Introduction
Sepsis-associated encephalopathy (SAE) is defined as
diffuse or multifocal cerebral dysfunction induced by the systemic
response to infection, without clinical or laboratory evidence of
direct brain infection (1). SAE is a
common complication of brain injury in sepsis, with an incidence of
9–71% in the intensive care unit (2). In a recent epidemiological multicenter
study, the incidence of delirium was 32.3%, in 497 patients with
brain injury, including 76 septic patients (3). SAE is not only associated with
increased mortality (4,5), but also permanent cognitive
dysfunction. Presently, the diagnosis of SAE is a clinical
challenge and involves the exclusion of other metabolic and
structural etiologies (5).
Conventional diagnostic techniques include electroencephalogram
(EEG), detection of biomarkers [neuron-specific enolase (NSE) and
S100-β] and magnetic resonance imaging (MRI). However, EEG findings
are not specific for SAE, and it is often unavailable or unreliable
for patients under sedation or with metabolic derangements
(1). There are a number of studies
suggesting that the diagnosis of SAE does not correlate with the
concentration of NSE in serum (6,7). Similar
studies indicate that the increase of S100-β in septic patients
does not correlate with the severity of neurological impairment or
with the outcome (8,9). Although MRI can identify structural
abnormalities of the brain and other diseases, including SAE, the
positive rates of SAE diagnosis are relatively low (10). Thus, MRI is not conducive to early
diagnosis and timely intervention of brain injury, because of the
deficiencies of conventional diagnostic techniques for SAE.
Magnetic resonance spectroscopy (MRS) is
well-established as a non-invasive technique for quantitative
analysis of metabolic compounds in living tissues. The degree of
brain damage was determined by quantitative detection of metabolic
compounds associated with brain injury. N-acetylaspartate (NAA) is
a marker of the functional integrity of neuronal mitochondrial
metabolism. Its reduction reflects neuronal loss or dysfunction,
and is associated with cognitive dysfunction and the degree of
brain injury (11). Choline (Cho) is
a precursor of the neurotransmitter acetylcholine and of
phosphatidylcholine, a major constituent of cell membranes.
Increases in Cho reflect increased membrane synthesis and/or
increased number of cells, and was shown to be associated with
recovery from neuronal injury (12).
In spite of its wide application in the diagnosis and assessment of
traumatic brain injury, hypoxic ischemic encephalopathy,
intracranial infections, stroke, cancer, metabolic disorders and
other diseases of the brain (13–15), few
researchers have explored its role in SAE. Therefore, in the
present study, we analyzed the association between the change of
MRS and brain injury in the rat model of sepsis, and confirm that
MRS technology represents a sensitive method for the early
diagnosis of brain injury caused by sepsis.
Materials and methods
Establishment of the sepsis model
This protocol was approved by the Institutional
Animal Care and Use Committee of Guangdong General Hospital
(Guangdong, China). Thirty-five adult Sprague-Dawley (SD) rats
(weight, 250–300 g) were randomly divided into 4 groups [5 in the
control group, and 10 each in the 6, 12 and 24 h
post-lipopolysaccharide (LPS)-injection groups]. Sepsis was induced
by a single intraperitoneal administration of LPS (lot. no.
B0013K030100; Sigma-Aldrich, St. Louis, MO, USA) at a dose of 30
mg/kg (16). Instead of LPS, rats in
the control group received intraperitoneal injections of normal
saline. After injection, all rats were housed in a room with
temperature of 22°C and humidity of 50%, with a 12-h light/dark
cycle and free access to food and water. Clinical symptoms and
survival rate were observed at the corresponding time-points (6, 12
and 24 h after establishing the sepsis model).
MRI and MRS
MRI and spectroscopy scans were performed in a 7
Tesla horizontal bore magnet (Bruker BioSpec 70/20 USR; Bruker,
Karlsruhe, Germany). Rats were deeply anesthetized by a single
intraperitoneal administration of 3% pentobarbital (2 ml/kg) at 6,
12 or 24 h (control group after 24 h) after inducing sepsis. The
heads of rats were then fixed in a body restrainer with a tooth-bar
and a cone shaped head holder. Body temperature was maintained at
37°C with a body heating pad connected to a water heat-exchange
system. Respiration was maintained in 800 ml/min oxygen via a
nosecone and monitored with a pressure pad.
Morphological images were acquired using a rapid
acquisition relaxation-enhanced (RARE) pulse sequence with T2
weighting (repetition time = 2,000 msec, TE = 36 msec, flip angle =
90°, RARE factor = 8, average number = 8, slice thickness = 0.8 mm,
number of slices = 16, field of view = 3.5×3.5 cm2 and
256×256 matrix).
Spectra were acquired on the region-of-interest of
the right hippocampus. The pointed-resolved surface coil
spectroscopy pulse sequence was used with the FieldMap with a
shimming method (TR = 2,500 msec, TE = 20 msec, voxel volume =
2×3×3 mm3, average number 512 and acquisition points
2,048). The MRS acquired via measured signal of free induction
decay were Fourier-transformed. The spectra were phased, and
zero-order baseline was corrected with the software. Spectral
windows where changes in signal intensity were identified contained
the peaks for NAA (2.02 ppm), Cho (3.21 ppm) and Cr (3.03 ppm). In
an effort to limit the sensitivity to phasing artifacts, we
computed metabolite ratios for analysis.
Blood sampling and brain tissue
preparation
Blood samples and brain tissue were collected
immediately after obtaining MRI and MRS data while rats were
maintained under anesthesia. A sternectomy was made to access the
heart. A 6-gauge needle (Beyotime, Shanghai, China) was inserted in
the left ventricle via the apex for blood sample collection, and
samples were then centrifuged at 2,500 × g for 10 min at 4°C. The
supernatant was then placed in an Eppendorf tube (Beyotime) and
stored at −70°C for detection of NSE and S100-β. A small opening
was cut in the right auricle to allow automatic blood flow. First,
rats were rapidly perfused through the left cardiac ventricle with
150 ml normal saline, and then perfusion speed was slowed down
until clear liquid flowed out of the right atrium (saline perfusion
time was not more than 5 min). Then the tissue was fixed by a
fixative solution [150 ml of 4% paraformaldehyde and 0.1%
glutaraldehyde in 0.1 M phosphate-buffered saline (PBS) (Biosharp,
Hefei, China)] until the liver turned yellow and toughened,
indicating that perfusion was successful. A craniotomy was
performed, in which the right brain tissue was removed for
dehydration and paraffin-embedding. Paraffin-embedded tissues were
cut coronally into 8-µm-thick sections for hematoxylin and eosin
(H&E) staining, Nissl staining (lot. no. 201308; Beyotime
Institute of Biotechnology, Jiangsu, China), and TUNEL staining
(lot. no. 11 684 817 910; Roche Diagnostics, Basel, Switzerland).
Pathological changes of hippocampal tissue were observed by
microscopy.
Measurement of serum markers
NSE and S100-β levels were analyzed by enzyme-linked
immunosorbent assay (ELISA) using commercially available kits (NSE,
lot. no. CSB-E07963r and S100-β, lot. no. CSB-E08066r) (both from
Cusabio Biotech Co., Ltd., Wuhan, China) according to the
manufacturer's instructions. Microtiter plates with the purified
antibody-coating in the solid phase were bound by anti-NSE.
Anti-S100-β antibody, standards and samples, biotinylated anti-NSE,
anti-S100-β protein antibody, and HRP-labelled avidin were then
added sequentially into micropores. After thorough washing, samples
were developed with 3,3′,5,5′-tetramethylbenzidine (TMB) substrate.
TMB was catalyzed into a blue color via peroxidase and finally
changed to yellow after acidification. The absorbance was measured
with a microplate reader at 450 nm [optical density (OD) value],
and sample concentrations were calculated according to the standard
curve.
Statistical analysis
Statistical analyses were performed using SPSS 17.0
statistical software (SPSS, Inc., Chicago, IL, USA). All
statistical assessments were two-sided, and the test statistics and
their corresponding P-values were calculated. A value of p<0.05
was considered to indicate a statistically significant difference.
Multiple comparisons of enumeration data were performed using the
Pearson's Chi-square test or Fisher's exact test. Analysis of
variance (ANOVA) was used for multiple comparisons of measurement
data where the data were normally distributed. The least
significance difference (LSD) test was utilized for the analysis of
differences between two groups. The Kruskal-Wallis test was used
for multiple comparisons of measurement data where the data were
non-normally distributed or exhibited homogeneity of variance. The
correlation between variables was analyzed by the Pearson's linear
regression test for normal distribution data or Spearman's
rank-coefficient test for non-normal distribution data.
Results
Clinical characteristics of the sepsis
model
Among the 30 non-control group SD rats, the
mortality rates were 30% (3/10), 40% (4/10), and 40% (4/10) in the
6, 12 and 24 h groups, respectively. All surviving rats showed
signs of sepsis, such as malaise, hair-bristle, hemorrhage in the
ear and nose, drowsiness and diarrhea. None of the aforementioned
symptoms occurred in the control group.
Right hippocampal changes after
LPS-induced sepsis
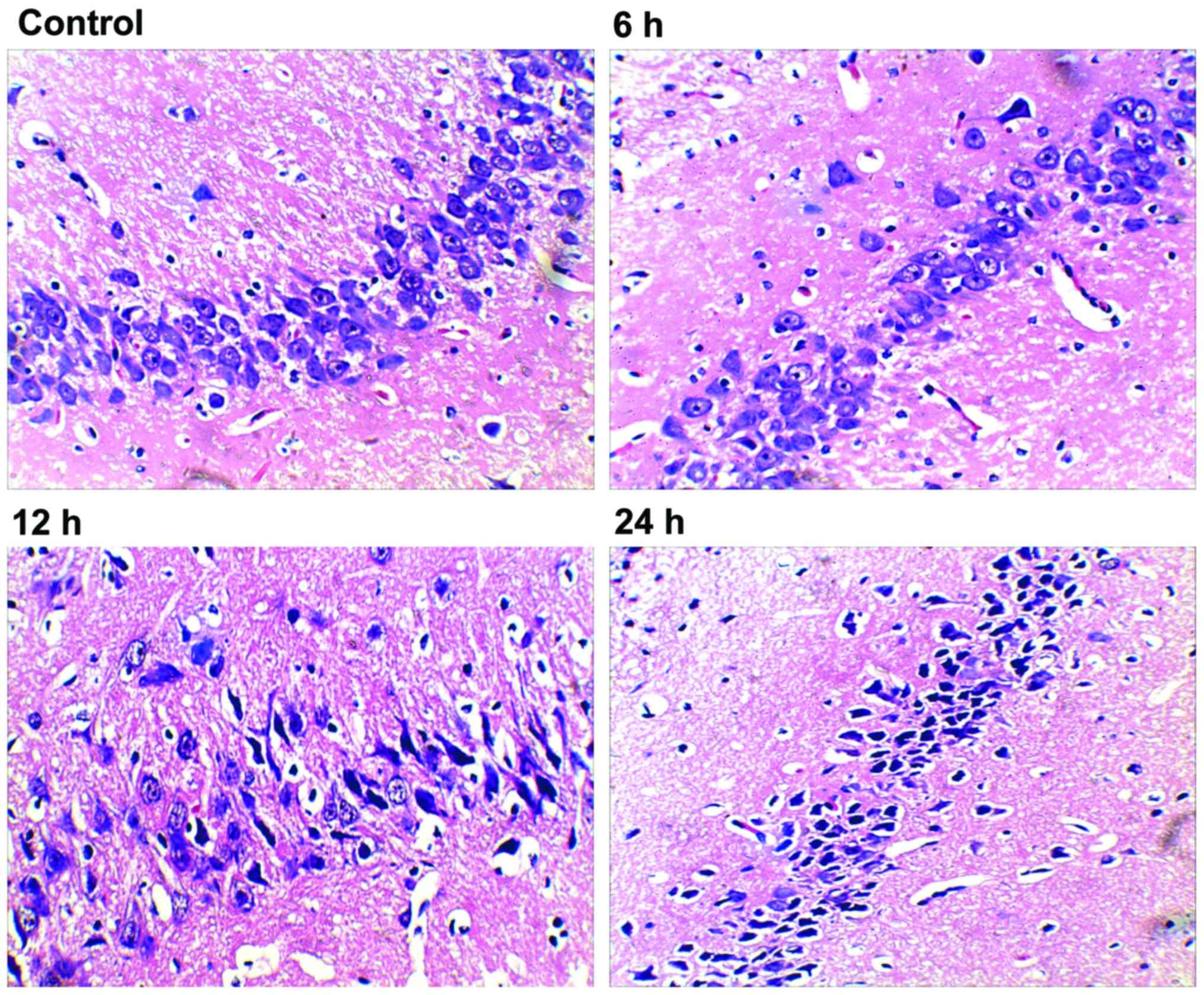
We first performed histopathological analysis of
H&E stained sections. After 24 h, hippocampal tissue in rats of
the control group showed normal neurons with intact cell structures
and nuclear membrane integrity (Fig.
1; control). The 6 h group exhibited neuronal pyknosis, but no
major changes in neural cell morphology or cell structures
(Fig. 1; 6 h). Both the 12 and 24
groups had intact histological structure, but showed obvious
neuronal pyknosis, swelling of organelles, loss of plasma membrane
integrity, and loss of intracellular content, which resulted in
dramatically decreased number of neurons and distinctive
perimicrovascular edema (Fig. 1; 12
and 24 h). Compared with the 6 h group, the 12 h group showed
increased neural damage, but was similar to that of the 24 h
group.
To quantify cell death, TUNEL staining was used in
the right hippocampal area. Examination of the control group
revealed no TUNEL-positive cells; whereas, there were a small
number of TUNEL-positive cells within the hippocampus of the 6 h
group. In contrast, both the 12 and 24 h groups showed obvious
TUNEL-positive cells (Fig. 2A), and
significantly increased formation of apoptotic bodies compared with
the control and 6 h groups (p<0.05) (Fig. 2B). The apoptotic cells had small cell
bodies in the plasma, and tan-color stained nuclei. In addition,
they had irregular shapes and were inconsistent in size, showing
nuclear condensation and fragmentation of some nuclei, as well as
formation of apoptotic bodies.
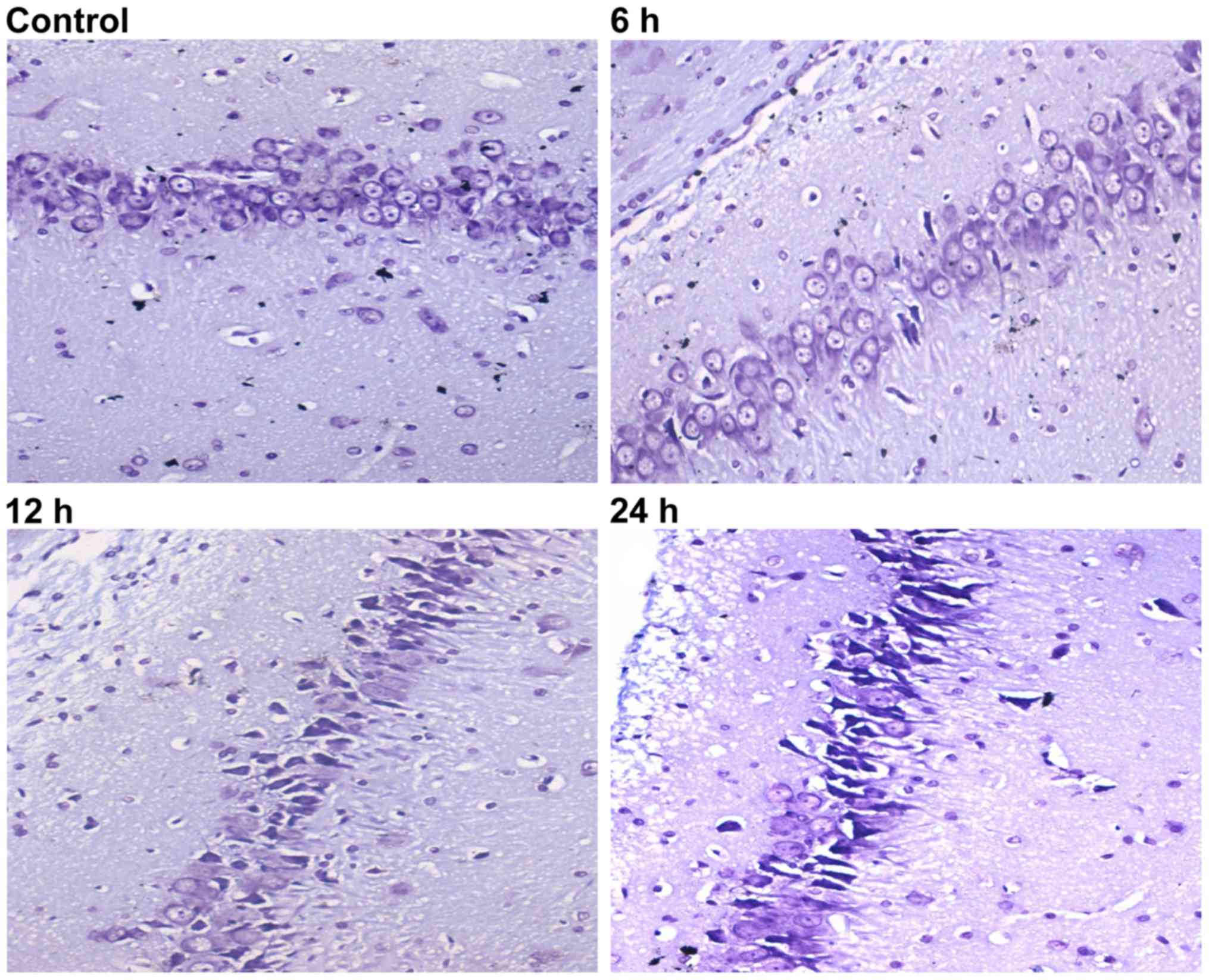
Nissl staining was then used to evaluate neuron
viability at the different time-points. When rats were subjected to
sepsis, injured neurons were characterized by cytoplasmic
shrinkage, nuclear pyknosis and hyperchromasia. Rats in the control
group (Fig. 3, control) did not
exhibit any damage or changes in neuronal morphology. Some
dead/dying neurons were observed as small, darkly stained, shrunken
cells formed in the 6 h group (Fig.
3; 6 h). Compared with the 6 h group, rats in the 12 and 24 h
groups had more extensive neuronal loss (Fig. 3; 12 and 24 h).
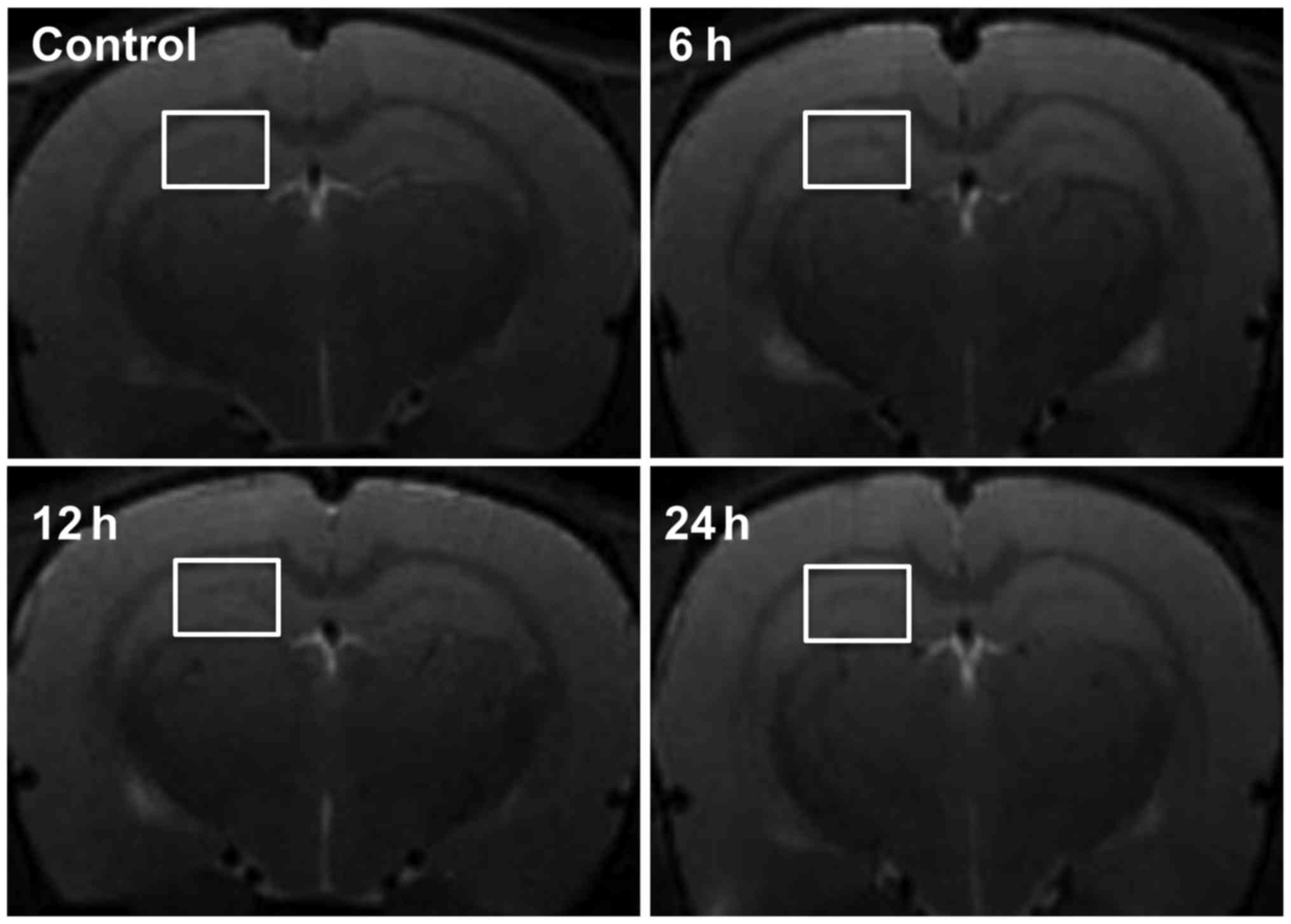
MRI
To evaluate the diagnostic value of MRI on brain
damage of septic rats, we observed the brain tissue of septic rats
at 6, 12 and 24 h after LPS-injection and in the control group
using T2-weighted axial imaging. There were no significant changes
at any of the time-points. No high-signal lesions (indicative of
cytotoxicity or vasogenic brain edema) or swelling of the lateral
ventricles were evident (Fig.
4).
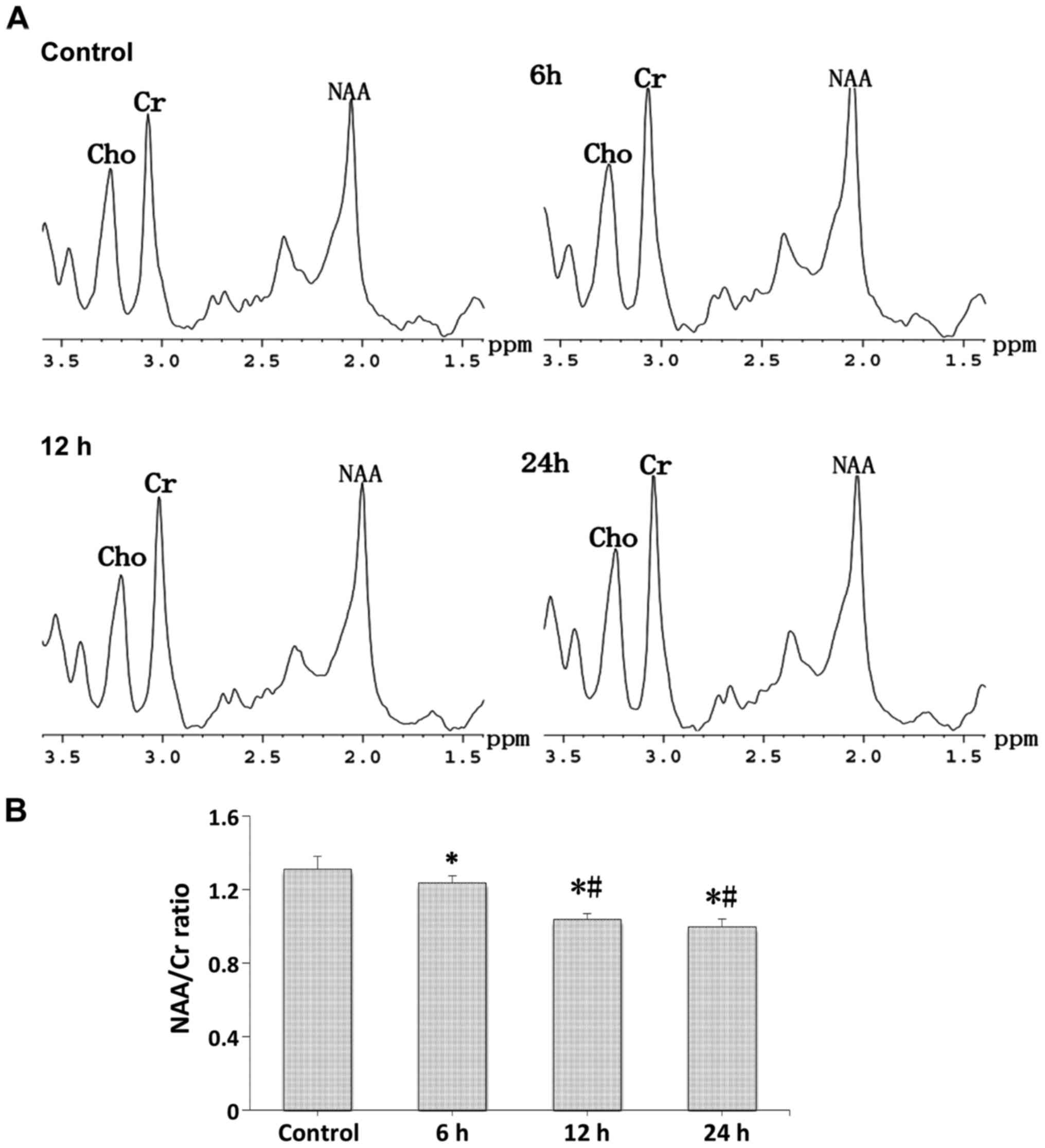
In vivo 1H MR spectra
Proton spectra (Fig.
5A) were acquired in a cubic volume of 18 µl in the right
hippocampus of the control and LPS-injection groups, and the
relative amounts of total NAA and Cr compounds were determined
after the identification of their respective peaks (Fig. 5B). Compared with the control group, a
strong decrease was seen in the N-acetylaspartate/choline (NAA/Cr)
ratio at the different time-points after LPS-injection, and the
NAA/Cr ratio in the 12 and 24 h groups showed a stronger decrease
compared with the 6 h group (p<0.05).
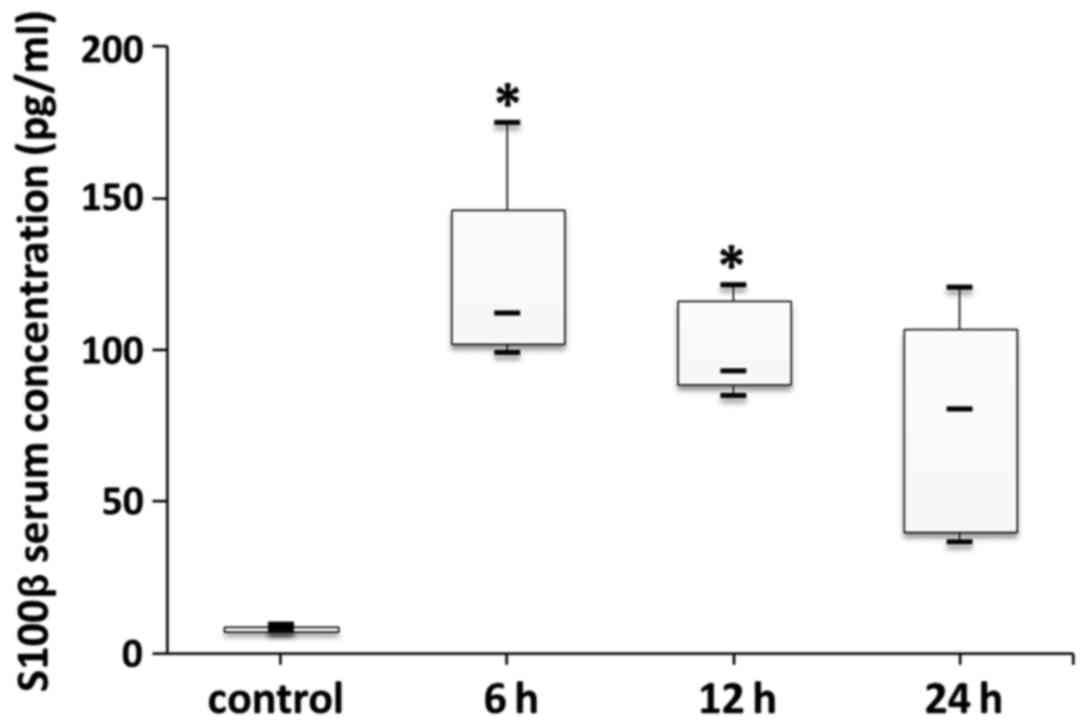
Measurement of NSE and S100-β
Both serum NSE and S100-β levels at the different
time-points after LPS-induced sepsis were higher than in the
control group (Figs. 6 and 7). There were statistically significant
differences in both serum NSE and S100-β levels between the control
and 6 h groups (p<0.05). In addition, there was a statistically
significant difference in serum S100-β levels between the control
and 12 h groups (p<0.05). However, there were no statistically
significant differences in serum NSE or S100-β levels between the
6, 12 h or 24 h groups (p>0.05).
Correlation analysis between two
variables
The correlation between apoptosis rate and the
NAA/Cr ratio was stronger than that between apoptosis rate and NSE
or S100-β (−0.925 vs. 0.434 vs. 0.517, respectively) (Table I).
 | Table I.Correlation between two variables. |
Table I.
Correlation between two variables.
| Items | r-values | P-values |
|---|
| Apoptosis rate and
NAA/Cr ratioa | −0.925 | 0.000 |
| Apoptosis rate and
NSE | 0.434 | 0.566 |
| Apoptosis rate and
S100-β | 0.517 | 0.483 |
Discussion
Although the pathogenesis of SAE is still unclear,
early diagnosis and treatment are critical to reduce mortality and
disability from brain damage in septic patients. However, the
methods of assessing brain injury such as EEG (1), T2WI (10), NSE (6,7) and
S100-β (8,9) have limitations. Brain MRS is a
non-invasive method to measure the concentrations of biochemical
compounds in MRI-specified areas of cerebral tissue (17). This technique offers the ability to
acquire in vivo data on biochemical compounds with
negligible influence on the disease process, and allows the
detection of even small deviations of normal brain function in
humans and animal models (18).
Here, we report the implementation of brain T2-weighted imaging
(T2WI), proton spectroscopy and serum markers including NSE and
S100-β, in LPS-induced septic rats. The results were compared with
histopathological analysis. Overall, compared with T2WI and serum
markers, the results obtained by MRS were closer to the
histopathological results of neuronal damage. The results of our
study contribute valuable and novel information for the early
diagnosis of brain injury caused by sepsis.
The histopathology presented herein indicates that
brain damage can be detected by any kind of dye used in the present
study in the early (up to 6 h) period of LPS-induced sepsis in
rats. In addition, compared with the control group, the damage in
the 12 and 24 h groups was most severe. Messaris et al
(19) confirmed the presence of
apoptosis in the hippocampus, and this process began 6 h after
initiation of experimental sepsis and increased in the late phases
of sepsis. The importance of apoptosis in the immunologic and
pathological mechanisms of sepsis has been recognized (20). However, in 1998, Mouihate and Pittman
(21) found no evidence of apoptosis
in the hippocampus and other brain regions of rats 5.5 h and 5 days
after intraperitoneal injection of LPS. The reasons for the
discrepancy in these results remain unclear. It may be related to
several factors, such as differences in species or age of rats,
different amounts of LPS, or the time-points of observation.
In the present study, little to no changes in T2WI
could be detected either in the early (6 h) or late (24 h) phase of
LPS-induced sepsis in rats. Although MRI can be utilized for the
exclusion of cerebral dysfunction from other cerebrovascular
conditions, it is not a specific test for SAE (1). In fact, we report that rats began to
have symptoms of brain disorder in the early period of sepsis, such
as mental fatigue and drowsiness, and pathological analysis also
indicated brain damage, but the results from MRI showed normal
brain. However, it was recently revealed that accumulation of
vasogenic edematic fluid at the base of the brain was observed by
T2WI at 6 and 24 h after cecal ligation and puncture (CLP)-induced
sepsis (18). This apparent
discrepancy may have been because of the differences in the
experimental models. The inflammatory processes mediated by CLP are
more intensely stimulated by polymicrobial sepsis.
MRS measurements indicated alterations in the NAA/Cr
ratio. These were clearly apparent in the hippocampus of septic
rats as early as 6 h after LPS-induction. Moreover, the decrease in
NAA/Cr ratio in the hippocampus was more significant at 12 and 24
h. Similar findings were reported by Bozza et al at 6 and 24
h after experimental sepsis (18).
However, our study confirms the presence of apoptosis by TUNEL
staining, and the results showed that the decrease in NAA/Cr ratio
was consistent with the apoptosis rate. This indicates that the
induction of apoptosis in the hippocampus is an important component
of the pathogenesis of brain dysfunction in sepsis, as confirmed
earlier by Sharshar et al (22). Taken together, our findings indicated
that neuronal damage was accompanied by decreased NAA levels in
septic rats (23–25).
Some researchers believed that NSE and S100-β may be
biomarkers of brain damage, and can be used as a testing index for
sepsis in clinic (1,26). Here, we compared the levels of NSE
and S100-β in the 6, 12 and 24 h, and control group. The results
showed that although the protein concentrations of NSE and S100-β
were different in the serum of each group, the statistical
differences were inconsistent with the pathological analyses. The
results of our study are consistent with those of others that
showed no correlation between serum concentrations of NSE and
S100-β and the severity of SAE (7,8).
Specifically, they asserted that the rise of S100-β concentration
may originate from outside the brain (the amount of S100-β in
chondrocytes and adipose cells is 1/4 that of the brain).
Despite the novelty of our findings, we acknowledge
some limitations to our study. In this study, we did not monitor
blood pressure during the MRS procedure. Therefore, we cannot rule
out the influence of hemodynamics on the complications of brain
damage after LPS-induced sepsis. However, it was shown that rats
subjected to CLP (with a mortality rate of 43%) did not exhibit
alterations in mean whole or local brain blood flow, and the
authors concluded that brain dysfunction is not a consequence of
changes of cerebral blood flow during severe sepsis (25). Moreover, there was an absence of EEG
monitoring because the MRS procedure requires a better condition.
These areas will be investigated in our future studies.
Taken together, relative to T2WI and serum
biomarkers, our observations indicate that MRS is a suitable
non-invasive method to investigate the complications of brain
damage in septic rats. Significant quantitative changes were
detected in the metabolic profiles of septic animals, which were
consistent with the apoptosis rate, and may ultimately result in
improvements of the diagnostic accuracy and severity assessment of
SAE in patients.
Acknowledgements
This study was supported by the Medical Science and
Technology Foundation of Guangdong Province (B2013015), and the
Science and Technology Foundation of Guangdong Province
(2013B022000081). The authors are thankful for the support received
from Dr Peter Hou and Dr Kun Xiao during the process of manuscript
development.
References
|
1
|
Cotena S and Piazza O: Sepsis-associated
encephalopathy. Transl Med UniSa. 2:20–27. 2012.PubMed/NCBI
|
|
2
|
Young GB, Bolton CF, Austin TW, Archibald
YM, Gonder J and Wells GA: The encephalopathy associated with
septic illness. Clin Invest Med. 13:297–304. 1990.PubMed/NCBI
|
|
3
|
Salluh JI, Soares M, Teles JM, Ceraso D,
Raimondi N, Nava VS, Blasquez P, Ugarte S, Ibanez-Guzman C, Centeno
JV, et al: Delirium Epidemiology in Critical Care Study Group:
Delirium epidemiology in critical care (DECCA): an international
study. Crit Care. 14:R2102010. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zampieri FG, Park M, Machado FS and
Azevedo LC: Sepsis-associated encephalopathy: not just delirium.
Clinics (Sao Paulo). 66:1825–1831. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pytel P and Alexander JJ: Pathogenesis of
septic encephalopathy. Curr Opin Neurol. 22:283–287. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rodríguez-Núñez A, Cid E, Rodríguez-García
J, Camiña F, Rodríguez-Segade S and Castro-Gago M: Concentrations
of nucleotides, nucleosides, purine bases, oxypurines, uric acid,
and neuron-specific enolase in the cerebrospinal fluid of children
with sepsis. J Child Neurol. 16:704–706. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van den Boogaard M, Ramakers BP, van Alfen
N, van der Werf SP, Fick WF, Hoedemaekers CW, Verbeek MM,
Schoonhoven L, van der Hoeven JG and Pickkers P:
Endotoxemia-induced inflammation and the effect on the human brain.
Crit Care. 14:R812010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Piazza O, Russo E, Cotena S, Esposito G
and Tufano R: Elevated S100B levels do not correlate with the
severity of encephalopathy during sepsis. Br J Anaesth. 99:518–521.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heizmann CW: S100B protein in clinical
diagnostics: assay specificity. Clin Chem. 50:249–251. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Piazza O, Cotena S, De Robertis E, Caranci
F and Tufano R: Sepsis associated encephalopathy studied by MRI and
cerebral spinal fluid S100B measurement. Neurochem Res.
34:1289–1292. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lim MK, Suh CH, Kim HJ, Cho YK, Choi SH,
Kang JH, Park W and Lee JH: Systemic lupus erythematosus: brain MR
imaging and single-voxel hydrogen 1 MR spectroscopy. Radiology.
217:43–49. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Castillo M, Kwock L and Mukherji SK:
Clinical applications of proton MR spectroscopy. AJNR Am J
Neuroradiol. 17:1–15. 1996.PubMed/NCBI
|
|
13
|
Cohen BA, Inglese M, Rusinek H, Babb JS,
Grossman RI and Gonen O: Proton MR spectroscopy and MRI-volumetry
in mild traumatic brain injury. AJNR Am J Neuroradiol. 28:907–913.
2007.PubMed/NCBI
|
|
14
|
Inder TE and Volpe JJ: Mechanisms of
perinatal brain injury. Semin Neonatol. 5:3–16. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mishra AM, Gupta RK, Jaggi RS, Reddy JS,
Jha DK, Husain N, Prasad KN, Behari S and Husain M: Role of
diffusion-weighted imaging and in vivo proton magnetic resonance
spectroscopy in the differential diagnosis of ring-enhancing
intracranial cystic mass lesions. J Comput Assist Tomogr.
28:540–547. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin LC, Chen YY, Lee WT, Chen HL and Yang
RC: Heat shock pretreatment attenuates sepsis-associated
encephalopathy in LPS-induced septic rats. Brain Dev. 32:371–377.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Van Zijl PC and Barker PB: Magnetic
resonance spectroscopy and spectroscopic imaging for the study of
brain metabolism. Ann N Y Acad Sci. 820:75–96. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bozza FA, Garteiser P, Oliveira MF, Doblas
S, Cranford R, Saunders D, Jones I, Towner RA and Castro-Faria-Neto
HC: Sepsis-associated encephalopathy: a magnetic resonance imaging
and spectroscopy study. J Cereb Blood Flow Metab. 30:440–448. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Messaris E, Memos N, Chatzigianni E,
Konstadoulakis MM, Menenakos E, Katsaragakis S, Voumvourakis C and
Androulakis G: Time-dependent mitochondrial-mediated programmed
neuronal cell death prolongs survival in sepsis. Crit Care Med.
32:1764–1770. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ayala A, Perl M, Venet F, Lomas-Neira J,
Swan R and Chung CS: Apoptosis in sepsis: mechanisms, clinical
impact and potential therapeutic targets. Curr Pharm Des.
14:1853–1859. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mouihate A and Pittman QJ:
Lipopolysaccharide-induced fever is dissociated from apoptotic cell
death in the rat brain. Brain Res. 805:95–103. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sharshar T, Gray F, de la Grandmaison
Lorin G, Hopkinson NS, Ross E, Dorandeu A, Orlikowski D, Raphael
JC, Gajdos P and Annane D: Apoptosis of neurons in cardiovascular
autonomic centres triggered by inducible nitric oxide synthase
after death from septic shock. Lancet. 362:1799–1805. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Davies DC: Blood-brain barrier breakdown
in septic encephalopathy and brain tumours. J Anat. 200:639–646.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Orihuela CJ, Fillon S, Smith-Sielicki SH,
El Kasmi KC, Gao G, Soulis K, Patil A, Murray PJ and Tuomanen EI:
Cell wall-mediated neuronal damage in early sepsis. Infect Immun.
74:3783–3789. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Papadopoulos MC, Lamb FJ, Moss RF, Davies
DC, Tighe D and Bennett ED: Faecal peritonitis causes oedema and
neuronal injury in pig cerebral cortex. Clin Sci (Lond).
96:461–466. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Undén J, Christensson B, Bellner J, Alling
C and Romner B: Serum S100B levels in patients with cerebral and
extracerebral infectious disease. Scand J Infect Dis. 36:10–13.
2004. View Article : Google Scholar : PubMed/NCBI
|