Introduction
Caveolae are 50–100-nm in size, non-clathrin-coated,
flask-shaped invaginations of the plasma membrane that are involved
in vesicular transport and signal transduction (1). Caveolins (CAVs) were identified as
essential proteins involved in the formation of invaginations
(2). Numerous studies have indicated
that CAVs associate with several signaling factors, including
heterotrimeric G-protein α-subunits, endothelial nitric oxide
synthase (eNOS), receptor and non-receptor tyrosine kinases and
protein kinase C (3–5). A previous study have suggested that
signaling interactions of CAVs with these factors are mediated by
the CAV scaffolding domain, which is a membrane-proximal region
(residues 82–101 in Cav-1) of the CAVs (6). Currently, Cav-1, −2 and −3 have been
identified as members of the CAV family (1). Cav-1 also exists in non-caveolar,
cellular or extracellular forms (1).
The Cav-1 isoform is particularly abundant in endothelial cells
(ECs), where it regulates various functions, including
transcytosis, permeability, vasculartone and angiogenesis (7). Previous results have demonstrated that
Cav-1 is a growth-inhibitory protein that may act as a tumor
suppressor (8,9). Cav-1 expression is downregulated in
some forms of cancer, including mesenchymal tumors and sarcomas
(8,10); however, in other cancer types; for
example, oral squamous cell carcinoma, Cav-1 expression is high
(11,12). These findings suggest that Cav-1 has
multiple actions in human cancer cells.
Angiogenesis is the process of generating novel
blood vessels derived as extensions from the existing vasculature
(13). The principal cells involved
are ECs, which line all blood vessels and constitute virtually the
entirety of capillaries (13).
Non-caveolar Cav-1 has an important role in the regulation of EC
proliferation, differentiation and tube formation (14). In addition, eNOS is a CAV-interacting
protein that has a central role in angiogenesis (15), and Cav-1 abundance and its cellular
distribution in ECs may be altered in nitric oxide (NO)-mediated
angiogenesis (16). Our previous
experimental study demonstrated that Cav-1 was important for
NO-mediated angiogenesis (17).
However, the exact molecular mechanisms of Cav-1 in the process of
angiogenesis have not been thoroughly explored.
Hepatocellular carcinoma (HCC) is one of the most
prevalent cancers worldwide, particularly in the Asia Pacific
region (18). Due to late diagnosis
and high rate metastasis of HCC, HCC is still associated with poor
survival rate (18). At present, the
5-year survival rate of individuals with HCC is very low at 34%
(19). The major risk factor for HCC
in China is infection with hepatitis B virus (HBV) (20).
The aim of the present study was to investigate the
expression and significance of Cav-1 in HBV-associated HCC. TRIzol
reagent was used for RNA extraction; semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR) and the mRNA
expression evels of Cav-1 mRNA were detected. Immunohistochemistry
analysis and microvessel counting was used for exploring the
expression of Cav-1, cluster of differentiation (CD)34 and vascular
endothelial growth factor (VEGF).
Materials and methods
Patients and tissue collection
The present study was approved by the Ethics
Committee of The Affiliated Drum Tower Hospital of Nanjing
University Medical School (Nanjing, China), and informed written
consent was obtained from all subjects. Tissue samples, including
HBV-associated HCC, non-tumor HBV-associated chronic hepatitis and
cirrhosis, were all obtained from 40 patients with HBV-associated
HCC (33 males and 7 females) who had consecutively undergone
surgical resections between June 2002 and June 2006 in our
hospital. The patients were selected according to the following
criteria: i) having primary HCC, ii) having a history of HBV
infection and tested positive for serum hepatitis B surface antigen
(HBsAg). All the 40 patients were diagnosed and histopathologically
confirmed with HCC (40 patients), including chronic hepatitis (11
patients) and cirrhosis (29 patients). The control normal liver
(non-tumor) specimens were obtained from patients (n=6; Group 1)
with metastatic liver carcinoma without HBV infection. The
corresponding non-tumor tissues were obtained from the same 40
patients with HBV-associated HCC, which were subsequently divided
into the HBV-associated chronic hepatitis group (n=11; Group 2) and
cirrhosis group (n=29; Group 3). The patients' clinical records and
histopathologic diagnoses were fully reviewed. The mean age of the
patients was 50±11 years. Tumor size varied from 2–15 cm in
diameter and the tumor diameter was determined as the longest
diameter of the specimen measured at the time of pathological
examination. Serum α-fetoprotein concentrations were measured using
an ELISA kit (EL0018; Huzhou Innoreagents Co., Ltd., Huzhou, China)
according to the manufacturer's instructions, with normal AFP
concentration defined as <20 ng/ml. Cancer tissues and adjacent
non-tumor liver tissues of the 40 patients with HBV-associated HCC
were excised from each surgical specimen immediately after liver
resection. Half of the tissue was flash-frozen in liquid nitrogen
for RT-PCR analysis. The other half of the tissue was fixed in 10%
neutral formalin for 2 weeks at 4°C and subjected to
histopathological examination and immunohistochemical study. All
the tissues were prepared from paraffin blocks as described below.
The scoring system of pathological grade and differentiation was
performed according to Edmonson Steiner grading system proposed in
1954. The following scoring was applied: Grade 1, minor
differentiation between tumor cells and hyperplastic liver cells;
Grade 2, tumor cells resemble normal hepatic cells while the nuclei
are larger and more hyperchromatic, and cell characteristics
indicate sharp, clear-cut borders; Grade 3, larger and more
hyperchromatic nuclei are present with a higher proportion of
nuclei to existing cytoplasm; and Grade 4, cells are filled with
nuclei that are intensely hyperchromatic. The diagnosis of tumors
with cancerous thrombi in the portal vein or intrahepatic
metastasis by computed tomography in HCC met the diagnosis criteria
of the American Association for the study of Liver Diseases
(21).
RNA extraction
Total RNA from frozen tissues was extracted using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The concentration of RNA
extracted was determined at wavelength of 260 nm using a
spectrophotometer (Eppendorf, Hamburg, Germany).
Semi-quantitative RT-PCR
The cycle number was set to 28, 30, 34 and 36 to
determine the plateau (22). cDNA
were synthesized using a Reverse Transcription System (Promega
Corp., Madison, WI, USA), according to the manufacturer's protocol.
Total RNA (1 µg) was reverse transcribed to first-strand cDNA in 20
µl of mixture containing 25 mM MgCl2 (4 µl), reverse
transcription 10X buffer (2 µl), 10 mM dNTP mixture (2 µl),
recombinant RNase inhibitor (0.5 µl), AMV reverse transcriptase (15
µl and Oligo (dT) primers (0.5 µg). The reaction conditions were as
follows: 42°C for 60 min, followed by 95°C for 5 min.
Semi-quantitative analysis for the expression of Cav-1 mRNA was
performed using RT-PCR technique and β-actin used as an internal
control (22). PCR was performed
using the following program: 94°C for 5 min, followed by 30 cycles
of 94°C for 30 sec, 58°C for 60 sec and 72°C for 45 sec. The
primers for RT-PCR were as follows: Cav-1, forward
5′-GACTTTGAAGATGTGATTGC-3′ and reverse 5′-AGATGGAATAGACACGGCTG-3′;
and β-actin, forward 5′-CTACAATGAGCTGCGTGTGGC-3′ and reverse
5′-CAGGTCCAGACGCAGGATGGC-3′. The PCR products were 254 and 275 bp,
respectively. RT-PCR was performed using a DNA thermal cycler MJ
Research PTC-200 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The PCR samples were analyzed using a 1% agarose gel and visualized
by ethidium bromide staining. The intensity of the bands was
measured by densitometry utilizing Tobias TBX1000 scanning
densitometer (Tobias Associates, Inc., Miami Beach, FL, USA).
Immunohistochemistry and microvessel
counting
A light microscope was utilized in the following
experiment. Serial sections (4-µm thick) from tumor and
corresponding non-tumor tissues tissue samples obtained from all
patients previously fixed in formalin were prepared from paraffin
blocks. Sections were deparaffinized and rehydrated in
Tris-buffered saline. Endogenous peroxidase activity was blocked
with 3% H2O2 for 10 min at room temperature.
Antigen retrieval was performed by microwave pretreatment with
Trisodium citrate 2.94 g, 0.2 M hydrochloric acid solution (22.0
ml), UltraPure sterile water, 0.1 M sodium hydroxide 1N solution
(pH=13, 0.1 M hydrochloric acid solution (pH=1, xylene and methanol
under 98°C prior to staining. Non-specific binding was blocked with
5% normal bovine serum (Jackson ImmunoResearch Laboratories, Inc.,
Shanghai, China) for 10 min at room temperature. The β-actin was
used as internal reference obtained from GenScript Co. Ltd.
(Nanjing, China; cat. no. A00702-100; 1:20). Subsequently, sections
were incubated with anti-CD 34 (1:30; cat. no. MS-363-P0; Leica
Microsystems Ltd., Milton Keynes, UK) at room temperature for 45
min, and anti-Cav-1 (1:100; cat. no. 610406; BD Biosciences;
Franklin Lakes, NJ, USA) and anti-vascular endothelial growth
factor (VEGF; 1:200; cat. no. SC-7269; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) at room temperature for 1 h. Subsequently,
corresponding secondary biotinylated immunoglobulin was applied and
then reacted with a streptavidin biotinylated horseradish
peroxidase complex (1:5,000; cat. no. ab96895; Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA) at 37°C for 30 min. The
sections were stained with a freshly prepared diaminobenzidine
solution and then counterstained with Mayer's hematoxylin. Negative
control was obtained by omitting the primary antibodies. A
semi-quantitative system was employed to evaluate the level of
Cav-1 and VEGF expression: Intensity was scored as absent (grade
0), weakly positive (grade 1), moderately positive (grade 2) or
strongly positive (grade 3) based on the proportion (percentage of
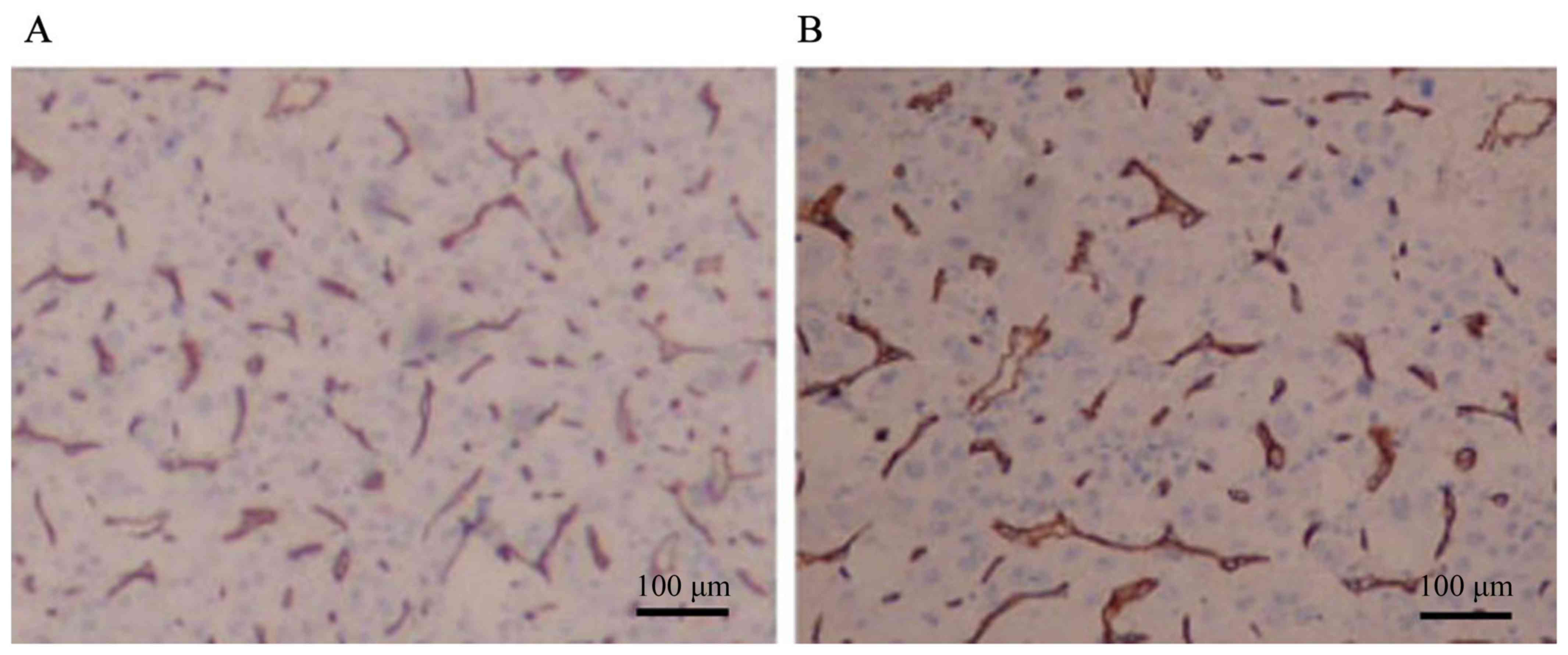
positive cells) and intensity, as described previously (23). Microvessel density (MVD) was
determined with CD34-stained slides using the procedure outlined by
Weidner et al (24).
Individual microvessels were counted in the area of highest
vascularity at magnification ×200 in three selected microscopic
fields. The microvessel count was expressed as the mean number of
vessels in the selected area.
Statistical analysis
All data were expressed as the mean ± standard
deviation as indicated. Data were analyzed using SPSS 16.0
statistical software (SPSS, Inc., Chicago, IL, USA). Statistical
comparisons were made between two groups using the Student's test
and between multiple groups with one-way analysis of variance
followed by Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Cav-1 mRNA expression levels
Cav-1 gene expression was evaluated in different
types of liver diseases Cav-1 mRNA expression was detected in 1/6
(16.7%) control non-tumor normal liver tissues from patients with
metastatic carcinoma without HBV infection, 34/40 (85.0%) non-tumor
liver tissues and 37/40 (92.5%) HBV-associated HCC. The Cav-1 mRNA
expression levels in the control normal liver tissues were negative
or very low (Figs. 1 and 2). However, the expression level of Cav-1
mRNA was increased in HBV-associated chronic hepatitis (Group 2),
HBV-associated cirrhosis (Group 3) and HBV-associated HCC compared
with non-tumor normal liver tissue from patients with metastatic
liver carcinoma without HBV infection (Group 1; Figs. 1 and 2). The expression levels of Cav-1 mRNA in
HBV-associated chronic hepatitis and cirrhosis were significantly
elevated compared with that in non-tumor normal liver tissue from
patients with metastatic carcinoma without HBV infection
(P<0.001); however, there was no significant difference between
HBV-associated chronic hepatitis and cirrhosis (P=0.076). The
expression levels of Cav-1 mRNA in HBV-associated HCC were
significantly decreased compared with that of corresponding
non-tumor tissues in HBV-associated chronic hepatitis and cirrhosis
(P=0.019 and P=0.045, respectively). But there was no significant
difference in the expression levels of Cav-1 mRNA in HBV-associated
HCC between Group 2 and Group 3 (P=0.41; Fig. 2).
Immunohistochemical analysis for Cav-1
and VEGF in HBV-associated HCC and their correlation with
angiogenesis
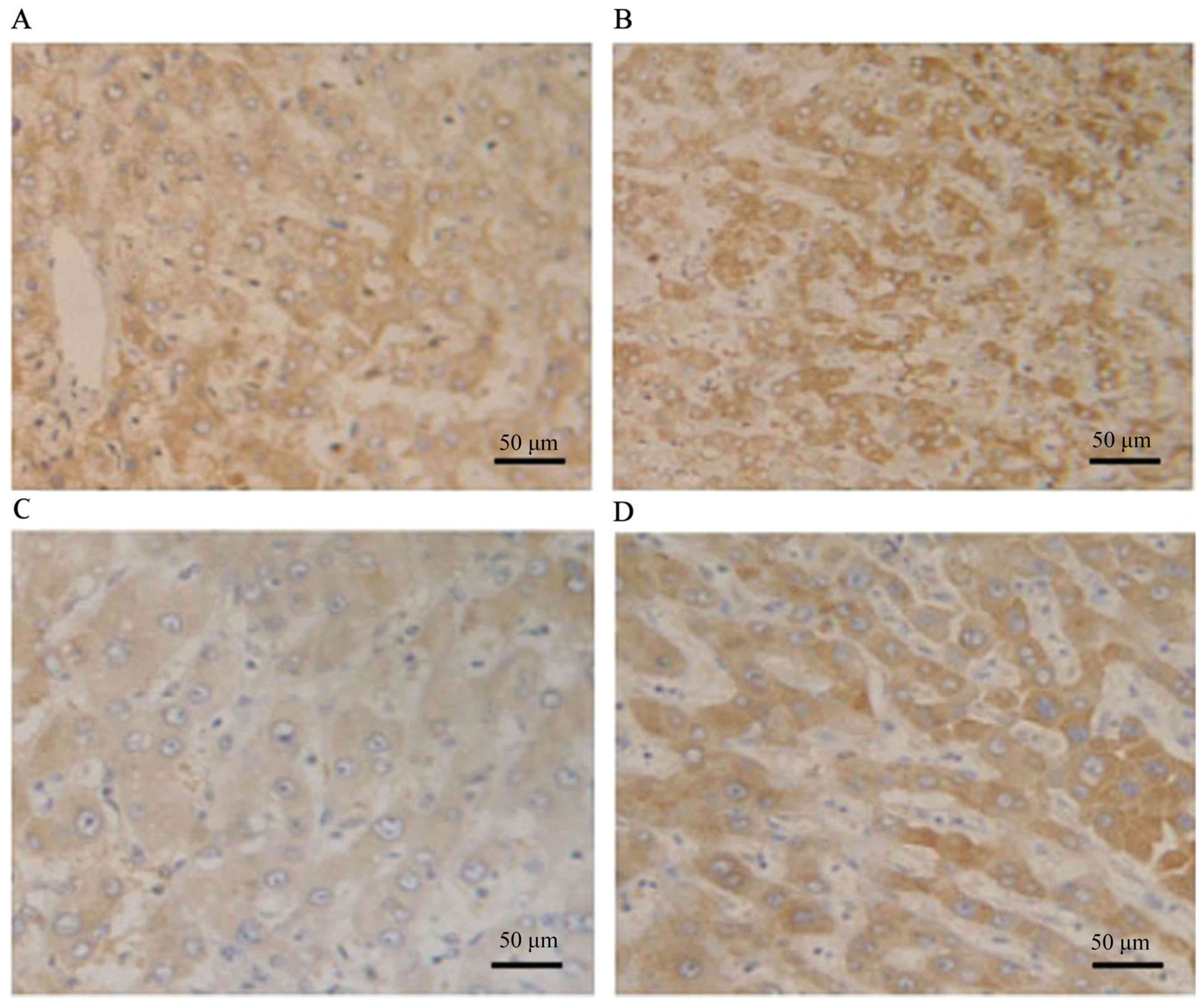
Cav-1 was expressed primarily in the cytoplasm of
tumor cells obtained from HBV-associated HCC tissues, as evidenced
by the presence of stained granular immunoreaction products. The
majority of tumors exhibited extensive staining for VEGF: seven
tumors (17.5%) were grade 0, nine (22.5%) were grade 1, 13 (32.5%)
were grade 2 and 11 (27.5%) were grade 3 (Fig. 3A and B). Cav-1 immunoreactivity was
indicated in 32/40 (80%) HBV-associated HCC tissues: 8 tumors (20%)
were grade 0, 11 (27.5%) were grade 1, 9 (22.5%) were grade 2 and
12 (30%) were grade 3 (Fig. 3C and
D). MVD was 145.2±16.2 (Fig. 4).
The expression levels of Cav-1 and VEGF significantly correlated
with MVD (rs=0.46, P=0.01; and rs=0.31,
P=0.05, respectively), which was not shown in figure.
Correlation of the expression of Cav-1
mRNA and MVD with clinicopathological characteristics of
HBV-associated HCC
The patients' clinical records and histopathologic
diagnoses were fully reviewed. As demonstrated in Table I, the expression levels of Cav-1 mRNA
and MVD exhibited a significant association with metastasis
(P=0.031 and P=0.046, respectively). However, no significant
association was noted between Cav-1 mRNA expression levels and MVD
and the other clinicopathological variables.
 | Table I.Association between Cav-1 mRNA
expression and MVD and clinicopathological features of the
patients. |
Table I.
Association between Cav-1 mRNA
expression and MVD and clinicopathological features of the
patients.
| Variables | n | Cav-1
expressiona | P-value | MVD | P-value |
|---|
| Age, years |
|
| 0.72 |
| 0.45 |
|
<60 | 26 |
0.72±0.18 |
|
148.4±14.1 |
|
|
>60 | 14 |
0.65±0.17 |
|
139.3±10.3 |
|
| Gender |
|
| 0.42 |
| 0.67 |
|
Male | 33 |
0.68±0.20 |
|
143.3±17.4 |
|
|
Female | 7 |
0.75±0.13 |
|
154.4±20.1 |
|
| α-fetoprotein,
µg/l |
|
| 0.12 |
| 0.98 |
|
<363 | 16 |
0.63±0.19 |
|
141.1±16.4 |
|
|
≥363 | 24 |
0.73±0.12 |
|
148.3±19.2 |
|
| Tumor diameter,
cm |
|
| 0.25 |
| 0.37 |
|
<5 | 23 |
0.65±0.21 |
|
138.1±18.8 |
|
| ≥5 | 17 |
0.75±0.11 |
|
152.6±17.1 |
|
| Edmondson grading
system |
|
| 0.09 |
| 0.05 |
|
I+II | 24 |
0.63±0.18 |
|
134.9±24.1 |
|
|
IIIa+IIIb | 16 |
0.78±0.12 |
|
163.2±14.7 |
|
|
Differentiation |
|
| 0.06 |
| 0.41 |
|
Well-differentiated | 6 |
0.60±0.10 |
|
141.7±20.4 |
|
|
Moderately + poorly
differentiated | 34 |
0.71±0.12 |
|
149.3±17.7 |
|
| Metastasis |
|
| 0.03 |
| 0.05 |
|
Yes | 19 |
0.80±0.18 |
|
164.8±33.9 |
|
| No | 21 |
0.59±0.11 |
|
127.2±16.5 |
|
Discussion
A study by Koleske et al (25) demonstrated that Cav-1 mRNA and
protein expression levels were reduced in NIH 3T3 cells transformed
by various oncogenes, and caveolae were also absent from these
transformed cells. A previous study has indicated that Cav-1 gene
mutations were identified in breast carcinomas and that the Cav-1
gene localizes to a suspected tumor suppressor locus on chromosome
7q31.1, which is commonly deleted in a variety of types of human
cancer (26). Prior reports have
suggested that Cav-1 may function as a tumor suppressor gene
(27,28). However, this finding is inconsistent
with the fact that Cav-1 is highly expressed in multiple cancer
types and cancer cell lines (29,30). In
the present study, positive Cav-1 mRNA expression was detected in
37/40 tumors, and the expression of Cav-1 was elevated in
HBV-associated HCC compared with non-tumor liver tissues from
patients with metastatic liver carcinoma without HBV infection. In
addition, 32/40 (80%) HBV-associated HCC specimens indicated Cav-1
immunoreactivity in tumor cells. These results suggested that
increased Cav-1 expression was detected in HBV-associated HCC
compared with the non-tumor tissue in group 1. Additionally, the
results of the present study also demonstrated that Cav-1
expression was downregulated in HBV-associated HCC tissues compared
with the corresponding non-tumor tissues. The exact reason of the
decreased expression levels in HBV-associated HCC compared with
corresponding non-tumor tissue was unclear; however, a previous
study demonstrated that Cav-1 gene disruption was involved in
promoting mammary tumor growth and enhancing cell metastasis
(31), which may explain the reduced
Cav-1 expression observed in the HBV-associated HCC specimens.
These effects of Cav-1 in different cancer specimens may be
mediated by different regions of the Cav-1 molecule (32). Although various studies have
suggested the correlation of Cav-1 with cancer, the exact role of
Cav-1 in cancer remains to be elucidated (32).
Numerous studies have demonstrated that Cav-1 may
have an important role in the carcinogenesis of HCC (33,34). A
study previously demonstrated that the expression of Cav-1 in
cirrhotic livers was markedly enhanced at the protein and mRNA
levels, whereas Cav-1 was almost undetectable in control liver
tissue (35). Macroregenerative and
dysplastic nodules (MDNs) are HCC precursor lesions and exhibit
distinct vascular profiles relative to adjacent cirrhotic liver
(36). It was determined that Cav-1
expression levels increased during the progression from normal to
cirrhotic liver, and further increased in MDNs, whereas hepatitis C
virus (HCV)-associated HCC liver exhibited similar or decreased
Cav-1 expression relative to adjacent non-neoplastic liver
(37). These findings suggested that
Cav-1 may have a direct role in malignant transformation of
hepatocytes. However, these studies were focused on HCV-associated
tissues. The present results demonstrated that the expression of
Cav-1 was upregulated in HBV-associated HCC and the percentage and
level of detectable Cav-1 mRNA was increased in non-tumor and
HBV-associated HCC liver tissues compared with normal liver
tissues. From the present results, it may be concluded that Cav-1
has a direct role in the malignant transformation of HBV-associated
HCC.
Cav-1 has been identified to be a
metastatic-associated gene with an independent prognostic value for
various types of cancer (29,33,38).
A study by Williams et al (39) revealed that loss of Cav-1 attenuated
prostate development by significantly reducing primary tumor burden
and metastatic disease in a transgenic prostate cancer model.
However, another study demonstrated that Cav-1 gene disruption
promoted mammary tumor growth and enhanced cell metastasis
(31). These studies indicate that
Cav-1 has tissue-or cell type-specific roles with regard to
tumorigenesis. In the present study, the expression of Cav-1 was
significantly correlated with metastasis, which suggests that Cav-1
may act in the progression of HBV-associated HCC.
The role of Cav-1 in angiogenesis has only been
partially defined. A previous study indicated that angiogenic
inhibition in pancreatic cancer was associated with the
upregulation of Cav-1 (40).
Furthermore, endothelial-specific expression of Cav-1 has been
suggested to impair eNOS activation, endothelial barrier function
and angiogenic responses to exogenous VEGF (41). Additionally, other research has shown
that Cav-1 was essential for capillary formation but had different
roles depending on the stage of angiogenesis (42).
HCCs are hypervascular tumors that exhibit
distinctive vascular profiles relative to the surrounding liver in
which they arise (36).
HCV-associated HCC, in which Cav-1 may be involved, was indicated
to be associated with angiogenesis in a previous study (36). A study by Mazzanti et al
(43) demonstrated that angiogenesis
was significantly varied in HCV-positive patients compared with
HBV-infected subjects or controls. In light of these findings, the
present study investigated the association between the expression
of Cav-1 and angiogenesis in HBV-associated HCC. It has been
demonstrated that VEGF-stimulated phosphorylation of extracellular
signal regulated kinase 1/2 and eNOS was abrogated in
Cav−/− ECs, but enhanced in the Cav+/+ mice
and ECs (41,44). Cav-1 expression is critical for
VEGF-induced angiogenesis (35).
MVD, an indicator of angiogenesis, has been correlated with Cav-1
expression in clear cell renal cell carcinoma (45). Similarly, the present study
demonstrated a strong association between Cav-1 expression and MVD
in HBV-associated HCC patients. As angiogenesis is fundamental to
the growth and metastasis of solid tumors, we speculate that the
increasing level of Cav-1 may act in the progression of
HBV-associated HCC by affecting angiogenesis.
In conclusion, the present results indicated that
Cav-1 may have an important role in the carcinogenesis and
progression of HBV-associated HCC. The results suggested that Cav-1
may be associated with angiogenesis of HCC, and therefore Cav-1 may
be an important target of anti-angiogenic therapy of HCC.
Acknowledgements
The authors would like to thank Professor Min Xie
(The Affiliated Drum Tower Hospital of Nanjing University Medical
School, Nanjing, China) for assistance with the experiments and
valuable discussion.
References
|
1
|
Cohen AW, Hnasko R, Schubert W and Lisanti
MP: Role of caveolae and caveolins in health and disease. Physiol
Rev. 84:1341–1379. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Busija AR, Patel HH and Insel PA:
Caveolins and cavins in the trafficking, maturation, and
degradation of caveolae: Implications for cell physiology. Am J
Physiol Cell Physiol. 312:C459–C477. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okamoto T, Schlegel A, Scherer PE and
Lisanti MP: Caveolins, a family of scaffolding proteins for
organizing ‘preassembled signaling complexes’ at the plasma
membrane. J Biol Chem. 273:5419–5422. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gupta VK, You Y, Klistorner A and Graham
SL: Shp-2 regulates the TrkB receptor activity in the retinal
ganglion cells under glaucomatous stress. Biochim Biophys Acta.
1822:1643–1649. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Trane AE, Hiob MA, Uy T, Pavlov D and
Bernatchez P: Caveolin-1 scaffolding domain residue phenylalanine
92 modulates Akt signaling. Eur J Pharmacol. 766:46–55. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen F, Barman S, Yu Y, Haigh S, Wang Y,
Black SM, Rafikov R, Dou H, Bagi Z, Han W, et al: Caveolin-1 is a
negative regulator of NADPH oxidase-derived reactive oxygen
species. Free Radic Biol Med. 73:201–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sowa G: Regulation of cell signaling and
function by endothelial caveolins: Implications in disease. Transl
Med (Sunnyvale). (Suppl 8):pii: 001. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wiechen K, Sers C, Agoulnik AI, Arlt K,
Dietel M, Schlag PM and Schneider U: Down-regulation of caveolin-1,
a candidate tumor suppressor gene, in sarcomas. Am J Pathol.
158:833–839. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi L, Chen XM, Wang L, Zhang L and Chen
Z: Expression of caveolin-1 in mucoepidermoid carcinoma of the
salivary glands: Correlation with vascular endothelial growth
factor, microvessel density, and clinical outcome. Cancer.
109:1523–1531. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Riwaldt S, Bauer J, Pietsch J, Braun M,
Segerer J, Schwarzwälder A, Corydon TJ, Infanger M and Grimm D: The
importance of caveolin-1 as key-regulator of three-dimensional
growth in thyroid cancer cells cultured under real and simulated
microgravity conditions. Int J Mol Sci. 16:28296–28310. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang CF, Yu GT, Wang WM, Liu B and Sun
ZJ: Prognostic and predictive values of SPP1, PAI and caveolin-1 in
patients with oral squamous cell carcinoma. Int J Clin Exp Pathol.
7:6032–6039. 2014.PubMed/NCBI
|
|
12
|
Auzair LB, Vincent-Chong VK, Ghani WM,
Kallarakkal TG, Ramanathan A, Lee CE, Rahman ZA, Ismail SM, Abraham
MT and Zain RB: Caveolin 1 (Cav-1) and actin-related protein 2/3
complex, subunit 1B (ARPC1B) expressions as prognostic indicators
for oral squamous cell carcinoma (OSCC). Eur Arch Otorhinolaryngol.
273:1885–1893. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Profirovic J, Strekalova E, Urao N,
Krbanjevic A, Andreeva AV, Varadarajan S, Fukai T, Hen R,
Ushio-Fukai M and Voyno-Yasenetskaya TA: A novel regulator of
angiogenesis in endothelial cells: 5-hydroxytriptamine 4 receptor.
Angiogenesis. 16:15–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nassar ZD, Hill MM, Parton RG, Francois M
and Parat MO: Non-caveolar caveolin-1 expression in prostate cancer
cells promotes lymphangiogenesis. Oncoscience. 2:635–645. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luo JD and Chen AF: Nitric oxide: A newly
discovered function on wound healing. Acta Pharmacol Sin.
26:259–264. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Phillips PG and Birnby LM: Nitric oxide
modulates caveolin-1 and matrix metalloproteinase-9 expression and
distribution at the endothelial cell/tumor cell interface. Am J
Physiol Lung Cell Mol Physiol. 286:L1055–L1065. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pan YM, Yao YZ, Zhu ZH, Sun XT, Qiu YD and
Ding YT: Caveolin-1 is important for nitric-oxide angiogenesis in
fibrin gels with human umbilical vein endothelial cells. Acta
Pharmacol Sin. 27:1567–1574. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Niu ZS, Niu XJ and Wang WH: Genetic
alterations in hepatocellular carcinoma: An update. World J
Gastroenterol. 22:9069–9095. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Riccardo Lencioni, Adrian M and Di
Bisceglie EASL: An update of angiogenesis in endothelial cells:
5-hypatocellular carcinoma. J Hepatology. 56:9082012.
|
|
20
|
Sarin SK, Kumar M, Lau GK, Abbas Z, Chan
HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, et al:
Asian-Pacific clinical practice guidelines on the management of
hepatitis B: A 2015 update. Hepatol Int. 10:1–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marone M, Mozzetti S, De Ritis D, Pierelli
L and Scambia G: Semiquantitative RT-PCR analysis to assess the
expression levels of multiple transcripts from the same sample.
Biol Proced Onlin. 3:19–25. 2001. View
Article : Google Scholar
|
|
23
|
Friedrichs K, Gluba S, Eidtmann H and
Jonat W: Overexpression of p53 and prognosis in breast cancer.
Cancer. 72:3641–3647. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weidner N, Carroll PR, Flax J, Blumenfeld
W and Folkman J: Tumor angiogenesis correlates with metastasis in
invasive prostate carcinoma. Am J Pathol. 143:401–409.
1993.PubMed/NCBI
|
|
25
|
Koleske AJ, Baltimore D and Lisanti MP:
Reduction of caveolin and caveolae in oncogenically transformed
cells. Proc Natl Acad Sci USA. 92:1381–1385. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hayashi K, Matsuda S, Machida K, Yamamoto
T, Fukuda Y, Nimura Y, Hayakawa T and Hamaguchi M: Invasion
activating caveolin-1 mutation in human scirrhous breast cancer.
Cancer Res. 61:2361–2364. 2001.PubMed/NCBI
|
|
27
|
Fu P, Chen F, Pan Q, Zhao X, Zhao C, Cho
WC and Chen H: The different functions and clinical significances
of caveolin-1 in human adenocarcinoma and squamous cell carcinoma.
Onco Targets Ther. 10:819–835. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Trimmer C, Sotgia F, Whitaker-Menezes D,
Balliet RM, Eaton G, Martinez-Outschoorn UE, Pavlides S, Howell A,
Iozzo RV, Pestell RG, et al: Caveolin-1 and mitochondrial SOD2
(MnSOD) function as tumor suppressors in the stromal
microenvironment: A new genetically tractable model for human
cancer associated fibroblasts. Cancer Biol Ther. 11:383–394. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang R, He W, Li Z, Chang W, Xin Y and
Huang T: Caveolin-1 functions as a key regulator of
17β-estradiol-mediated autophagy and apoptosis in BT474 breast
cancer cells. Int J Mol Med. 34:822–827. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sugie S, Mukai S, Yamasaki K, Kamibeppu T,
Tsukino H and Kamoto T: Significant association of caveolin-1 and
caveolin-2 with prostate cancer progression. Cancer Genomics
Proteomics. 12:391–396. 2015.PubMed/NCBI
|
|
31
|
Williams TM, Medina F, Badano I, Hazan RB,
Hutchinson J, Muller WJ, Chopra NG, Scherer PE, Pestell RG and
Lisanti MP: Caveolin-1 gene disruption promotes mammary
tumorigenesis and dramatically enhances lung metastasis in vivo.
Role of Cav-1 in cell invasiveness and matrix metalloproteinase
(MMP-2/9) secretion. J Biol Chem. 279:51630–51646. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gai X, Lu Z, Tu K, Liang Z and Zheng X:
Caveolin-1 Is up-regulated by GLI1 and contributes to GLI1-driven
EMT in hepatocellular carcinoma. PLoS One. 9:e845512014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu WR, Jin L, Tian MX, Jiang XF, Yang LX,
Ding ZB, Shen YH, Peng YF, Gao DM, Zhou J, et al: Caveolin-1
promotes tumor growth and metastasis via autophagy inhibition in
hepatocellular carcinoma. Clin Res Hepatol Gastroenterol.
40:169–178. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang W, Feng X, Zhang S, Ren Z, Liu Y,
Yang B, Lv B, Cai Y, Xia J and Ge N: Caveolin-1 confers resistance
of hepatoma cells to anoikis by activating IGF-1 pathway. Cell
Physiol Biochem. 36:1223–1236. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yokomori H, Oda M, Yoshimura K, Nomura M,
Wakabayashi G, Kitajima M and Ishii H: Elevated expression of
caveolin-1 at protein and mRNA level in human cirrhotic liver:
Relation with nitric oxide. J Gastroenterol. 38:854–860. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pan YM, Yao YZ, Zhu ZH, Sun XT, Qiu YD and
Ding YT: Caveolin-1 is important for nitric oxide-mediated
angiogenesis in fibrin gels with human umbilical vein endothelial
cells. Acta Pharmacol Sin. 27:1567–1574. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yerian LM, Anders RA, Tretiakova M and
Hart J: Caveolin and thrombospondin expression during
hepatocellular carcinogenesis. Am J Surg Pathol. 28:357–364. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang WS, Wang RJ, Ding JL, Liu CY and
Wang JH: Caveolin-1: A novel biomarker for prostate cancer.
Zhonghua Nan Ke Xue. 18:635–638. 2012.(In Chinese). PubMed/NCBI
|
|
39
|
Williams TM, Hassan GS, Li J, Cohen AW,
Medina F, Frank PG, Pestell RG, Di Vizio D, Loda M and Lisanti MP:
Caveolin-1 promotes tumor progression in an autochthonous mouse
model of prostate cancer: Genetic ablation of Cav-1 delays advanced
prostate tumor development in tramp mice. J Biol Chem.
280:25134–25145. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bocci G, Fioravanti A, Orlandi P, Di
Desidero T, Natale G, Fanelli G, Viacava P, Naccarato AG, Francia G
and Danesi R: Metronomic ceramide analogs inhibit angiogenesis in
pancreatic cancer through up-regulation of caveolin-1 and
thrombospondin-1 and down-regulation of cyclin D1. Neoplasia.
14:833–845. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bauer PM, Yu J, Chen Y, Hickey R,
Bernatchez PN, Looft-Wilson R, Huang Y, Giordano F, Stan RV and
Sessa WC: Endothelial-specific expression of caveolin-1 impairs
microvascular permeability and angiogenesis. Proc Natl Acad Sci
USA. 102:204–209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu J, Razani B, Tang S, Terman BI, Ware
JA and Lisanti MP: Angiogenesis activators and inhibitors
differentially regulate caveolin-1 expression and caveolae
formation in vascular endothelial cells. Angiogenesis inhibitors
block vascular endothelial growth factor-induced down-regulation of
caveolin-1. J Biol Chem. 274:15781–15785. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mazzanti R, Messerini L, Monsacchi L,
Buzzelli G, Zignego AL, Foschi M, Monti M, Laffi G, Morbidelli L,
Fantappié O, et al: Chronic viral hepatitis induced by hepatitis C
but not hepatitis B virus infection correlates with increased liver
angiogenesis. Hepatology. 25:229–234. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sonveau X, Martinive P, DeWever J, Batova
Z, Daneau G, Pelat M, Ghisdal P, Grégoire V, Dessy C, Balligand JL
and Feron O: Caveolin-1 expression is critical for vascular
endothelial growth factor-induced ischemic hindlimb
collateralization and nitric oxide-mediated angiogenesis. Circ Res.
95:154–161. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Joo HJ, Oh DK, Kim YS, Lee KB and Kim SJ:
Increased expression of caveolin-1 and microvessel density
correlates with metastasis and poor prognosis in clear cell renal
cell carcinoma. BJU Int. 93:291–296. 2004. View Article : Google Scholar : PubMed/NCBI
|