Introduction
As a common renal disease in clinical practice,
acute renal injury is caused by renal ischemia-reperfusion.
Ischemia/reperfusion is the determinant factor for the early
functional recovery of renal transplantation (1,2). As a
highly perfused organ, kidney is vulnerable to ischemic injury.
Studies have shown that renal ischemia for more than 20 min will
cause damage, ischemia for 20–40 min is reversible, but reperfusion
after ischemia for more than 40 min can cause permanent damage
(3). Studies have shown that the
development of tolerance of organs to ischemia can inhibit or
reduced the ischemia-reperfusion injury (4). Takahashi-Sato et al (5) carried out a study to explore the
effects of different preconditioning and postconditioning methods
on ischemia/reperfusion in the heart. They found that
postconditioning had a protective effect on reperfusion after
cardiac ischemia (6–8). However, there is no research on the
effect of preconditioning and postconditioning on renal
ischemia-reperfusion. It was found (9) that Bcl-2 and Bax were involved in the
process of ischemia-reperfusion in the heart, and Bcl-2 and Bax may
be related to the protective effect of postconditioning on
ischemia-reperfusion injury in the heart. In this study, rat
ischemia-reperfusion model was established to investigate the
protective effects of preconditioning on rats with
ischemia-reperfusion, and to test whether Bcl-2 and Bax were
involved in that process. This study aimed to provide new insights
into the pathogenesis and clinical treatment of renal
ischemia-reperfusion injury.
Materials and methods
Instruments and materials
Creatinine (Cr) assay kit (R&D Systems,
Minneapolis, MN, USA); blood urea nitrogen (BUN) assay kit (R&D
Systems); xanthine oxidase assay kit used to determine the activity
of superoxide dismutase (SOD; R&D Systems); TUNEL kit (R&D
Systems); dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO,
USA), TRIzol kit (Invitrogen, Carlsbad, CA, USA), reverse
transcription kit (Invitrogen); rabbit anti-Bax-beta, rabbit
anti-Bax and rabbit anti-GAPDH primary antibodies, and horseradish
peroxidase labeled anti-rabbit secondary antibody (Cell Signaling
Technology, Danvers, MA, USA); ECL luminescent substrate
(Invitrogen); color developing powder (Invitrogen); pipettes
(Eppendorf, Hamburg, Germany); PCR instrument (Applied Biosystems,
Inc., Foster City, CA, USA); UV imaging system (Biometra GmbH,
Göttingen, Germany); electronic balance (Sartorious BP121S;
Sartorius AG, Goettingen, Germany); −80°C refrigerator (Thermo
Fisher Scientific, Schwerte, Germany); low temperature centrifuge
(Thermo Fisher Scientific) and frozen slicers (Leica Microsystems,
Wetzlar, Germany). The sources of other related instruments and
reagents are described in the relevant section.
Experimental animals and grouping
Male Sprague-Dawley (SD) rats (220–250 g) were
purchased from the Shanghai Medical Laboratory Animal Center.
Laboratory animal certificate number: SCXK (Shanghai) 2013–0015.
Rats were raise in quiet environment with free access to food and
water following the circadian rhythm for 1 week to adapt to the
environment. Thirty-six SD rats were randomly divided into three
groups (n=12) including sham operation (S) group,
ischemia-reperfusion group (I/R) group and ischemic preconditioning
(IP) group.
Establishment of renal
ischemia-reperfusion model
Rats were fasted for 12 h before surgery. After
anesthesia with intraperitoneal injection of 4% chloral hydrate at
a dose of 10 ml/kg, abdominal hair was removed and an incision was
made on abdomen along the mediventral line to open abdominal
cavity. Renal capsule was bluntly separated to separate the
kidneys. Arterial clip was then used to clip bilateral kidney
pedicles. The dark red color of kidneys indicated the successfully
established ischemia model. In I/R group, bilateral renal pedicles
were clipped for 45 min, followed by perfusion for 6 h to establish
I/R model. Both kidneys in rats of S group were separated and
exposed for 45 min, but renal pedicles were not clipped. In IP
group, bilateral renal pedicles were clipped for 5 min, followed by
perfusion for 5 min, this procedure was repeated 3 times, after
that rats were subjected to the treatment described in the I/R
group.
Sample collection and preparation
Peripheral blood was collected after reperfusion for
6 h. Blood samples were centrifuged 2,650 × g for 15 min to collect
supernatant. Supernatant was transferred to tubes and stored at
−80°C. After blood sample collection, rats in each group were
sacrificed, and both kidneys were separated and fixed in 10%
formalin for subsequent pathologic examination and other
studies.
Renal tissue injury scoring
After H&E staining, pathological changes were
observed under optical microscope (BX-42; Olympus, Tokyo, Japan)
and scores of renal pathological damage were evaluated. Scoring was
performed according to the standard of renal injury: normal renal
tissue, 0 point; area of renal tissue damage in renal tubular area
<25%, 1 point; area of renal tissue damage in renal tubular
between 25 and 50%, 2 points; area of renal tissue damage in renal
tubular between 50 and 75%, 3 points; area of renal tissue damage
in renal tubular between >75%, 4 points.
Determination of levels of Cr and BUN
and activity of SOD in serum
Contents of Cr and BUN in serum of rats in each
group were determined according to the instructions of the Cr assay
kit and BUN assay kit. Cr content was expressed as number + µmg/ml
and BUN content expressed as number + mmol/ml. SOD activity in
serum of rats in each group was determined according to the
instructions of SOD activity assay kit and expressed as number +
U/mg.
TUNEL to detect the apoptosis of rat
renal cells
TUNEL assay was performed according to the
instructions of kit and apoptotic cells were observed under an
optical microscope. Cells with brown nucleus were positive cells.
Five visual fields were randomly selected (magnification, ×400) to
count the number of apoptotic cells. The proportion of the number
apoptotic cells to the number of total cells was used as apoptotic
index.
Semi-quantitative PCR to detect the
expression of related mRNA
Total RNA was extracted from renal tissue using the
TRIzol kit. The quality of total RNA was checked by agarose gel
electrophoresis. Only RNA samples with satisfactory qualities were
used in subsequent experiments. Reverse transcription was performed
using a kit to synthesize cDNA and expression levels of Bax and
Bcl-2 were detected by semi-quantitative PCR with GAPDH as
endogenous control. Reaction conditions were: 95°C for 4 min,
followed by 35 cycles of 95°C for 30 sec, 64°C for 25 sec and 72°C
for 30 sec. Primers were synthesized by Tiangen Biotech Co., Ltd.
(Beijing, China). All primers are listed in Table I. PCR products were subjected to
agarose gel electrophoresis and results were observed using a UV
imaging system. The relative expression levels of Bax and Bcl-2
were represented by the ratio of Bax/GAPDH and Bcl-2/GAPDH.
 | Table I.Primers used in PCR. |
Table I.
Primers used in PCR.
|
| Sequences |
|---|
| Bax | Forward:
5′-ATCCAGAGACAAGACATGTAC-3′ |
|
| Reverse:
5′-TTCAGATGTTCTAAGCCTACGG-3′ |
| Bcl-2 | Forward:
5′-TGGCGGTTTGCGGTGGAC-3′ |
|
| Reverse:
5′-CCAGTGCAGGGTCCGAGGT-3′ |
| GAPDH | Forward:
5′-GATGATTGGCATGGCTTT-3′ |
|
| Reverse:
5′-CACCTTCCGTTCCAGTTT-3′ |
Western blot analysis to detect the
expression of related proteins
Renal tissue was mixed with RIPA lysate with a ratio
of 100 mg:1 ml. The mixture was centrifuged (10,500 × g) at 4°C for
10 min to collect the supernatant. Protein concentration was
measured using the DAB Protein Quantitative kit (Invitrogen). After
that, protein samples were subjected to SDS-PAGE gel
electrophoresis, followed by transmembrane to PVDF membrane. After
blocking with 5% skim milk, membranes were incubated with primary
rabbit polyclonal Bax antibody (dilution, 1:500; cat. no. ab53154)
and rabbit monoclonal Bcl-2 antibody (dilution, 1:500; cat. no.
ab32124) overnight at 4°C. After washing with TBST 3 times, 5 min
for each time, membranes were incubated with secondary goat
anti-rabbit (HRP) IgG antibody (dilution, 1:2000; cat. no. ab6721)
for 2 h. All antibodies were all purchased from Abcam (Cambridge,
MA, USA). After washing with TBST 3 times, proper amount of ECL
luminescent substrate (ratio of A and B was 1:1) was added and
incubated with the membranes in the dark. The results were scanned
and processed by ImageJ to calculate the expression level of each
protein.
Statistical analysis
Data were expressed as mean ± standard deviation,
and processed using the SPSS 19.0 software (SPSS, Inc., Chicago,
IL, USA). Comparisons between 2 groups were performed using t-test,
and comparisons among multiple groups were performed using variance
analysis. If the variance was homogeneous, the comparisons between
two groups were performed using Bonferronic method. If the variance
was not homogeneous, Welch method was used. Multiple comparisons
were performed using Dunnett's T3 method. P<0.05 was considered
to be statistically significant.
Results
Changes in renal function in rats
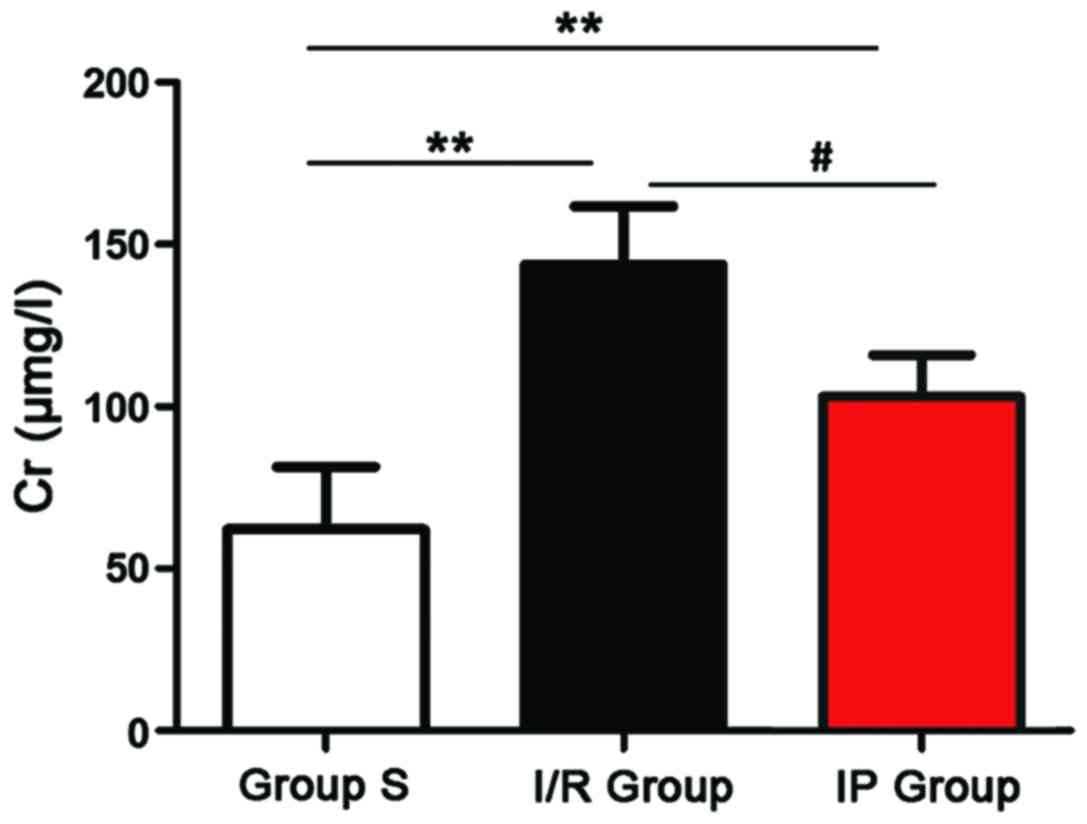
Levels of Cr and BUN in peripheral blood of rats in
each group were detected by Cr and BUN detection kit. As shown in
Figs. 1 and 2, compared with group S, levels of Cr and
BUN were significantly increased in I/R group and IP group
(P<0.01). Compared with IP group, levels of Cr and BUN were
significantly increased in the I/R group (P<0.01).
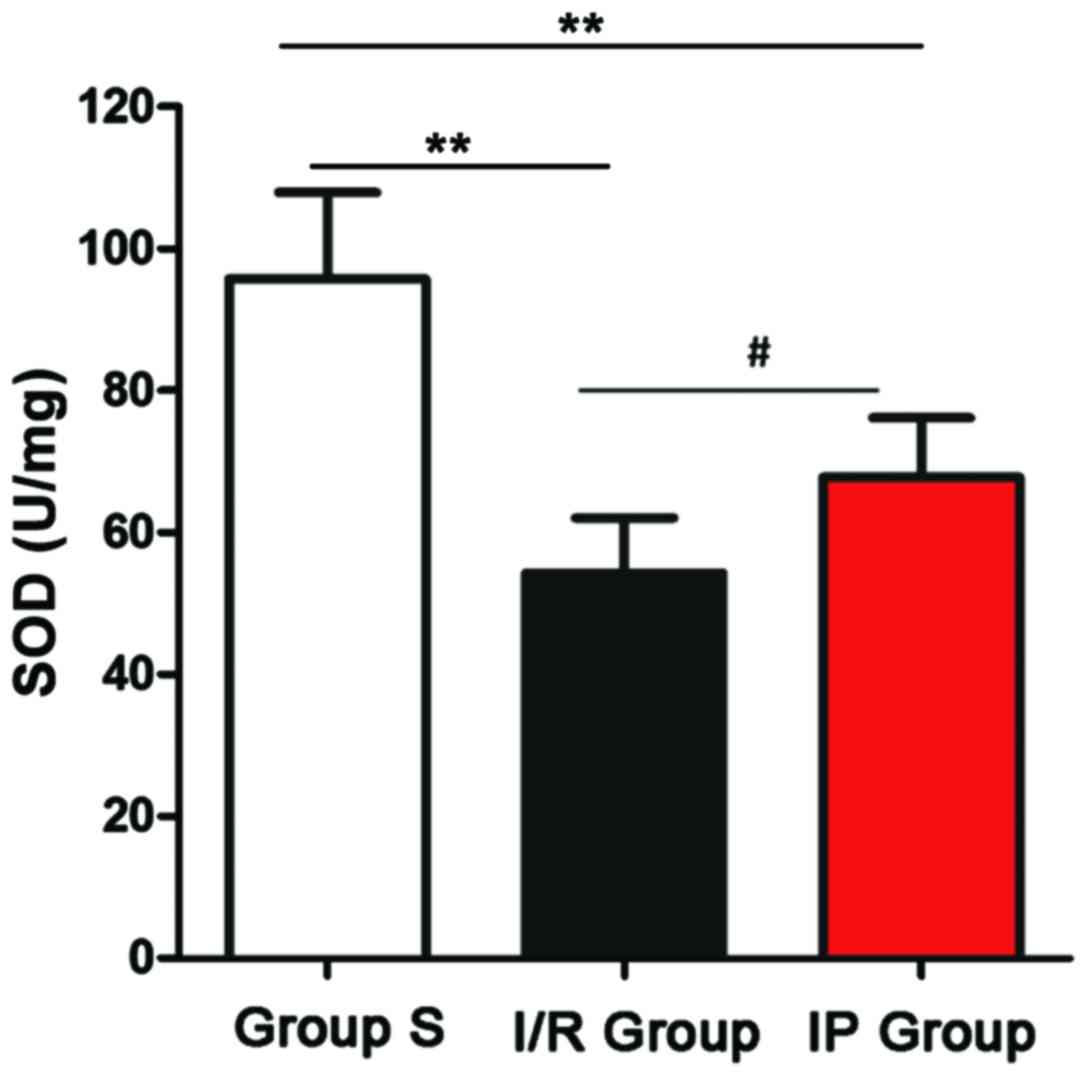
Changes in SOD activity
Activity of SOD was detected by SOD activity test
kit. As shown in Fig. 3, activity of
SOD in the I/R and IP groups was significantly lower than in the S
group (P<0.01), and activity of SOD in the I/R group was
significantly lower than in the IP group (P<0.05).
Pathological changes of the kidneys in
rats
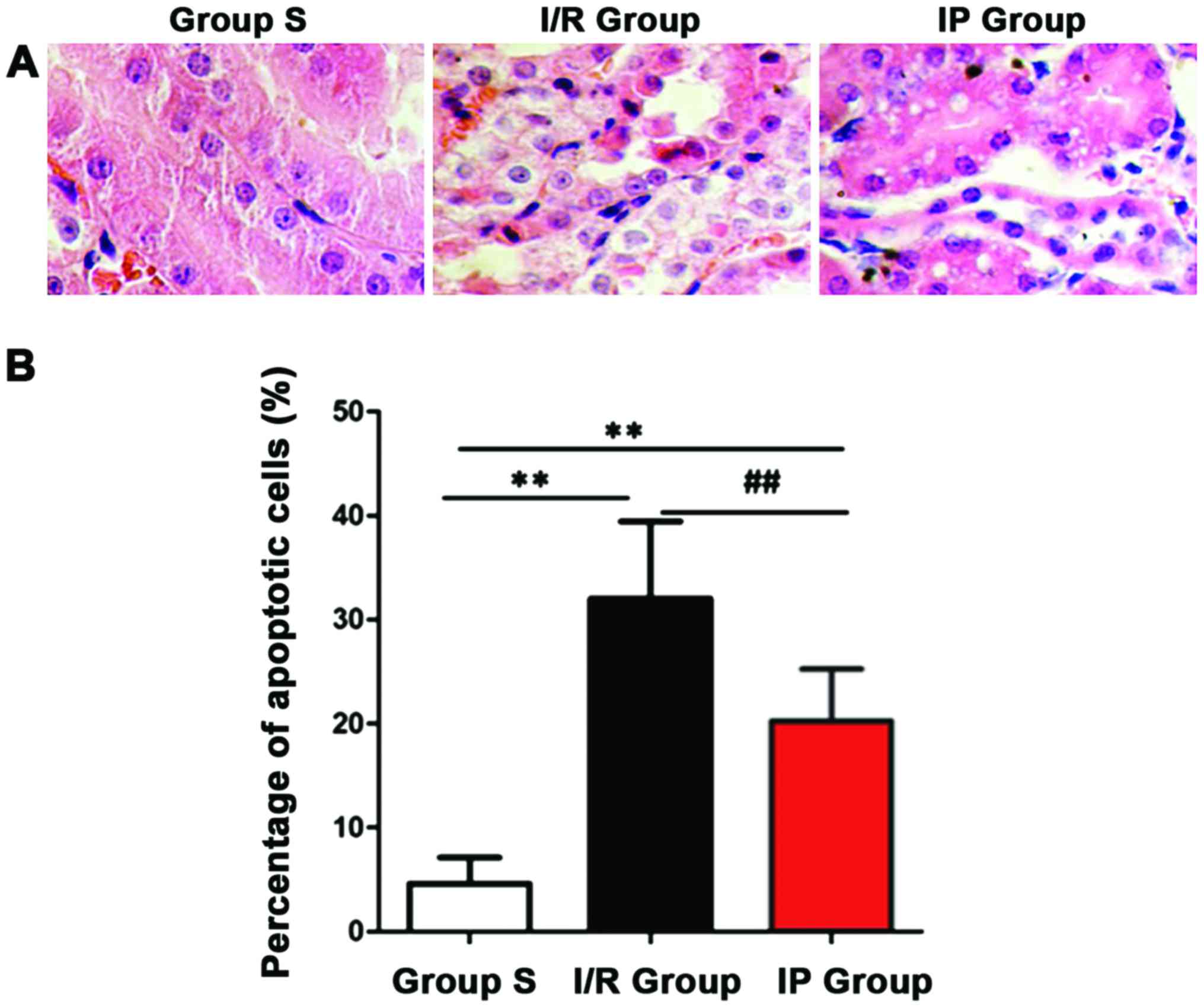
Pathological changes of renal tissue in each group
after H&E staining were observed under an optical microscope.
Structure of renal tissue in group S was clear, renal tubular and
glomerular were normal, degeneration and atrophy were not observed
in renal tubular epithelial cells, and the cavity was not expanded.
In the I/R and IP groups, renal tissue glomerular telangiectasia
and congestion, renal tubular epithelial cell edema, and granular
degeneration or vacuolar degeneration were observed and the cavity
was narrowed. After scoring (Table
II), renal injury was found to be more serious in the I/R and
IP groups than in the S group (P<0.01), and renal injury was
more serious in the I/R group than in the IP group (P<0.05).
 | Table II.Pathological changes of kidney in rats
of each group. |
Table II.
Pathological changes of kidney in rats
of each group.
| Groups | Renal injury
score |
|---|
| S group | 0.68±0.19 |
| I/R group |
2.39±0.29a |
| IP group |
1.89±0.33a,b |
Renal cell apoptosis
Apoptotic cells were detected using TUNEL kit.
Number of positive cells was counted and the apoptotic index was
calculated. As shown in Fig. 4,
compared with S group, number of apoptotic cells in I/R group and
IP group was significantly increased (P<0.01). Compared with IP
group, the number of apoptotic cells in I/R group was significantly
increased (P<0.01).
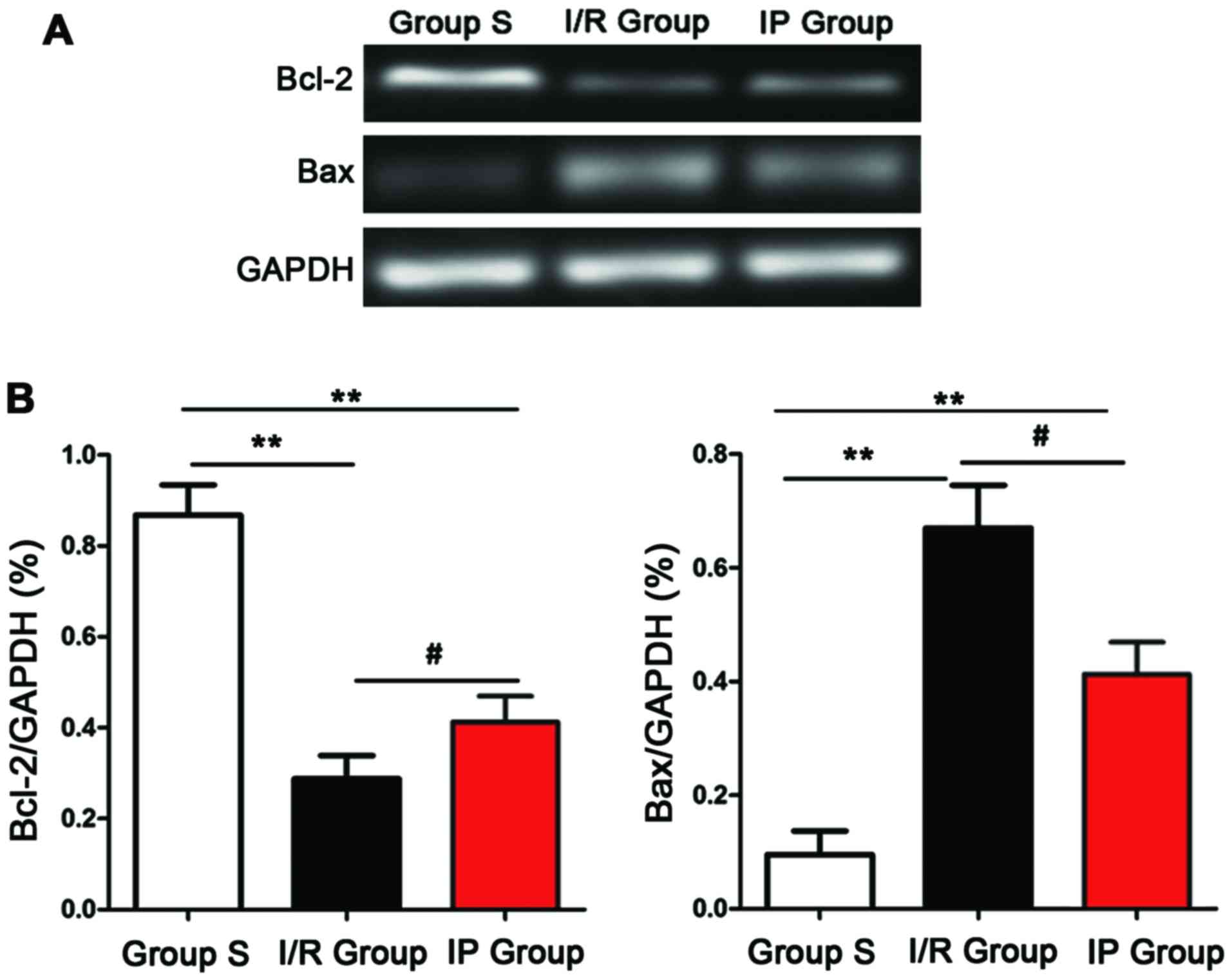
mRNA expression detected by
semi-quantitative PCR
Expression of Bcl-2 and Bax mRNA was detected by
semi-quantitative PCR. As shown in Fig.
5, compared with S group, expression level of Bcl-2 mRNA was
significantly decreased, expression level of Bax mRNA was
significantly increased, and the ratio of Bcl-2/Bax was
significantly decreased in IP group and I/R group (P<0.01).
Compared with I/R group, expression level of Bcl-2 mRNA was
significantly increased, expression level of Bax mRNA was
significantly deceased, and the ratio of Bcl-2/Bax was
significantly increased in IP group (P<0.05).
Protein expression detected by western
blot analysis
Expression of Bcl-2 and Bax protein was detected by
western blot analysis. As shown in Fig.
6, compared with S group, expression level of Bcl-2 protein was
significantly decreased, expression level of Bax protein was
significantly increased, and the ratio of Bcl-2/Bax was
significantly decreased in IP group and I/R group (P<0.01).
Compared with I/R group, expression level of Bcl-2 protein was
significantly increased, expression level of Bax protein was
significantly deceased, and the ratio of Bcl-2/Bax was
significantly increased in IP group (P<0.01).
Discussion
Renal blood flow needs to be blocked in renal
transplantation, nephrolithotomy and surgical resection of renal
tumors and other complex surgeries, and renal reperfusion after
blood flow blocking can cause renal damage (10). Numerous studies have shown that
ischemia-reperfusion injury may be related to the production of
oxygen free radicals, adenine nucleotide metabolic disorders and
cell apoptosis (11). Drugs
(ulinastatin and propofol), low temperature, preconditioning and
postconditioning can play a protective role in ischemia-reperfusion
injury, and all these methods can be used to reduce
ischemia-reperfusion injury to the kidneys (12–14).
In this study, rat ischemia-reperfusion model was
established and levels of Cr and BUN in serum were detected to
evaluate the degree of renal injury. Results showed that levels of
Cr and BUN in the serum of rats in I/R group were significantly
higher than those of S group, and the difference was statistically
significant (P<0.01), indicating the successful establishment of
rat ischemia-reperfusion model. Ischemia-reperfusion model was
established by clamping bilateral renal pedicle. Reperfusion time
and clamping sites can seriously affect the stability of the model.
Preliminary experiment showed that reperfusion could significantly
affect renal function and the mode was stable. This study showed
that preconditioning performed by clamping bilateral renal pedicle
for 5 min and reperfusion for 5 min could improve the renal
ischemia-reperfusion injury in rats. Levels of Cr and BUN in IP
group were higher than those in the I/R group (P<0.01). Ischemic
preconditioning can induce the production of endogenous protective
substances in the body to resist ischemia and reperfusion injury to
organs and tissues, and this protective effect in mulple organs
have been proved by various studies (15,16). Li
and Liu (17) found that ischemic
preconditioning can effectively reduce the effects of
ischemia-reperfusion on myocardial function. Lipid peroxidation
caused by oxygen free radical is one of the factors that cause
ischemia-reperfusion injury. SOD activity can be used to indirectly
assess the ability of the body to scavenge oxygen free radicals
(18). In this study, compared with
S group and IP group, SOD activity in I/R group was significantly
reduced, indicating that ischemia-reperfusion could cause renal
injury and ischemic preconditioning can protect the body. As the
currently most commonly used method to study cell apoptosis, TUNEL
can be used to directly observe apoptotic cells, and tissue or cell
apoptosis can be evaluated by calculating apoptosis index. Bcl gene
family, which contains anti-apoptotic gene Bcl-2 and pro-apoptotic
gene Bax, is closely related to cell apoptosis. Bcl-2 can inhibit
cell apoptosis by inhibiting the production of free radicals,
intracellular calcium overload, reducing the permeability of
mitochondrial membranes and maintaining the oxidative function of
mitochondria, while Bax has opposite functions (19,20).
This study found that, compared with S group, cell apoptosis was
significantly increased in I/R group, and results of
semi-quantitative PCR and western blot analysis showed that,
compared with S group, expression levels of Bcl-2 mRNA and protein
were significantly decreased, expression levels of Bax mRNA and
protein were significantly increased, and the ratio of Bcl-2/Bax
was significantly decreased in IP group and I/R group. Compared
with I/R group, expression level of Bcl-2 was significantly
increased, expression level of Bax was significantly deceased, and
the ratio of Bcl-2/Bax was significantly increased in IP group.
Those results suggest that ischemia-reperfusion can lead to
increased cell apoptosis, while ischemic preconditioning can
increase the membrane stability and protect the kidneys by
upregulating the expression of Bcl-2 and downregulating the
expression of Bax in renal tissue.
In conclusion, ischemic preconditioning can protect
rats with renal ischemia-reperfusion injury possibly by increasing
the expression level of Bcl-2 and decreasing the expression level
of Bax. The repeated short-term ischemia-reperfusion treatment can
be applied in clinical practice to induce the injury to kidneys
caused by ischemia-reperfusion.
References
|
1
|
Kim Y, Ge Y, Tao C, Zhu J, Chapman AB,
Torres VE, Yu AS, Mrug M, Bennett WM, Flessner MF, et al:
Consortium for Radiologic Imaging Studies of Polycystic Kidney
Disease (CRISP): Automated segmentation of kidneys from MR images
in patients with autosomal dominant polycystic kidney disease. Clin
J Am Soc Nephrol. 11:576–584. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fox CS, Matsushita K, Woodward M, Bilo HJ,
Chalmers J, Heerspink HJ, Lee BJ, Perkins RM, Rossing P, Sairenchi
T, et al: Chronic Kidney Disease Prognosis Consortium: Associations
of kidney disease measures with mortality and end-stage renal
disease in individuals with and without diabetes: A meta-analysis.
Lancet. 380:1662–1673. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haynes R, Staplin N, Emberson J,
Herrington WG, Tomson C, Agodoa L, Tesar V, Levin A, Lewis D, Reith
C, et al: SHARP Collaborative Group: Evaluating the contribution of
the cause of kidney disease to prognosis in CKD: Results from the
Study of Heart and Renal Protection (SHARP). Am J Kidney Dis.
64:40–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ta MH, Schwensen KG, Foster S, Korgaonkar
M, Ozimek-Kulik JE, Phillips JK, Peduto A and Rangan GK: Effects of
TORC1 inhibition during the early and established phases of
polycystic kidney disease. PLoS One. 11:e01641932016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takahashi-Sato K, Murakawa M, Kimura J,
Ito MA and Matsuoka I: Loss of ectonucleotidases from the coronary
vascular bed after ischemia-reperfusion in isolated rat heart. BMC
Cardiovasc Disord. 68:2577–2584. 2016.
|
|
6
|
Chua S, Lee FY, Tsai TH, Sheu JJ, Leu S,
Sun CK, Chen YL, Chang HW, Chai HT, Liu C, et al: Inhibition of
dipeptidyl peptidase-IV enzyme activity protects against myocardial
ischemia-reperfusion injury in rats. J Transl Med. 19:285–297.
2016.
|
|
7
|
Wang K, Zhang J, Liu J, Tian J, Wu Y, Wang
X, Quan L, Xu H, Wang W and Liu H: Variations in the protein level
of Omi/HtrA2 in the heart of aged rats may contribute to the
increased susceptibility of cardiomyocytes to ischemia/reperfusion
injury and cell death: Omi/HtrA2 and aged heart injury. Age
(Dordr). 30:266–271. 2016.
|
|
8
|
Huang P, Sun Y, Yang J, Chen S, Liu AD,
Holmberg L, Huang X, Tang C, Du J and Jin H: The ERK1/2 signaling
pathway is involved in sulfur dioxide preconditioning-induced
protection against cardiac dysfunction in isolated perfused rat
heart subjected to myocardial ischemia/reperfusion. Int J Mol Sci.
206:26–32. 2016.
|
|
9
|
ONeill KL, Huang K, Zhang J, Chen Y and
Luo X: Inactivation of prosurvival Bcl-2 proteins activates Bax/Bak
through the outer mitochondrial membrane. Genes Dev. 30:973–988.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lindqvist LM, Heinlein M, Huang DC and
Vaux DL: Prosurvival Bcl-2 family members affect autophagy only
indirectly, by inhibiting Bax and Bak. Invest New Drugs.
34:713–725. 2016.
|
|
11
|
Zhang WZ, Li R, Liu S, Zhang JD, Ning XF
and Cai SL: Effects of renal ischemic postconditioning on
myocardial ultrastructural organization and myocardial expression
of Bcl-2/Bax in rabbits. BioMed Res Int. 2016:93494372016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen S, Wang JF, Wu JQ, Zhou JX, Meng SD,
Ma J, Zhu CL, Deng GG and Liu D: GC/MS-based metabolomic analysis
of alleviated renal ischemia-reperfusion injury induced by remote
ischemic preconditioning. Eur Rev Med Pharmacol Sci. 21:765–774.
2017.PubMed/NCBI
|
|
13
|
Tawfik MK: Renoprotective activity of
telmisartan versus pioglitazone on ischemia/reperfusion induced
renal damage in diabetic rats. Eur Rev Med Pharmacol Sci.
16:600–609. 2012.PubMed/NCBI
|
|
14
|
Park SY, Shrestha S, Youn YJ, Kim JK and
Kim SY: Role of Toll-like receptor-4 in renal graft
ischemia-reperfusion injury. Asian Pac J Trop Med. 9:2–9. 2016.
|
|
15
|
Kierulf-Lassen C, Kristensen ML, Birn H,
Jespersen B and Nørregaard R: No effect of remote ischemic
conditioning strategies on recovery from renal ischemia-reperfusion
injury and protective molecular mediators. BMC Cancer. 16:686–691.
2016.PubMed/NCBI
|
|
16
|
Chen YT, Tsai TH, Yang CC, Sun CK, Chang
LT, Chen HH, Chang CL, Sung PH, Zhen YY, Leu S, et al: Exendin-4
and sitagliptin protect kidney from ischemia-reperfusion injury
through suppressing oxidative stress and inflammatory reaction. J
Transl Med. 11:2702013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y and Liu S: The effect of
dexmedetomidine on oxidative stress response following cerebral
ischemia-reperfusion in rats and the expression of intracellular
adhesion molecule-1 (ICAM-1) and S100B. Med Sci Monit. 23:867–873.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Batinic-Haberle I, Tovmasyan A, Roberts
ER, Vujaskovic Z, Leong KW and Spasojevic I: SOD therapeutics:
Latest insights into their structure-activity relationships and
impact on the cellular redox-based signaling pathways. Antioxid
Redox Signal. 20:2372–2415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Gan W and An G: Influence of
Tanshinone IIa on heat shock protein 70, Bcl-2 and Bax expression
in rats with spinal ischemia/reperfusion injury. Neural Regen Res.
7:2882–2888. 2012.PubMed/NCBI
|
|
20
|
Ma Z, Wei Q, Dong G, Huo Y and Dong Z: DNA
damage response in renal ischemia-reperfusion and ATP-depletion
injury of renal tubular cells. Biochim Biophys Acta.
1842:1088–1096. 2014. View Article : Google Scholar : PubMed/NCBI
|