.
Effects of rhGDF-5 on periodontal
specific differentiation of iPSC-MSCs encapsulated in HA hydrogels
in vivo
The capability of rhGDF-5 to support the periodontal
specific differentiation of iPSC-MSCs was further evaluated in
vivo. The cells and rhGDF-5 were embedded in HA hydrogels for
subcutaneous transplantation in nude mice. After 6 weeks, the
implants were retrieved and no adverse local responses, such as
inflammation, were observed. Immunohistochemistry results
demonstrated strong positive staining for OCN, periostin and CAP in
the newly formed tissues by the composite of iPSC-MSCs, rhGDF-5 and
HA hydrogels (Fig. 3A-C). However,
very low expression was detected in the hydrogels incorporating
iPSC-MSCs (Fig. 3D-F) or rhGDF-5
alone (Fig. 3G-I).
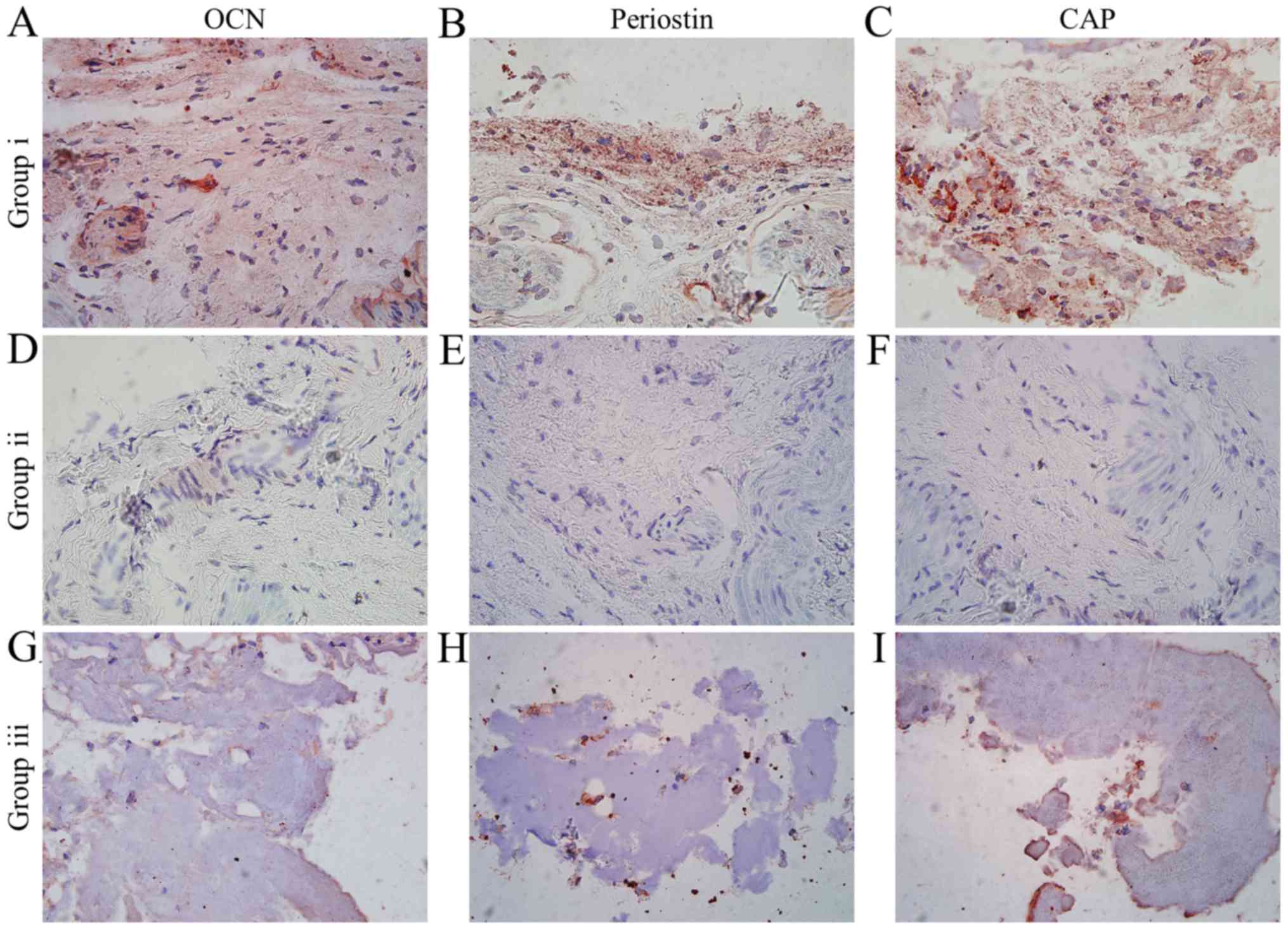 | Figure 3.Immunohistochemical analysis
(magnification, ×40) of engineered hyaluronic acid hydrogels 6
weeks after subcutaneous transplantation in the three intervention
groups. Staining of (A) OCN, (B) periostin and (C) CAP in Group i.
Staining of (D) OCN, (E) periostin and (F) CAP in Group ii.
Staining of (G) OCN, (H) periostin and (I) CAP in Group iii. Group
i, iPSC-MSCs + rhGDF-5 + hydrogel; Group ii, iPSC-MSCs + hydrogel;
Group iii, rhGDF-5 + hydrogel; rhGDF-5, recombinant human
growth/differentiation factor-5; iPSC-MSCs, induced pluripotent
stem cell-derived mesenchymal stem cells; OCN, osteocalcin; CAP,
cementum attachment protein. |
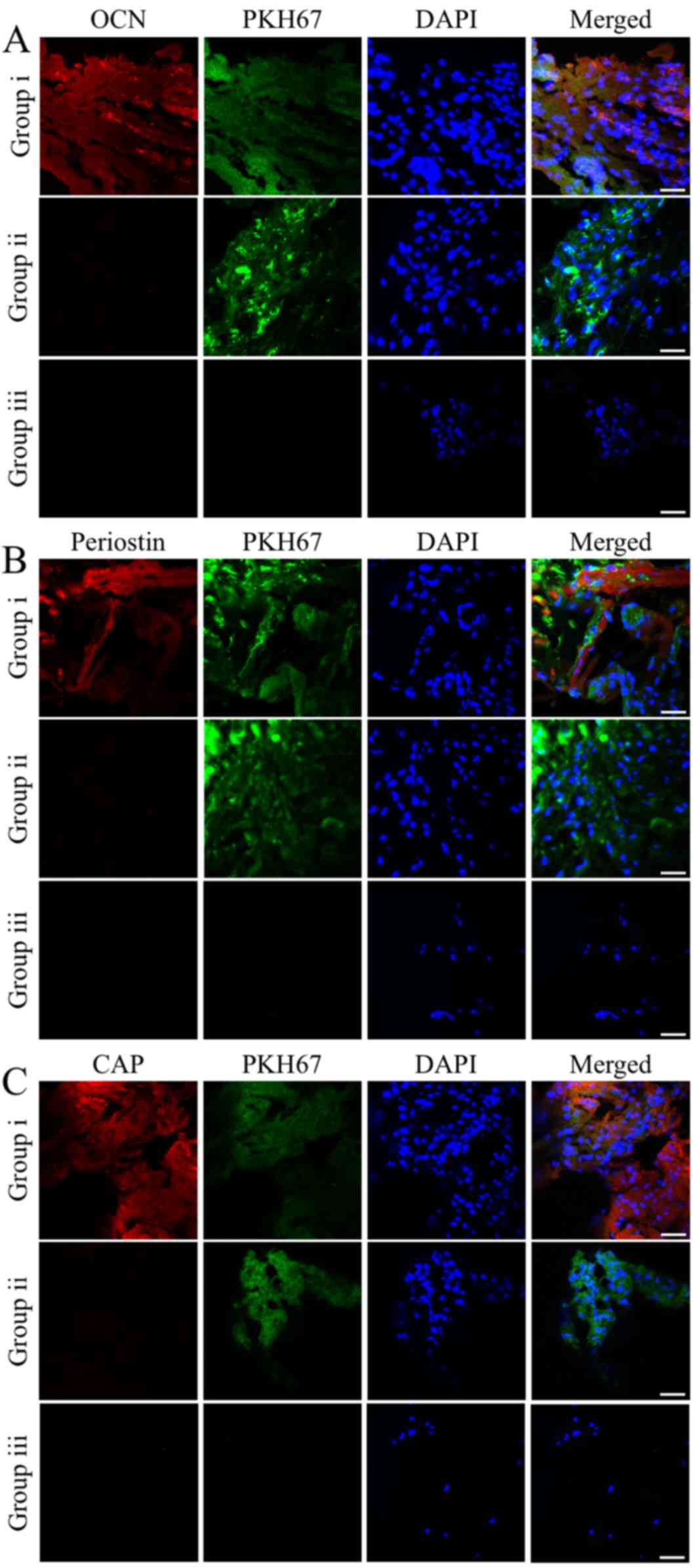
To track the donor cells in the specimens, the
iPSC-MSCs pre-labeled with PKH67 were also examined by
immunofluorescence staining. Co-localization of the markers with
PKH67 correlated with the immunohistochemical analysis. The
majority of the iPSC-MSCs implanted with rhGDF-5 exhibited strong
expression levels of OCN, periostin and CAP. The OCN-, periostin-
and CAP-positive cells accounted for 90.17±9.98, 77.29±10.65 and
83.73±10.33%, respectively, of the PKH67-labeled donor cells
(Fig. 4). By contrast, expression of
the three markers was not detected in the iPSC-MSCs or the ingrown
indigenous cells of the groups containing hydrogels incorporating
either iPSC-MSCs or rhGDF-5 alone (Fig.
4).
Discussion
Periodontal regeneration remains a substantial
challenge in management of periodontitis due to the complex
architecture and function of periodontium. Tissue engineering
approaches employing multipotent progenitor cells, signaling
molecules and bioactive scaffolds have been recognized as promising
therapeutics for reliable and predictable periodontal regeneration
(4,5). The development and repair of
periodontal tissues involve an orchestrated process of
proliferation and differentiation of periodontal progenitor cells
for osteogenesis, fibrogenesis and cementogenesis (4,24). To
regenerate the three major components of periodontal tissues, it is
therefore crucial to identify an optimum cell source with the
capacity to differentiate into functional periodontal cells, and to
provide a microenvironment in favor of effective periodontal
specific differentiation of the implanted cells.
Previous studies have adopted multiple induction
conditions to prompt periodontal specific differentiation of adult
stem cells and characterize their differentiation capacity
(25,26). iPSC-MSCs have been recognized as a
promising cell source for periodontal regeneration (14). In the present study, iPSC-MSCs were
generated and their differentiation capacity under rhGDF-5
treatment was examined. OCN is an osteoblast-specific protein
implicated in bone mineralization, and has been commonly used as a
marker for the osteogenic differentiation of progenitor cells at
the late stage (24,27). Periostin is highly expressed in
periodontal ligament fibroblasts and has an important role in
periodontal tissue integrity (28).
CAP is a cementoblast-related marker and its production is
restricted to cementum (29). In the
present study, iPSC-MSCs incubated with rhGDF-5 for 1–3 weeks
displayed significantly higher gene expression levels of OCN,
periostin and CAP compared with the untreated control groups. The
immunostaining results corresponded with the RT-qPCR data. This is
consistent with a previous observation on the overexpression of
OCN, periostin and CAP in rhGDF-5-treated iPSCs (20). Notably, the rhGDF-5 treatment failed
to enhance the expression of periostin and CAP in the BMSCs,
despite significantly increased expression of OCN compared with the
untreated control. Previous research has demonstrated that GDF-5
was able to stimulate osteogenic differentiation of BMSCs in a
subcutaneous rat model (30).
Although BMSCs may aid periodontal regeneration, a recent
meta-analysis indicated that BMSCs have no favorable effect on
periodontal ligament formation as determined by subgroup analysis
(6). In addition, in the present
study, the 4-week rhGDF-5 incubation greatly induced mineralization
in both the iPSC-MSCs and BMSCs, whereas there were barely
discernible mineralized deposits in the untreated controls.
Collectively, these data highlight the potential of rhGDF-5 to
induce periodontal specific differentiation of iPSC-MSCs, and
suggest that iPSC-MSCs may have a greater capacity than BMSCs to
differentiate into periodontal cells in response to rhGDF-5.
Furthermore, the present study evaluated the
differentiation potential of iPSC-MSCs delivered by HA hydrogels
with or without rhGDF-5 in a subcutaneous murine model. HA
hydrogels have been demonstrated to enhance periodontal treatment
outcomes when applied alone or in conjugation with cells/factors
(31,32). In the present study, the engineered
hydrogels demonstrated successful biocompatibility 6 weeks after
transplantation. The newly formed tissues by the HA hydrogels
containing iPSC-MSCs and rhGDF-5 displayed strong production of
OCN, periostin and CAP; however, the markers were not detected in
the hydrogels incorporating either iPSC-MSCs or rhGDF-5 alone.
Furthermore, the introduction of rhGDF-5 induced expression of the
markers in the majority of the implanted iPSC-MSCs. This further
indicates that rhGDF-5 may promote the differentiation of iPSC-MSCs
into periodontal cells in vivo.
In conclusion, iPSC-MSCs displayed a high capacity
of periodontal specific differentiation in response to rhGDF-5 both
in vitro and in vivo. With reference to BMSCs,
iPSC-MSCs may be a more effective cell source for use in
periodontal treatment. The incorporation of iPSC-MSCs and rhGDF-5
in HA hydrogel is likely to offer a promising therapeutic approach
for periodontal regeneration. Further investigations are warranted
to elucidate the mechanisms underlying the effects of GDF-5 and to
evaluate the regenerative potential of iPSC-MSCs/rhGDF-5/HA
hydrogel composites in more clinically relevant models.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81470739).
References
|
1
|
Pihlstrom BL, Michalowicz BS and Johnson
NW: Periodontal diseases. Lancet. 366:1809–1820. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chapple IL: Time to take periodontitis
seriously. BMJ. 348:g26452014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kassebaum NJ, Bernabé E, Dahiya M,
Bhandari B, Murray CJ and Marcenes W: Global burden of severe
periodontitis in 1990–2010: A systematic review and
meta-regression. J Dent Res. 93:1045–1053. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen FM, Sun HH, Lu H and Yu Q: Stem
cell-delivery therapeutics for periodontal tissue regeneration.
Biomaterials. 33:6320–6344. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hynes K, Menicanin D, Gronthos S and
Bartold PM: Clinical utility of stem cells for periodontal
regeneration. Periodontol 2000. 59:203–227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan XZ, Yang F, Jansen JA, de Vries RB and
van den Beucken JJ: Cell-based approaches in periodontal
regeneration: A systematic review and meta-analysis of periodontal
defect models in animal experimental work. Tissue Eng Part B Rev.
21:411–426. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang Y, Rossi FM and Putnins EE:
Periodontal regeneration using engineered bone marrow mesenchymal
stromal cells. Biomaterials. 31:8574–8582. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Requicha JF, Viegas CA, Muñoz F, Azevedo
JM, Leonor IB, Reis RL and Gomes ME: A tissue engineering approach
for periodontal regeneration based on a biodegradable double-layer
scaffold and adipose-derived stem cells. Tissue Eng Part A.
20:2483–2492. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seo BM, Miura M, Gronthos S, Bartold PM,
Batouli S, Brahim J, Young M, Robey PG, Wang CY and Shi S:
Investigation of multipotent postnatal stem cells from human
periodontal ligament. Lancet. 364:149–155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mooney DJ and Vandenburgh H: Cell delivery
mechanisms for tissue repair. Cell Stem Cell. 2:205–213. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jung Y, Bauer G and Nolta JA: Concise
review: Induced pluripotent stem cell-derived mesenchymal stem
cells: Progress toward safe clinical products. Stem Cells.
30:42–47. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X,
Zhang Y, Lam FF, Kang S, Xia JC, Lai WH, et al: Functional
mesenchymal stem cells derived from human induced pluripotent stem
cells attenuate limb ischemia in mice. Circulation. 121:1113–1123.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moslem M, Eberle I, Weber I, Henschler R
and Cantz T: Mesenchymal stem/stromal cells derived from induced
pluripotent stem cells support CD34(pos) hematopoietic stem cell
propagation and suppress inflammatory reaction. Stem Cells Int.
2015:8430582015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hynes K, Menicanin D, Han J, Marino V,
Mrozik K, Gronthos S and Bartold PM: Mesenchymal stem cells from
iPS cells facilitate periodontal regeneration. J Dent Res.
92:833–839. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lam J, Truong NF and Segura T: Design of
cell-matrix interactions in hyaluronic acid hydrogel scaffolds.
Acta Biomater. 10:1571–1580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bae MS, Ohe JY, Lee JB, Heo DN, Byun W,
Bae H, Kwon YD and Kwon IK: Photo-cured hyaluronic acid-based
hydrogels containing growth and differentiation factor 5 (GDF-5)
for bone tissue regeneration. Bone. 59:189–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee J and Wikesjö UM:
Growth/differentiation factor-5: Pre-clinical and clinical
evaluations of periodontal regeneration and alveolar
augmentation-review. J Clin Periodontol. 41:797–805. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reynolds MA and Aichelmann-Reidy ME:
Protein and peptide-based therapeutics in periodontal regeneration.
J Evid Based Dent Pract. 12 (3 Suppl):S118–S126. 2012. View Article : Google Scholar
|
|
19
|
Moore YR, Dickinson DP and Wikesjö UM:
Growth/differentiation factor-5: A candidate therapeutic agent for
periodontal regeneration? A review of pre-clinical data. J Clin
Periodontol. 37:288–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yin X, Li Y, Li J, Li P, Liu Y, Wen J and
Luan Q: Generation and periodontal differentiation of human
gingival fibroblasts-derived integration-free induced pluripotent
stem cells. Biochem Biophys Res Commun. 473:726–732. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu J, Wang Y, Jiao J, Liu Z, Zhao C, Zhou
Z, Zhang Z, Forde K, Wang L, Wang J, et al: Patient-specific
cardiovascular progenitor cells derived from integration-free
induced pluripotent stem cells for vascular tissue regeneration.
Biomaterials. 73:51–59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu J, Smith LA, Feng K, Liu X, Sun H and
Ma PX: Response of human embryonic stem cell-derived mesenchymal
stem cells to osteogenic factors and architectures of materials
during in vitro osteogenesis. Tissue Eng Part A. 16:3507–3514.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Amin HD, Olsen I, Knowles JC, Dard M and
Donos N: Effects of enamel matrix proteins on multi-lineage
differentiation of periodontal ligament cells in vitro. Acta
Biomater. 9:4796–4805. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sowmya S, Chennazhi KP, Arzate H,
Jayachandran P, Nair SV and Jayakumar R: Periodontal specific
differentiation of dental follicle stem cells into osteoblast,
fibroblast, and cementoblast. Tissue Eng Part C Methods.
21:1044–1058. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Inanç B, Elçin AE and Elçin YM: In vitro
differentiation and attachment of human embryonic stem cells on
periodontal tooth root surfaces. Tissue Eng Part A. 15:3427–3435.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD,
Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, et al:
Endocrine regulation of energy metabolism by the skeleton. Cell.
130:456–469. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Padial-Molina M, Volk SL, Taut AD,
Giannobile WV and Rios HF: Periostin is down-regulated during
periodontal inflammation. J Dent Res. 91:1078–1084. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Han P, Ivanovski S, Crawford R and Xiao Y:
Activation of the canonical wnt signaling pathway induces cementum
regeneration. J Bone Miner Res. 30:1160–1174. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shimaoka H, Dohi Y, Ohgushi H, Ikeuchi M,
Okamoto M, Kudo A, Kirita T and Yonemasu K: Recombinant
growth/differentiation factor-5 (GDF-5) stimulates osteogenic
differentiation of marrow mesenchymal stem cells in porous
hydroxyapatite ceramic. J Biomed Mater Res Part A. 68:168–176.
2004. View Article : Google Scholar
|
|
31
|
Fawzy E, l-Sayed KM, Mekhemar MK,
Beck-Broichsitter BE, Bähr T, Hegab M, Receveur J, Heneweer C,
Becker ST, Wiltfang J and Dörfer CE: Periodontal regeneration
employing gingival margin-derived stem/progenitor cells in
conjunction with IL-1ra-hydrogel synthetic extracellular matrix. J
Clin Periodontol. 42:448–457. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Eick S, Renatus A, Heinicke M, Pfister W,
Stratul SI and Jentsch H: Hyaluronic acid as an adjunct after
scaling and root planing: A prospective randomized clinical trial.
J Periodontol. 84:941–949. 2013. View Article : Google Scholar : PubMed/NCBI
|